Phosphorylation of a monomeric antenna protein correlates with increased photoprotective energy dissipation in monocots and is controlled by distinct enzymes compared with trimeric major antennae.
Abstract
Phosphorylation of the photosystem II antenna protein CP29 has been reported to be induced by excess light and further enhanced by low temperature, increasing resistance to these stressing factors. Moreover, high light-induced CP29 phosphorylation was specifically found in monocots, both C3 and C4, which include the large majority of food crops. Recently, knockout collections have become available in rice (Oryza sativa), a model organism for monocots. In this work, we have used reverse genetics coupled to biochemical and physiological analysis to elucidate the molecular basis of high light-induced phosphorylation of CP29 and the mechanisms by which it exerts a photoprotective effect. We found that kinases and phosphatases involved in CP29 phosphorylation are distinct from those reported to act in State 1-State 2 transitions. In addition, we elucidated the photoprotective role of CP29 phosphorylation in reducing singlet oxygen production and enhancing excess energy dissipation. We thus established, in monocots, a mechanistic connection between phosphorylation of CP29 and nonphotochemical quenching, two processes so far considered independent from one another.
In eukaryotic photosynthesis, light-dependent reactions are performed by two supramolecular complexes, PSII and PSI, which catalyze light harvesting and electron transport from water to NADP+. To this aim, water is oxidized by PSII, which, in turn, is oxidized by PSI, which becomes a reductant for ferredoxin-NADP(+) oxidoreductase and NADP+ (Nelson and Ben-Shem, 2004). The two photosystems are functionally connected by the plastoquinone (PQ) and cytochrome (cyt) b6/f, which catalyze the building of the transthylakoid proton gradient, which is dissipated by ATP synthase (ATPase) activity for ATP synthesis from ADP and inorganic phosphate (Pi). PSII and PSI have clearly distinct absorption spectra, with PSI-light-harvesting complex I (LHCI) complexes being enriched in red-shifted absorption forms (Gobets and van Grondelle, 2001). Within canopies, this leads to differential excitation depending on available light quality. This effect needs to be compensated to avoid imbalance of electron transport rates, yielding into either photoinhibition or decrease of photon use efficiency. Two major regulatory mechanisms counteract these effects. (1) State 1-State 2 transitions are active in limiting light conditions (Rintamäki et al., 2000) and inhibited by reduction of a disulfide bridge in high light (HL; Lemeille et al., 2009). This mechanism is activated by overreduction of PQ to plastoquinol (PQH2) through activation of a thylakoid bound kinase, STN7, acting on LHCII (Depège et al., 2003; Bellafiore et al., 2005). This causes a fraction of PSII antenna system, mainly Lhcb2 (Leoni et al., 2013), to be transferred to PSI in stroma-exposed membranes. The consequent increase in PSI antenna size (Galka et al., 2012) bursts the electron transfer rate and reequilibrates PQ/PQH2 redox poise, thus causing feedback inactivation of kinase activity. A phosphatase, PPH1-TAP38 (Pribil et al., 2010; Shapiguzov et al., 2010), dephosphorylates LHCII, allowing for its return to PSII in grana partitions. (2) The process of nonphotochemical quenching (NPQ) is rather aimed to photoprotection from excess light (Horton and Ruban, 2005; de Bianchi et al., 2010). In this condition, saturation of downstream metabolic reactions causes depletion of ADP and Pi and inhibition of ATPase activity, which normally brings back protons from lumen to the stromal compartment. This causes accumulation of protons in the lumen, triggering dissipation into heat of the energy absorbed in excess by PSII (de Bianchi et al., 2010; Niyogi and Truong, 2013). Thus, the synergic, although independent, activities of State-1-State 2 transitions and NPQ cover the needs for regulation of photosynthesis over the wide dynamic range of light intensity experienced by plants when shaded or by daily light changes. However, a number of experimental results do not fit within this scheme: phosphorylation of PSII antenna proteins, namely CP29 (LHCB4) has been reported to be induced in very HL and further enhanced by low temperature (Bergantino et al., 1995), implying inhibition of LHC protein phosphorylation by HL is not a general feature of plant photosynthesis. Moreover, CP29 phosphorylation has been shown to be protective in condition of HL combined with low temperature (Mauro et al., 1997), a major stressing factor limiting crop productivity. HL-induced CP29 phosphorylation has been specifically found in monocots, either C3 or C4 (Bergantino et al., 1998), which include the large majority of food crops, thus making the study of this process of interest for both basic and applied research. In dicots, CP29 phosphorylation has been detected at very low level (Fristedt and Vener, 2011) and targeted to different sites within the N-terminal domain with respect to monocots (Testi et al., 1996). Although early research focused on biochemical and physiological characterization of CP29 phosphorylation (Croce et al., 1996; Mauro et al., 1997; Hwang et al., 2003), genetic dissection of this regulation process has been hampered by lack of genetic resources. More recently, rice (Oryza sativa) has become a model organism for monocots, and knockout collections have become available. In this study, we have used reverse genetics coupled to biochemical and physiological analysis to elucidate the molecular basis of HL-induced phosphorylation of CP29 and the mechanisms by which it exerts a photoprotective effect. We found that a different set of kinases and phosphatases is involved in CP29 reversible phosphorylation with respect to that reported to act in State 1-State 2 transitions and that the photoprotection effect is mediated by an enhancement of excess energy dissipation. These results establish, for the first time, a mechanistic connection between thylakoid protein phosphorylation and NPQ, so far believed to be independent processes.
RESULTS
Kinetics of CP29 Phosphorylation and Recovery
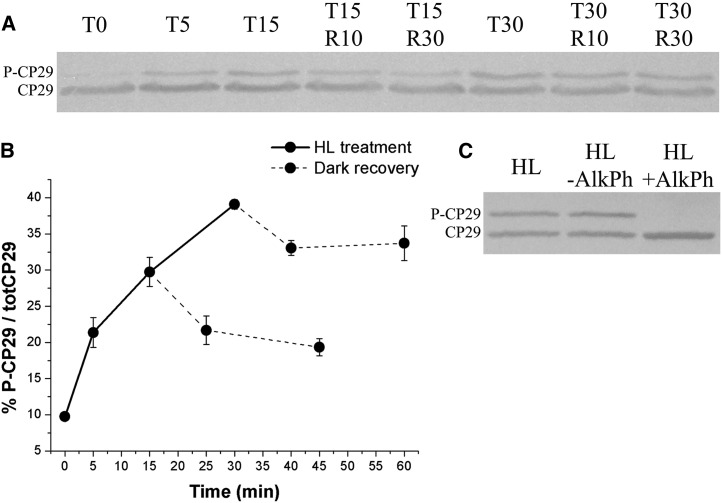
Rice leaves from plants grown in a greenhouse for 8 weeks and incubated in the dark for 6 h were exposed to white light of 1,000 μmol photons m–2 s–1 for different periods. After 15- or 30-min of illumination, leaves were further incubated in the dark. Samples were harvested at different times and cooled in ice water slurry, and chloroplasts were isolated. SDS-PAGE analysis of thylakoid proteins, followed by immunoblots with anti-CP29 antibody, detected two bands with apparent molecular mass of 30 and 34 kD (Fig. 1A). At t = 0, only the faster migrating band was detected. Upon light exposure, a slow migrating band appeared and accumulated with time, while the intensity of the fast-migrating band decreased. Densitometric analysis showed that the 34-kD band was accumulated to 30% and 40% upon 15 and 30 min of light exposure, respectively (Fig. 1B). Upon dark recovery, the relative amplitude of the 34-kD band decreased by 7% to 8% in the first 15 min, after which the decay was slower. Alkaline phosphatase-treated thylakoids only showed the 30-kD band (Fig. 1C), implying that the change in mobility of CP29 was due to phosphorylation, as previously shown in corn (Zea mays) and barley (Hordeum vulgare; Bergantino et al., 1998). These results show that rice CP29 was rapidly phosphorylated in HL and slowly dephosphorylated in the dark.
Figure 1.
CP29 phosphorylation kinetic in rice. A, Immunoblot of rice-isolated chloroplasts assayed with anti-CP29 polyclonal antibody. Before chloroplast isolation, leaves have been treated with HL (1,000 µmol photons m–2 s–1) for different time lengths (Tx indicates minutes of illumination after 6-h dark adaptation) and then dark incubated (Rx indicates minutes of dark incubation upon HL treatment). Tris-Gly SDS-PAGE 15% plus Urea 3M; 1 µg of total chlorophyll (Chl) per lane. B, Densitometrical analysis of immunoblot in A, determining the amount of P-CP29 with respect to the total amount of CP29 (totCP29). Average values have been obtained considering two independent biological replicates. C, Alkaline phosphatase (AlkPh) treatment on rice-isolated monomeric antenna complexes (obtained according to Betterle et al., 2009) from HL-treated samples. P-CP29 dephosphorylation has been evaluated as in A. One-quarter microgram of Chl per lane.
Identification of the CP29 Phosphorylation Site
Previous work with the monocot corn (Testi et al., 1996) showed HL-dependent phosphorylation of CP29 occurring at Thr-83 of the mature protein, a conserved site in many plant species (Chen et al., 2013). Different phosphorylation sites were reported for Arabidopsis (Arabidopsis thaliana) CP29 (AtCP29; Fristedt and Vener, 2011). To verify whether rice CP29 (OsCP29) was phosphorylated in corn- or Arabidopsis-like site(s) upon HL treatment, we analyzed the two CP29-reactive polypeptides by mass spectrometry (MS) analysis for the detection of phosphopeptides. To this aim, thylakoids from dark-adapted and HL-treated wild-type rice leaves were solubilized by n-Dodecyl β-d-maltoside and fractionated by Suc gradient ultracentrifugation (Supplemental Fig. S1A). The Suc bands 2 (B2) containing monomeric LHCB subunits, including CP29, was further fractionated by SDS-PAGE, and the presence of Phosphorylated CP29 (P-CP29) was verified by western blot (Supplemental Fig. S1B). Densitometry of the Coomassie Blue-stained gel yielded consistent results with western-blot analysis, implying that the antibody had similar reactivity for the phosphorylated and unphosphorylated forms of the polypeptide (Supplemental Fig. S2). The SDS-PAGE bands corresponding to CP29 (from both dark-adapted and HL-treated samples) and P-CP29 were excised and analyzed by liquid chromatography-MS analysis in triplicate. No phosphorylated fragments were detected from the 30-kD band (CP29), while a phosphorylated Thr residue was detected in a fragment from the 34-kD band (Supplemental Table S1). This phosphorylated site, Thr-82 in the sequence of the mature protein, was the same site previously identified on corn P-CP29 as Thr-83. Any other HL-related phosphorylated residues were not detected in rice P-CP29 from fractions isolated from the wild type. In low and HL, we found no evidence of phosphorylation at Thr-112 or Thr-114, which were previously identified as phosphorylation sites in the dicot Arabidopsis (Hansson and Vener, 2003), or the Thr-33 and Ser-103 residues identified as phosphorylation sites in Chlamydomonas reinhardtii (Lemeille et al., 2009). These results, together with the recovery of the 30-kD apparent molecular mass upon alkaline phosphatase treatment (Fig. 1C), imply that OsCP29 is phosphorylated into Thr-82 upon HL treatment only. Also, this posttranslational modification was responsible for the change in mobility of CP29 in SDS-PAGE gels and was the same modification occurring in corn (Testi et al., 1996). Moreover, we show that quantitative assessment of phosphorylated versus unphosphorylated forms of a specific protein, often difficult, becomes accessible in the case of CP29 because a single phosphorylation event causes change of SDS-PAGE mobility, thus allowing quantitative detection of phosphorylation based on Coomassie Blue stain or immunoblotting.
Roles of STN7 Kinase and PPH1 Phosphatase in CP29 Phosphorylation and Dephosphorylation
Previous work with Arabidopsis and C. reinhardtii showed that the LHC antenna proteins of PSII are phosphorylated by the STN7 kinase and dephosphorylated by the PPH1 phosphatase during State 1-State 2 transitions and that these gene products control the physiological changes associated, including the far-red light-induced changes of room temperature fluorescence, changes in 77-K emission spectra, and binding of LHCII to the PSI-LHCI complex (Depège et al., 2003; Bellafiore et al., 2005; Pribil et al., 2010; Shapiguzov et al., 2010).
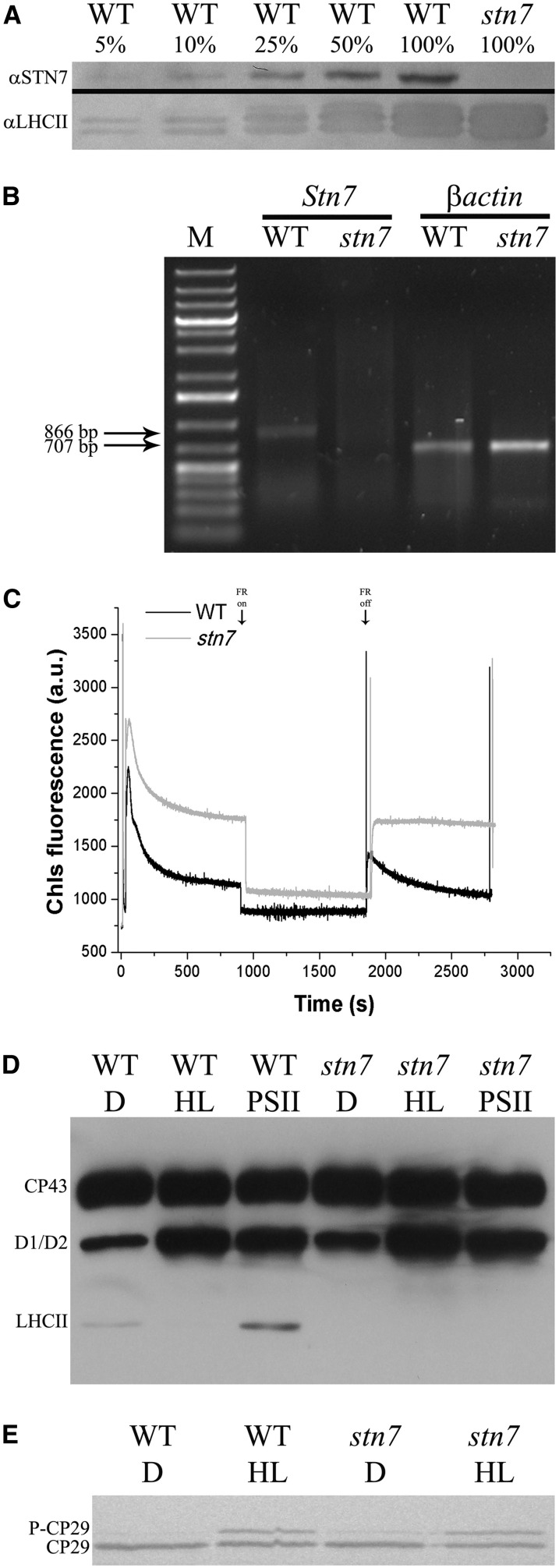
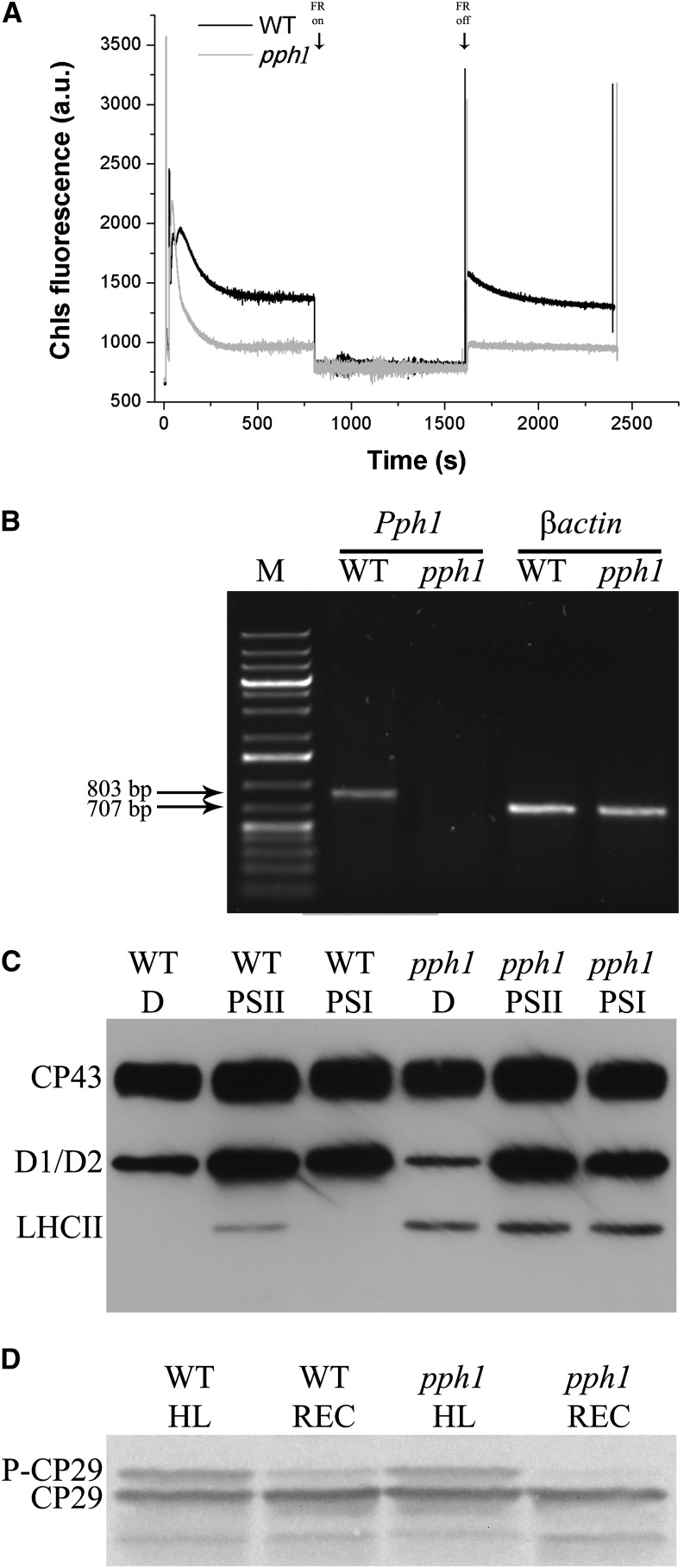
To investigate the possible involvement of STN7 kinase and PPH1 phosphatase in rice CP29 phosphorylation, we identified a mutant of rice lacking STN7 from Oryza Tag Line (Centre de Coopération Internationale en Recherche Agronomique pour le Développement [CIRAD]; rice ssp. japonica ‘Nipponbare,’ no. AJTH05) by screening the seeds by western blot using anti-STN7 antibody. In Figure 2A, we compared selected stn7 mutant with the wild type by immunoblotting analysis at different dilution: The signal for STN7 protein was detectable in the wild type but not in the mutant lanes even upon 20-fold dilution, suggesting that the level of STN7 kinase in the stn7 mutant was at least 20 times lower than in the wild type. The insertion site in this stn7 mutant was within an intronic region (OryGenesDB rice mutant database, http://orygenesdb.cirad.fr/; LOC_Os05g47560; Supplemental Fig. S3). We then proceeded to assess whether there was any level of leakiness in this genotype by analyzing the transcription of STN7 gene (Fig. 2B). It is shown that no Stn7 mRNA could be amplified in the mutant, while it was evident in the wild type. The phenotype of the stn7 mutant (Fig. 2C) was also characterized by a stable fluorescence level upon removal of far-red light. When compared to the contrasting behavior of the wild type, undergoing a fast fluorescence decline in the same conditions, this result strongly supports the mutant phenotype corresponded to the original stn7 mutant (Bellafiore et al., 2005). In addition, stn7 mutant exhibited a higher stationary fluorescence, implying plastoquinone pool was overreduced with respect to the wild type, likely due to a decreased excitation of PSI compared with the wild type. A knockout PPH1 (TAP38) mutant was obtained from Rice Mutant Database (rice ssp. japonica ‘Zhonghua 15,’ no. 04Z11OK94). In the absence of an antibody able to recognize rice PPH1 protein, we screened lines by pulse amplitude-modulated fluorometry (Fig. 3A). Mutant plants were characterized by a faster fluorescence decrease upon exposure to blue light with respect to the wild type (Fig. 3A) and by a stable level of fluorescence upon removal of far-red light, implying that State 1-State 2 transitions, once induced by blue light, did not relax within the time of the measurement. Even in the case of pph1 mutant, the insertion site was mapped in an intronic sequence (Supplemental Fig. S3; http://orygenesdb.cirad.fr/; Loc_Os01g37130), and the analysis of the transcription of the gene, however, revealed the mutant level of the mRNA below detection (Fig. 3B).
Figure 2.
Isolation and characterization of rice stn7 mutant. A, Immunoblot analysis using anti-STN7 antibody (αSTN7). Thylakoids have been isolated from wild-type (WT) and stn7 rice plants and then loaded on Tris-Tricine SDS-PAGE 10%. Wild-type 100% and stn7 100% correspond to 2 µg of Chl. An immunoblot analysis using anti-LHCII antibody αLHCII has been performed as an internal control. B, Reverse transcription (RT)-PCR measurement of gene-specific transcripts. Sequences of the oligonucleotides used are reported in “Materials and Methods.” The expected sizes of the PCR products are as follows: Stn7, 866 bp; and βactin, 707 bp. M indicates molecular mass marker (1-kb Plus Ladder, Thermo Scientific). C, Analysis of state transition in stn7 mutant. Chlorophyll fluorescence emission was measured upon treatment with blue light and blue light supplemented with far-red (FR) light, which induce transition to State 2 and State 1, respectively. a.u., Arbitrary unit. D, Analysis of thylakoid phosphoproteins using anti P-Thr (Cell Signaling) antibody. Rice wild-type and stn7 mutant leaves were either dark adapted (D) or treated with HL (1,500 µmol photons m–2 s–1, 30 min) or PSII-specific light (PSII; 100 µmol photons m–2 s–1, 1 h, orange filter), and then thylakoids have been collected (Suorsa et al., 2004). Tris-Gly SDS-PAGE 15% plus Urea 3M; 0.75 µg of Chl per lane. E, Evaluation of rice stn7 mutant capacity to phosphorylate CP29 upon HL induction (1,500 µmol photons m–2 s–1, 30 min). Wild-type and stn7 rice leaves were illuminated, and then thylakoids have been collected and loaded on SDS-PAGE. Immunoblot analysis as in Figure 1A. One microgram of Chl per lane.
Figure 3.
Isolation and characterization of rice pph1/tap38 mutant. A, Analysis of state transition in pph1 mutant. Chlorophyll fluorescence emission was measured upon treatment with blue light and blue light supplemented with far-red (FR) light, which induce transition to State 2 and State 1, respectively. a.u., Arbitrary unit. B. RT-PCR measurement of gene-specific transcripts. Sequences of the oligonucleotides used are reported in “Materials and Methods.” The expected sizes of the PCR products are as follows: Pph1, 803 bp, and βactin, 707 bp. M indicates molecular mass marker (1-kb Plus Ladder, Thermo Scientific). C, Analysis of thylakoid phosphoproteins using anti P-Thr (Cell Signaling) antibody. Rice wild-type (WT) and pph1 mutant leaves were either dark adapted (D) or treated with PSII-specific light (PSII; 50 µmol photons m–2 s–1, 1 h, orange filter) or PSII-specific light followed by far-red illumination (PSI; 30 min), and then thylakoids have been collected (Suorsa et al., 2004). Tris-Gly SDS-PAGE 15% plus Urea 3M; 0.75 µg of Chl per lane. D, Evaluation of rice pph1 mutant capacity to dephosphorylate P-CP29 upon dark incubation. Wild-type and pph1 rice leaves, before thylakoid isolation, were illuminated (1,000 µmol photons m–2 s–1, 30 min) and then dark incubated for P-CP29 dephosphorylation (30 min) and loaded on SDS-PAGE. Immunoblot analysis as in Figure 1A. One microgram of Chl per lane.
The relation between the fluorescence phenotype and the phosphorylation pattern in the two mutants was assessed by using antiphospho-Thr antibodies to detect changes in polypeptide phosphorylation pattern upon treatments with light conditions either favoring PQ reduction or oxidation and HL (stn7, Fig. 2D; pph1, Fig. 3C).
In dark-adapted wild-type leaves, P-LHCII could not be detected, while a clear signal appeared upon treatment with PSII light. An excess light treatment (1,000 μmol photons m–2 s–1) made P-LHCII signal disappear again (Fig. 2D). In addition to P-LHCII signal, two more P-Thr reactive bands were detected in all samples with mobility corresponding to CP43 and D1/D2 proteins. While the level of P-CP43 was high in all conditions, the P-D1/D2 signal was lower in the dark-adapted samples and highest in those treated with PSII light and excess light. Consistent with the role of STN7 and PPH1 gene products assessed in Arabidopsis, the stn7 mutant lacked P-LHCII signal in all conditions (Fig. 2D), while the pph1 mutant had constitutive levels of P-LHCII (Fig. 3C).
The above results show that stn7 mutation was effective in preventing the onset of State 1-State 2 transitions of LHCII phosphorylation, while pph1 mutation prevented the State 2-State 1 reversion, implying no redundancy of their respective enzymatic activities occurred in rice as previously reported in Arabidopsis. On this basis, we proceeded to assess the effect of these two mutations in CP29 phosphorylation. Figures 2E and 3D show the results of anti-CP29 immunoblotting of thylakoids from wild-type, stn7, and pph1 rice plants incubated in the dark, treated with excess light, or further recovered in the dark for 30 min. The three genotypes showed the same behavior as for the CP29 immunoreactive band pattern, implying that STN7 and PPH1 were not involved in CP29 phosphorylation in HL and recovery in the dark. Because the effect of stn7 and pph1 mutations had a complete control over LHCII phosphorylation and de-phosphorylation (Figs. 2D and 3C), this implies that these two phosphorylation/dephosphorylation events are controlled by distinct kinase/phosphatase systems.
An in Vitro Assay for LHCB4 Phosphorylation
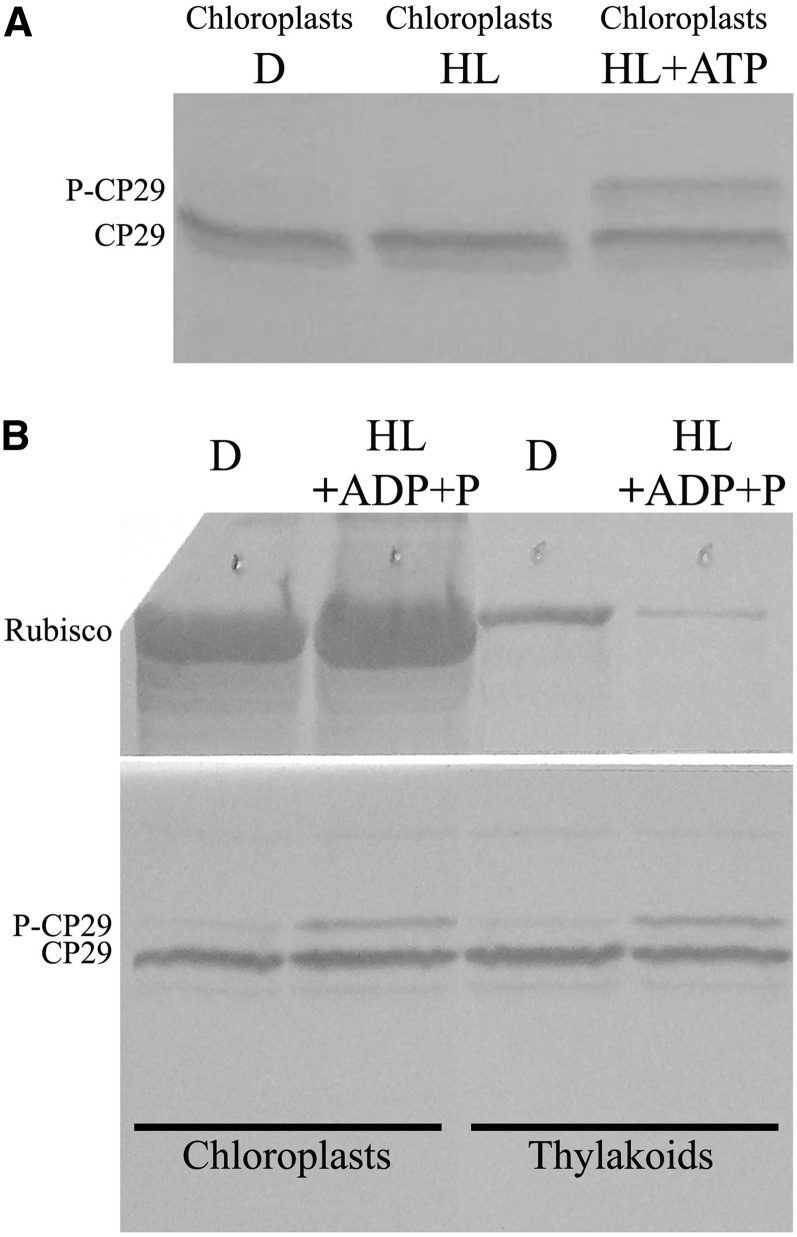
To gain additional information of the mechanisms controlling CP29 phosphorylation, we verified the possibility of reproducing phosphorylation in vitro. To this aim, we isolated fully active chloroplasts from dark-adapted wild-type rice leaves that were incubated in the dark or exposed to HL (1,000 µmol m–2 s–1) for 30 min with or without the addition of ATP. Aliquots were then submitted to SDS-PAGE and immunoblotting for detection of CP29 versus P-CP29 (Fig. 4A). Dark and HL-ATP samples only showed the unphosphorylated CP29 band, while HL plus ATP conditions led to the appearance of the 34-kD band corresponding to P-CP29. Similar results could be obtained in HL in presence of ADP plus Pi rather than ATP (Fig. 4B). The level of P-CP29 obtained was similar among functional chloroplasts and thylakoids, suggesting the kinase responsible for this reaction was associated with the thylakoid membranes.
Figure 4.
In vitro assay for CP29 phosphorylation. A, In vitro phosphorylation of CP29 in isolated functional chloroplasts (Casazza et al., 2001) illuminated with white light of 1,000 µmol photons m–2 s–1 for 30 min and in the presence of exogenous ATP. Immunoblot analysis as in Figure 1A. One microgram of Chl per lane. B, Determination of CP29 kinase localization by inducing CP29 phosphorylation in isolated functional chloroplasts or thylakoids, illuminated with white light of 1,000 µmol photons m–2 s–1 for 30 min in the presence of exogenous ADP and P+. Immunoblot analysis has been performed using anti-CP29 and anti-Rubisco antibody. One microgram of Chl per lane.
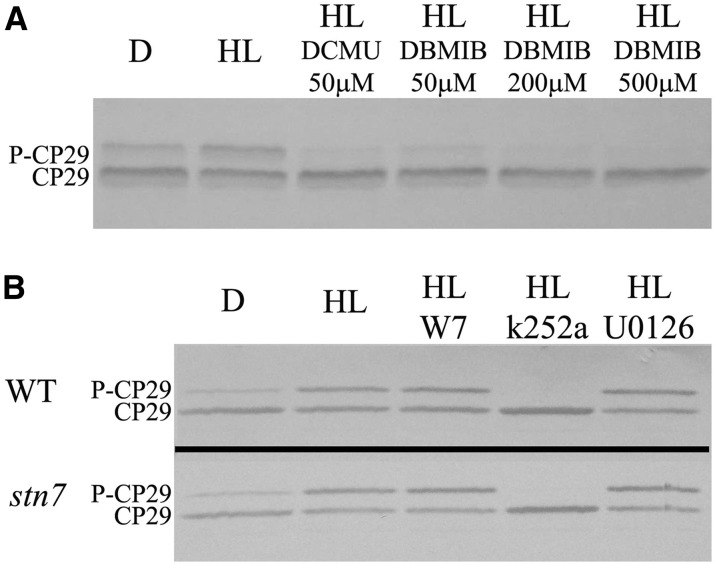
We then utilized the in vitro assay for performing a pharmacological analysis of CP29 phosphorylation on isolated chloroplasts. We first proceeded to verify the effect of controlling the redox state of the PQ pool and cyt b6/f complex. To this aim, chloroplasts isolated from dark-adapted rice leaves were illuminated (1,000 µmol m–2 s–1, 30 min) in the presence of 50 µm 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU; Fig. 5A), leading to a strong reduction of P-CP29 accumulation. Also, the addition of 50 µm 2,5-dibromo-6-isopropyl-3-methyl-1,4-benzoquinone (DBMIB) yielded a strong reduction of P-CP29 level, which became undetectable when increasing the inhibitor concentration to 200 or 500 µm. Because DCMU and DBMIB respectively decrease and increase the redox state of PQH2, these results indicate that the CP29 kinase is activated by the reduction PQH2 and/or cyt b6/f.
Figure 5.
Pharmacological analysis of CP29 kinase activation. A, In vitro redox regulation of CP29 phosphorylation by DCMU and DBMIB and chemicals controlling plastoquinone reduction state (Casazza et al., 2001). Functional chloroplasts were illuminated for 30 min with white light of 1,000 µmol photons m–2 s–1 in the presence of these redox regulators and then loaded on SDS-PAGE. Immunoblot analysis as in Figure 1A. Three-quarter microgram of Chl per lane. D, Dark-adapted samples. B, Effect of kinase inhibitors on CP29 phosphorylation in isolated chloroplasts. Inhibitors used were W7 (CDPK inhibitors), U0126 (MAPK inhibitor), and k252a (Ser/Thr kinase inhibitor; Chen et al., 2009). Functional chloroplasts were illuminated for 30 min with white light of 1,000 µmol photons m–2 s–1 in the presence of these chemicals and then loaded on SDS-PAGE. Immunoblot analysis as in Figure 1A. Three-quarter micrograms of Chl per lane. WT, Wild type.
The study of P-CP29 function requires a system for selective inhibition of CP29 phosphorylation or the availability of a mutant lacking the CP29 kinase, so far not identified. Alternatively, selective prevention of P-CP29 accumulation could be obtained by the use of kinase inhibitors (Chen et al., 2009) that could be tested in the in vitro phosphorylation assay, thus avoiding the problems of permeability often encountered in vivo. As described by Chen et al. (2009), we tested different kinase inhibitors: U0126, an inhibitor of Mitogen-activated protein kinase (MAPK), W7, an inhibitor of Ca2+-dependent protein kinase, and k252a, an alkaloid kinase inhibitor derived from the fungus Nocardiopsis spp. (Ruegg et al., 1989). When rice chloroplasts from dark-adapted leaves were treated with HL in the presence of U0126 and W7 inhibitors, we obtained the same P-CP29 level as in the control sample, treated with HL but without inhibitor (Fig. 5B). Differently, in the presence of k252a, no P-CP29 was detected, irrespective from light conditions, implying this alkaloid is a strong inhibitor of the CP29 kinase in agreement with previous results (Chen et al., 2009). We used 25 μm for U0126, 100 μm W7, and 20 μm K252a (Chen et al., 2009). The inhibitory concentration value for CP29 phosphorylation in rice chloroplast (chlorophyll concentration of 40 μg mL–1) by k252a was determined at 2 µm (Supplemental Fig. S4). It is interesting to note that in the presence of k252a, P-CP29 level is even lower than in dark-adapted thylakoids, implying that, upon blocking of the CP29 kinase, a phosphatase specific for CP29 can proceed. It is also interesting to note that k252a strongly reduces the intensity of the two high molecular mass bands in the immunoblotting with antiphospho-Thr (Supplemental Fig. S5), suggesting this is also an inhibitor of the kinase(s) involved in PSII core complex phosphorylation.
PSII Photoprotection and P-CP29
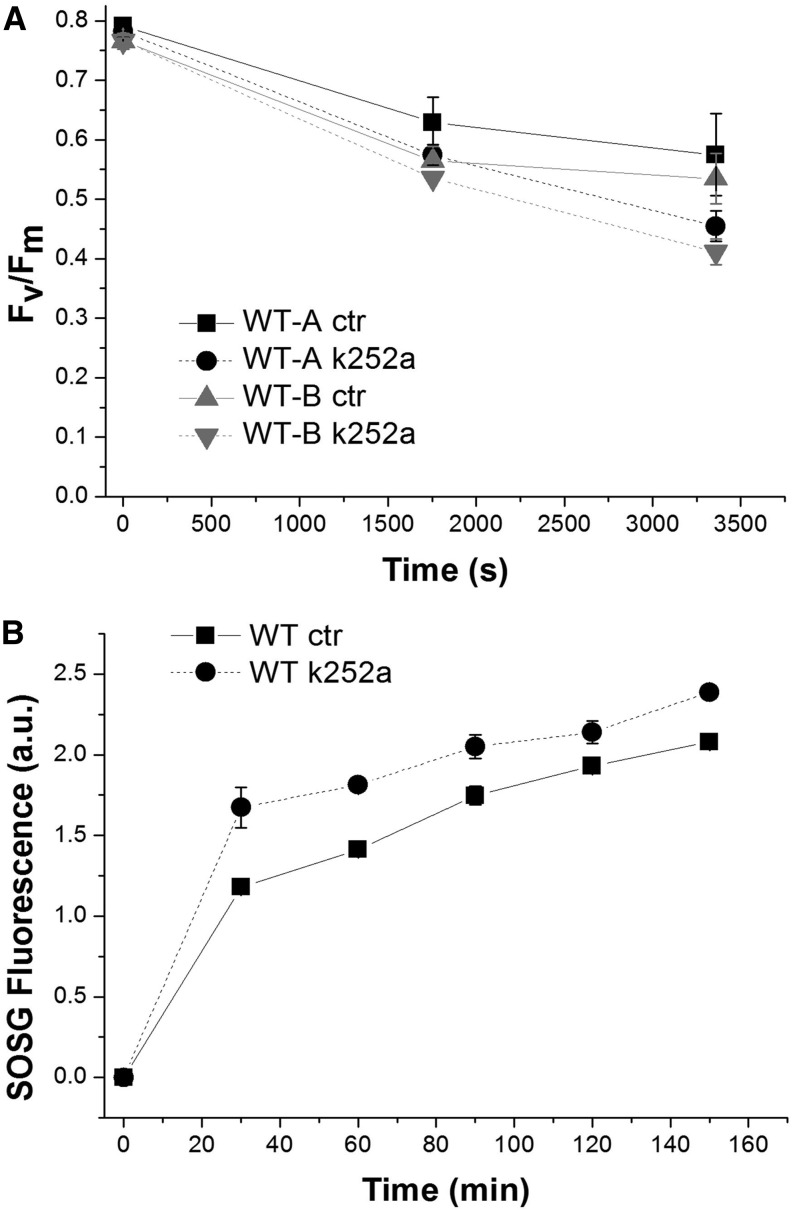
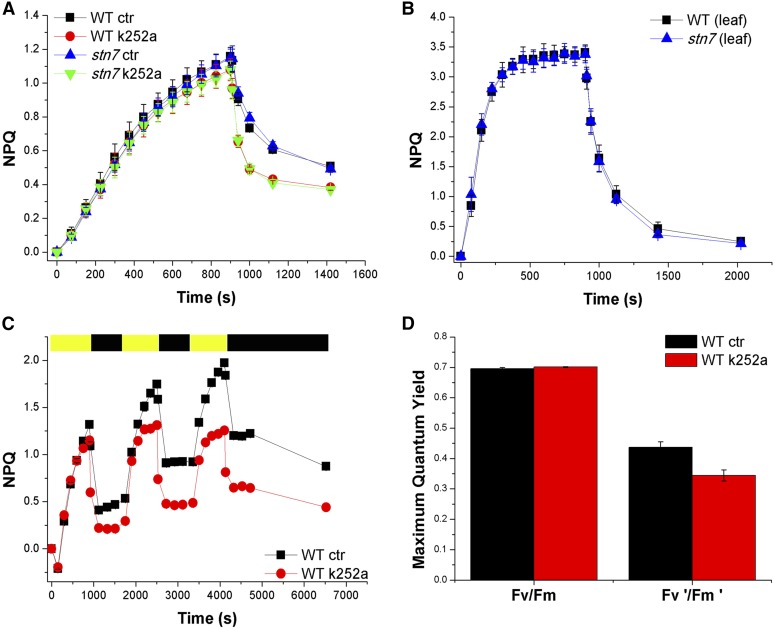
The availability of an inhibitor for P-CP29 accumulation allows for experimental verification of the physiologic effect of this phosphorylation event. Previously, a photoprotective effect was suggested based on the differential behavior of near-isogenic lines (Bergantino et al., 1995). To this aim, we exposed isolated chloroplasts at 1,000 µmol m–2 s–1 in the presence of and without k252a assessing photoinhibition by measuring the maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm). As reported in Figure 6A, the inhibitor caused a faster decrease of Fv/Fm during HL treatment, supporting the idea of a photoprotective activity for P-CP29. Photoinhibition is induced by ROS accumulation (Havaux et al., 2007; Dall’Osto et al., 2010): we measured the production of singlet oxygen during illumination of isolated chloroplasts by using a fluorescent probe (Flors et al., 2006; Dall’Osto et al., 2007; Betterle et al., 2010) and observed that the presence of k252a led to an increased production of 1O2 (Fig. 6B). NPQ dissipates light energy absorbed in excess, thus decreasing the production of 1O2 while CP29 was shown to have a major role in NPQ (de Bianchi et al., 2011) by activating zeaxanthin radical cation (Holt et al., 2005; Ahn et al., 2008). To verify the hypothesis that accumulation of P-CP29 was correlated to an increased NPQ activity, we measured NPQ in isolated chloroplasts in the presence or absence (control [CTR]) of k252a inhibitor (Fig. 7A). The chloroplast preparation obtained from rice showed a PSII quantum yield of 0.795 ± 0.05. NPQ measured in CTR was reproducible, yielding values of 1.15 ± 0.04 with an energy quenching (qE) component of 0.65 ± 0.03, as measured after 10-min recovery in the dark. Maximum NPQ amplitude was only slightly lower in presence of k252a (1.06 ± 0.04), while a strong effect was observed on dark relaxation kinetics, twice as faster in k252a-treated samples compared with CTR. Thus, the presence of P-CP29 could be associated with an increased inhibitory quenching (qI). Because the difference in NPQ activity increased with time during light exposure, we repeated the 15-min actinic light treatment three times with 10-min dark intervals in between. Upon three cycles of exposure to actinic light, CTR chloroplasts reached an NPQ value of 2.0 compared with an NPQ value of 1.25 in k252a-treated samples (Fig. 7C), implying that CP29 phosphorylation led to a 60% increase in NPQ activity, due to both an increase of qI and qE. It should be noticed that the increase of qI cannot be ascribed to photoinhibition because Fv/Fm was higher in CTR with respect to the k252a sample (Fig. 7D). It is interesting to note that the comparison between the inhibition of CP29 phosphorylation and the reduction in NPQ and qI yielded k252a concentration-dependent curves with a similar behavior (Supplemental Fig. S4).
Figure 6.
Effect of CP29 kinase inhibitor on photoprotection of intact chloroplasts. A, Fv/Fm in functional rice wild-type (WT) chloroplasts during illumination with white light of 1,000 µmol photons m–2 s–1. These chloroplasts have been treated in the presence or absence of Ser/Thr inhibitor k252a, and two independent batches of them have been analyzed. Ctr, Control samples. B, Singlet oxygen production in functional chloroplasts in the presence or absence of Ser/Thr inhibitor k252a. Functional chloroplasts have been treated with red light of 1,500 µmol photons m–2 s–1, and singlet oxygen production has been measured at different time points using the fluorescent probe Singlet Oxygen Sensor Green (SOSG; Betterle et al., 2010). a.u., Arbitrary unit.
Figure 7.
Effect of the CP29 kinase inhibitor k252a on NPQ activity of chloroplasts from wild-type (WT) and stn7 rice genotypes. A, Measurements of NPQ kinetics on rice chloroplasts in the presence or absence of Ser/Thr inhibitor k252a. Chloroplasts have been isolated from wild-type and stn7 rice dark-adapted leaves and then illuminated with actinic white light of 1,000 µmol photons m–2 s–1 at 23°C. Symbols and error bars show means ± sd (n = 4). Ctr, Control samples. B, Measurements of NPQ kinetics on wild-type and stn7 rice leaves illuminated with actinic white light of 1,000 µmol photons m–2 s–1 at 23°C. Symbols and error bars show means ± sd (n = 4). C, NPQ kinetics of wild-type and stn7 rice chloroplasts during three consecutive periods of illumination with white light (1,000 µmol photons m–2 s–1, 15 min, 23°C) with a 10-min period of darkness in between, as indicated by the yellow and black bars. Chloroplasts have been treated in the presence or absence of Ser/Thr inhibitor k252a. Symbols and error bars show means ± sd (n = 4). D, Fv/Fm in functional chloroplasts before (Fv/Fm) and after (Fv'/Fm') the treatment with actinic light 1,000 µmol m–2 s–1 for 1.5 h.
Is LHCII/PSII Core Phosphorylation or Zeaxanthin Accumulation Involved in Quenching Reactions?
In rice, thylakoid protein phosphorylation involves not only CP29 but also LHCII and PSII core. To dissect the effect of these different phosphorylation events, we first considered the case of LHCII. In principle, the light intensity used in our experiments was well above the inhibition level for STN7 kinase (Rintamäki et al., 2000; Lemeille et al., 2009), suggesting P-LHCII should not be involved. NPQ measurements with Os-stn7 mutant showed no difference with respect to the wild type, irrespective from whether the measurement was performed in isolated chloroplasts or in vivo (Fig. 7, A and B). This was consistent with Figure 3C showing no P-LHCII signal in HL-treated wild-type samples.
The case of PSII core phosphorylation was more difficult to tackle because, as reported in Supplemental Figure S5, k252a significantly decreased phosphorylation of PSII core subunits, in addition to preventing P-CP29, potentially leading to a (still unknown) quenching effect. To verify whether this was the case, we studied the k252a effect on Arabidopsis, a species where CP29 is not phosphorylated or to a very low level under the control of STN7 kinase (Bergantino et al., 1998; Bellafiore et al., 2005; Tikkanen et al., 2006; Wunder et al., 2013) and for which knockout mutants are available for both Stn7 and Stn8 genes encoding kinases involved in LHCII and PSII core phosphorylation, respectively (Bellafiore et al., 2005; Bonardi et al., 2005). Supplemental Figure S6 shows the NPQ kinetics during three consecutive actinic light exposures (1,000 µmol m–2 s–1) separated by dark recovery, as for rice in Figure 7C. The kinetics of NPQ rise and recovery were identical for chloroplasts from wild-type, stn7, and stn8 plants, and the addition of k252a did not produce any change in quenching activity in any of these genotypes, none of which is active in CP29 phosphorylation. It was important to verify that k252a was effective in blocking STN7 and STN8 kinases in Arabidopsis (Supplemental Fig. S7), leading to a reduction of CP43, D1, D2, and LHCII phosphorylation level in HL-treated functional chloroplasts alike in rice samples shown in Supplemental Figure S5. The results in Supplemental Figure S7 show that HL treatment led to an increased level of CP43 and D1/D2 phosphorylations, which were instead prevented in the presence of k252a to a larger extent with time of treatment. Another PSII core subunit well known to be phosphorylated, PSII reaction center protein H (PsbH; Aro et al., 2004) was not resolved in our gel system due to its low Mr (approximately 10 kD). These results are consistent with the differences in quenching activities of CTR versus k252a-treated rice (Fig. 7) being caused by differences in CP29 phosphorylation only, without contribution by phosphorylation of PSII core subunits.
We finally considered the hypothesis that differences in accumulation of zeaxanthin could be involved in HL-dependent NPQ enhancement. An enhancing effect of zeaxanthin on quenching reactions has been reported (Niyogi et al., 1998; Dall’Osto et al., 2005; Nilkens et al., 2010; Ruban and Johnson, 2010), thus opening the possibility that k252a-dependent differences in zeaxanthin accumulation during HL might cause at least part of the differences in quenching reported in Figure 7A for rice. To verify this hypothesis, we measured both P-CP29 accumulation and zeaxanthin accumulation in control and k252-treated rice chloroplasts during 15-min HL treatment and subsequent dark relaxation (Supplemental Figs. S5 and S8). We observed that a low level of P-CP29 was present in the dark (percentage P-CP29 per total CP29), which then increased in HL. The level of P-CP29 in k252a-treated sample, instead, decreased in the light and remained low over the subsequent dark treatment, suggesting that the CP29 kinase was active in the dark in the presence of added ATP, while its inhibition allowed for phosphatase activity to proceed. Zeaxanthin accumulation was the same in CTR and in the presence of k252a, implying the difference in zeaxanthin accumulation was not the reason for differential quenching in rice chloroplasts (Supplemental Fig. S8).
Localization of P-CP29 in Thylakoid Domains
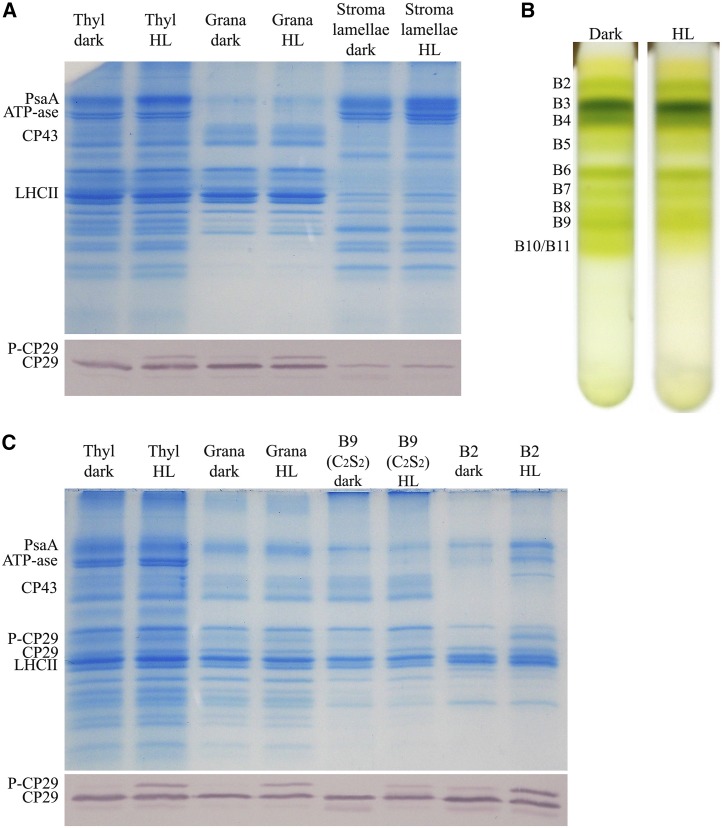
Previous work in C. reinhardtii has shown that CP29 phosphorylation leads to its migration from grana membranes, where it participates to PSII supercomplexes and stroma membranes to become part of a PSI-LHCI-LHCII-CP29 supercomplex (Kargul et al., 2005; Drop et al., 2014). In rice, dissociation of PSII supercomplexes with migration of P-CP29 to stroma-exposed membranes was also reported (Liu et al., 2009; Chen et al., 2013). To verify whether phosphorylation changed the localization of CP29 in the rice photosynthetic apparatus, we isolated thylakoid membranes from rice plants either dark adapted or exposed to HL (1,000 µmol m–2 s–1, 30 min). These thylakoids were then fractionated into grana membranes and stroma lamellae by detergent treatment and differential centrifugation (Barbato et al., 2000; Sirpiö et al., 2007; Morosinotto et al., 2010) and analyzed by SDS-PAGE and immunoblotting (Fig. 8A). Figure 8A shows the Coomassie Blue-stained SDS-PAGE gel loaded with the thylakoid, grana, and stroma lamellae preparations: a small portion of PSI contaminants is present in grana membranes, although a strong enrichment of PSII and PSI/ATPase in, respectively, grana membranes and stroma lamellae is evident. P-CP29 was detected in whole thylakoids and the grana fraction with P-CP29 per total CP29 ratio of 30% in both fractions. Instead, no P-CP29 could be detected in the stroma membrane fraction. Pigment analysis showed that the chlorophyll a/b ratio of thylakoids, grana membranes, and stroma lamellae was, respectively, 3.25 ± 0.03, 2.35 ± 0.05, and 6.70 ± 0.1, irrespective of whether the samples derived from dark-adapted or HL-treated plants. Also, the Chl amount recovered in the fraction from the two conditions was equal. With the aim of verifying whether CP29 phosphorylation did affect association of CP29 with PSII core, we solubilized the grana membrane obtained above with n-Dodecyl α-d-maltoside and fractionated the pigment-binding complexes by Suc gradient ultracentrifugation (Caffarri et al., 2009). This procedure yielded a free carotenoid upper band (B1) and three green bands containing, respectively, monomeric Lhcb proteins (B2), trimeric LHCII (B3), and the CP29-CP24 (LHCII)3 supercomplex (B4; Fig. 8B). Higher apparent molecular mass bands contained PSII core (B5) and PSII-LHCII supercomplexes of different size (Caffarri et al., 2009). Clearly, the abundance of larger PSII supercomplexes (B9 and B10/B11) was lower in HL-treated samples. When bands were harvested and analyzed by SDS-PAGE and immunoblotting, P-CP29 was found to be enriched in the fraction B2, while its level in the B9 supercomplex was low: 7.3% versus 45% observed in grana membranes (Fig. 8C). These results show that while P-CP29 remained in grana membranes, its phosphorylation caused dissociation of large supercomplexes into complexes with smaller size.
Figure 8.
Localization of P-CP29 in thylakoid membranes. A, Fractionation of thylakoid membranes for the isolation of grana and stroma lamellae membranes (see “Materials and Methods”). Thylakoids (Thyl) have been collected from dark-adapted and HL-treated rice leaves (white light, 1,000 µmol photons m–2 s–1, 30 min). These fractions have been loaded on Tris-Gly SDS-PAGE for Coomassie Blue gel staining or immunoblot analysis using anti-CP29 antibody. PsaA, PSI-A core protein of PSI. B, PSII supercomplex fractionation from grana membranes according to Caffarri et al. (2009). Thylakoids have been collected from dark-adapted and HL-treated rice leaves (white light, 1,000 µmol photons m–2 s–1, 30 min). C, Thylakoids, grana membranes, PSII-C2S2, and monomeric antenna fractions have been loaded on Tris-Gly SDS-PAGE for Coomassie Blue gel staining or immunoblot analysis using anti-CP29 antibody.
DISCUSSION AND CONCLUSION
P-CP29 has been previously reported in several species from green algae to monocots. In the case of C. reinhardtii, CP29 phosphorylation is dependent on State Transition7 Kinase (STT7) kinase and has been reported to be involved in state transitions (Kargul et al., 2005; Allorent et al., 2013). In Arabidopsis, a dicot, P-CP29 is weakly detectable upon treatment with PSII light, and its phosphorylation is under the control of STN7 kinase, homologous to STT7 (Tikkanen et al., 2006; Fristedt and Vener, 2011). Within monocots, CP29 phosphorylation has been reported in rice (C3; Hwang et al., 2003), barley (Bergantino et al., 1998), and corn (C4; Mauro et al., 1997; Bergantino et al., 1998), and yet in these species, no association was established with State 1-State 2 transitions. Rather, a photoprotective effect was reported against cold stress (Bergantino et al., 1995) and, more recently, to other environmental stresses, such as water and salt stresses in monocots (Chen et al., 2009; Liu et al., 2009). Also, an effect in modulating the size of PSII-LHCII supercomplexes was suggested (Chen et al., 2009; Fristedt and Vener, 2011).
In this study, we investigated whether the HL-induced reversible phosphorylation of CP29 in monocots is catalyzed by the same set of enzymes that were previously found to be active in the major PSII antenna LHCII. Moreover, we assessed the nature of the photoprotective activity consequent to CP29 phosphorylation.
CP29 Kinase and Phosphatase Activities
Isolation of rice insertional mutants inactivating stn7 and pph1 genes was instrumental in assessing the dependence of CP29 phosphorylation on the activity of LHCII kinase and phosphatase involved in State 1-State 2 transitions. stn7 and pph1 mutations were effective in preventing LHCII phosphorylation and dephosphorylation in rice (Figs. 2D and 3C) as well as fluorescence changes associated with this process (Figs. 2C and 3A), in agreement with previous reports in the dicot Arabidopsis (Bellafiore et al., 2005; Pribil et al., 2010; Shapiguzov et al., 2010). However, these mutations were ineffective in preventing the accumulation of P-CP29 or its dephosphorylation in the dark (Figs. 2E and 3D).
We conclude that neither STN7 nor PPH1 are involved in reversible CP29 phosphorylation in rice. This is likely the case with corn and barley, as suggested by the similar behavior of P-CP29 in these species in being promoted by excess light conditions and enhanced by concomitant stress conditions rather than inhibited at relatively low irradiance (Rintamäki et al., 1997). These results imply that, in addition to regular State 1-State 2 transitions active in green algae (Allorent et al., 2013) and dicots (Bellafiore et al., 2005), an additional phosphorylation-dependent regulation mechanism evolved in monocots for response to excess light conditions. Thus, reversible phosphorylation of the two subunits of PSII antenna system, LHCII and CP29, are independent in rice, at variance with the case of Arabidopsis, where STN7 activity was responsible for both LHCII phosphorylation and the low level of CP29 phosphorylation found (Tikkanen et al., 2006; Fristedt and Vener, 2011). In C. reinhardtii, CP29 was strongly phosphorylated (Kargul et al., 2005; Allorent et al., 2013) and yet was found to be STT7 dependent. Thus, the lack of STT7/STN7 involvement in monocot CP29 phosphorylation is consistent with the different conditions activating this process: STN7 activity is up-regulated by a reduced plastoquinone pool (Zito et al., 1999; Tikkanen et al., 2011) induced by PSII light or unbalanced excitation of PSII and PSI. However, STN7 activity is also inhibited by reduced ferrodoxin/thioredoxin (Vainonen et al., 2005; Lemeille et al., 2009), leading to the inhibition of LHCII phosphorylation in HL (Fig. 2D). We show that CP29 kinase is associated with thylakoid membranes (Fig. 4B) and is activated upon reduction of cyt b6/f (Fig. 5A), similar to STN7 kinase in Arabidopsis (Wunder et al., 2013). Although sharing with STN7 the activation by reduced PQ, CP29 kinase is not inactivated by HL, which is required for CP29 phosphorylation. This strongly suggests involvement of a kinase different from STN7, consistent with the results by Pursiheimo et al. (2003; Fig. 2E). The alignment of STN7 kinase sequences from Arabidopsis and rice (Supplemental Fig. S9) showed high level of conservation. The one CP29 sequence from rice and the three CP29 isoforms (LHCB4) from Arabidopsis are reported in Supplemental Figure S9: apart from some differences at the chloroplast transit peptide domains, the four sequences were highly conserved, with the exception of the C terminus for LHCB4.3, which was significantly shorter. Supplemental Table S1 shows that the phosphorylation site in rice P-CP29 was at Thr-82 in the mature protein: this residue is conserved in Arabidopsis LHCB4.1 and LHCB4.2 but not in LHCB4.3, suggesting that the different phosphorylation behavior of CP29 in rice and Arabidopsis depends on the kinase rather than to differences in the substrate sequence. At present, the identity of CP29 kinase is still unknown. One candidate is STN8. This hypothesis is supported by the report that STN8 activity is not inhibited by HL (Bonardi et al., 2005; Vainonen et al., 2005) and is the only Ser/Thr kinase enzyme associated with thylakoid in corn through MS analysis, in addition to STN7 (Friso et al., 2010). Moreover, the highest P-CP29 accumulation level was reported in cold and HL (Bergantino et al., 1995), a condition known to induce overreduction of electron transport chain due to a decreased demand for reducing equivalent by the Calvin-Benson cycle (Savitch et al., 2011). The alignment of STN8 protein of Arabidopsis and rice (Supplemental Fig. S9) shows that the rice homolog has a significant longer N terminus compared with the Arabidopsis one, consistent with a different substrate binding activity. Although we could not have access to a monocot stn8 mutant, the STN8 hypothesis is supported by the results of the pharmacological analysis using intact isolated chloroplasts. This approach was attempted before (Bergantino et al., 1995; Chen et al., 2009) using drug delivery by leaf infiltration, yielding results of difficult interpretation likely due to problems with diffusion of the chemicals in the leaf tissue to the target site. We found the isolated chloroplast method much more reliable, and among the three kinase inhibitors assayed in this work, only one, namely k252a (an alkaloid kinase inhibitor), was effective in preventing CP29 phosphorylation in HL (Fig. 5B), in agreement with previous results (Chen et al., 2009). K252a also caused a decreased CP43 phosphorylation level (Supplemental Fig. S5), suggesting the two reactions were caused by the same kinase, while phosphorylation of PSII core subunits has been shown to be catalyzed by STN8 (Bonardi et al., 2005; Nath et al., 2013). Interestingly, k252a treatment shows that the effect of CP29 phosphatase was complete when CP29 kinase has been inactivated. The level of P-CP29 decreased upon direct (by k252a; Fig. 5B) or indirect (see DCMU and DBMIB; Fig. 5A) kinase inactivation during HL treatment with respect to the starting level in dark samples.
Physiological Effect of CP29 Phosphorylation
Previous work with near-isogenic lines in corn reported an increased resistance to photoinhibitory treatment in the presence of P-CP29 (Mauro et al., 1997). Moreover, dissociation of PSII supercomplexes and migration of CP29 from grana to stroma-exposed membranes has been reported (Liu et al., 2009), which might potentially increase PSI activity and alleviate PQ overreduction, similar to previous reports with Chlamydomonas spp. (Kargul et al., 2005; Drop et al., 2014). Our results with rice are in contrast with these latter reports and strongly indicate that CP29 does not migrate to stroma lamellae upon phosphorylation but remains in the grana partitions. Although we cannot offer a clear explanation for the discrepancy of our results with respect to those reported (Liu et al., 2009), we notice that properties of fractions putatively deriving from grana partitions, grana margins, and stroma lamellae in Liu et al. (2009) only exhibit small differences in the content of D1 and LHCII, which is unexpected in the light of the extreme lateral heterogeneity of thylakoid membranes (Anderson et al., 2012), possibly as a consequence of the drought stress applied in that study. In our hands, extreme lateral separation was obtained for PSII plus LHCII versus PSI plus ATPase, respectively, in grana versus stroma lamellae (Fig. 8A). CP29 signal in stromal fraction was very low and did not increase upon HL treatment, irrespective from its phosphorylation state (Fig. 8A). Further analysis of grana membranes showed that PSII-LHCII supercomplexes were maintained (Fig. 8B, bands B6–B9), even in presence of P-CP29 in HL-treated samples, with the exception of the larger complexes (Fig. 8B, bands B10/B11), which are composed by C2S2M and C2S2M2 PSII dimers, as previously reported (Caffarri et al., 2009). These results imply that while P-CP29 caused dissociation of largest supercomplexes into complexes with smaller size, it remained in grana membranes. This suggests that whatever the physiological effect of P-CP29, it is exerted within grana domains and within the inner layer of the PSII antenna system close to the PSII core. It is worth noting that upon HL treatment, a phosphorylation of PSII core subunits was also observed, as reported in Supplemental Figure S5, thus we cannot exclude that CP43, D1/D2, and/or PsbH phosphorylation might also have a role in PSII destabilization upon HL as, previously reported for Arabidopsis (Tikkanen et al., 2008).
Figure 1 shows that P-CP29 accumulation is a very rapid process upon exposure to excess light. In these conditions, NPQ is also activated, allowing for fast heat dissipation of the excess energy absorbed (Li et al., 2000; Külheim et al., 2002; Horton et al., 2008). A relation between NPQ and CP29 phosphorylation has been previously suggested (Mauro et al., 1997). Here, we exploited the k252a inhibitor for studying the relation between accumulation of P-CP29 and NPQ in fully functional isolated chloroplasts exposed to excess light. A clear effect was detected on NPQ rise kinetic and relaxation kinetic in the dark (Fig. 7, A and B). Dark-adapted, P-CP29-depleted chloroplast underwent a small NPQ increase and a strong delay in its relaxation. Due to the slow dephosphorylation kinetic (Fig. 1), repeated light exposures led to progressively increasing NPQ amplitude (Fig. 7C). Thus, these NPQ kinetic changes in both the rise and decay were abolished by k252a and were absent in the Arabidopsis wild type as well as in stn7 and stn8 mutants (Supplemental Fig. S6), where CP29 did not accumulate. Because k252a did not affect the synthesis of zeaxanthin (Supplemental Fig. S8), an NPQ enhancer (Niyogi et al., 1998), we conclude that P-CP29 had an enhanced quenching capacity versus CP29 and that this process is physiologically active in monocots, but not in the dicot Arabidopsis plants. The photoprotective effect of the additional NPQ induced in P-CP29 is evident from the slower rate of NPQ dark relaxation of both the wild type and stn7 rice mutants (Fig. 7A) and by the decreased rate of singlet oxygen produced (Fig. 6B). The use of the Arabidopsis wild type and stn7 and stn8 mutants as controls for the treatment with k252a inhibitor allowed us to correlate NPQ and photoprotection phenotypes with the phosphorylation of CP29 in rice. Exposure of Arabidopsis chloroplasts to k252a did not induce any significant NPQ variation. We cannot exclude that in monocots, phosphorylation of CP29 and PSII core subunits cooperatively yields the increased photoprotection phenotype observed, and a deeper investigation of PSII core phosphorylation is required. However, the comparison of the Arabidopsis wild type and stn7/stn8 mutants and the rice wild type treated or untreated with k252a inhibitors strongly suggests a peculiar role of P-CP29 in photoprotection.
The Mechanism of Quenching in P-CP29
Enhanced quenching in the presence of P-CP29 is consistent with the decrease in amplitude and slower NPQ kinetic in plants lacking CP29 (de Bianchi et al., 2011) and with the rate-limiting activity of CP29 in transferring excitation energy from outer antenna to PSII core complex (Caffarri et al., 2011). It is interesting to consider the consequences of CP29 phosphorylation: MS analysis showed that mature rice CP29 is phosphorylated at the Thr-82 residue (Supplemental Table S1), located at the N terminus, exposed to the stromal surface of thylakoids. This position is homologous to Thr-83, previously reported for ZmCP29. No other phosphorylated residues were detected in OsCP29, implying that the changes in conformation previously detected (Testi et al., 1996) are specially associated with this phosphorylation event. CP29 spectral properties are also modulated by zeaxanthin binding (Croce et al., 1996; Crimi et al., 2001). Previous work on the isolated P-CP29 demonstrated that phosphorylation does not induce the protein to undergo a dissipative state in detergent solution (Crimi et al., 2001): on the basis of this result, we propose that phosphorylation-dependent conformational change of CP29 in monocots affects the overall PSII supercomplexes conformation, as evidenced by the partial disassembly of PSII-LHCII supercomplexes in smaller complexes (Fig. 8C), leading to formation of quenched states in a zeaxanthin-like long-living mechanism. In this context, we cannot exclude that protein-protein interaction in PSII-LHCII supercomplexes leads to formation of some specific quenching sites in P-CP29, which are inactivated when the protein is extracted by detergent treatment. For example, previous work in thylakoid membranes purified from Arabidopsis showed the formation of carotenoid radical cations responsible for energy dissipation upon NPQ induction in a high fraction (33%) of monomeric proteins (Holt et al., 2005): upon investigation of isolated proteins, the yield of carotenoid radical cation formation dramatically decreased to less than 1% (Avenson et al., 2008). A similar protein environment-dependent activation of quenching sites in P-CP29 cannot be excluded.
Among monomeric LHCB proteins, CP26 and CP24 showed the highest rates of zeaxanthin binding upon exposure to HL (Betterle et al., 2010), while CP29 has lower binding rates (Morosinotto et al., 2002). We propose that monocots developed an additional response to fast and repeated exposure to excess light by pretriggering the energy-dissipative activity through a change of conformation in CP29, caused by N-terminal phosphorylation. This mechanism adds to the zeaxanthin-dependent NPQ mechanisms, which have its preferential target in CP26 (Dall’Osto et al., 2005) and CP24 (Betterle et al., 2010) and is likely to be more efficient than violaxanthin-to-zeaxanthin exchange in conditions of low temperature when the rate of the lipid diffusion step required for violaxanthin deepoxidation and binding to the L2 site of Lhc proteins is decreased. Also, zeaxanthin formation requires ascorbate, a reducing cofactor, which is also involved in a number of redox reactions and might become limiting due to its use in concomitant pathways such as the water-water cycle (Asada, 1999). In the cold, ATP level is strongly increased by reduced requirement by the Calvin-Benson cycle, a condition favoring kinase activity.
MATERIALS AND METHODS
Plant Materials and Growth Condition
Rice (Oryza sativa) lacking STN7 was obtained from Oryza Tag Line (CIRAD; rice japonica ‘Nipponbare,’ no. AJTH05; Larmande et al., 2008), whereas rice lacking PPH1/TAP38 was obtained from the Rice Mutant Database (rice japonica ‘Zhonghua 15,’ no. 04Z11OK94; Zhang et al., 2006). Plants were grown in a greenhouse at 28°C/35°C with natural light during warmer seasons or artificial light (400 μmol photons m–2 s–1) during late autumn and winter (14-h-day/10-h-night photoperiod).
RT-PCR Analysis
For RT-PCR, total RNA was isolated from 10-week-old plants with the Spectrum Plant Total RNA Kit (Sigma-Aldrich). RT was performed using M-MLV reverse transcriptase with oligo(dT) primer and 1 µg of total RNA and was followed by 37 cycles of PCR amplification. In parallel, amplification of the housekeeping gene βactin transcript with primers collected from the literature (Bi et al., 2011) has been performed from the same complementary DNAs as the loading control. The primers used for Stn7 (LOC_Os05g47560) and Pph1 (Loc_Os01g37130) genes were as follows: STN7 (forward) AACGGACAGCAGCCTCATAC; STN7 (reverse) TGTGCCATGGGTTTCTTGTA; PPH1 (forward) CTTGTTGTCTCGCACATTGG; and PPH1 (reverse) AATATCTTGGCCAGCAGGAG.
Pigment Analysis
Pigments were extracted and then separated and quantified by HPLC as previously described (Gilmore and Yamamoto, 1991).
Membrane Isolation
Functional chloroplasts/thylakoids were isolated as previously described (Casazza et al., 2001). Experiments with functional chloroplasts/thylakoids were performed at a chlorophyll concentration of 40 μg mL–1 in the presence of 10 µm methylviologen, 0.5 mm ATP, and 15 mm Na-ascorbate. To investigate the mechanism of CP29 phosphorylation, functional chloroplasts were treated with U0126 (MAPK inhibitor, Sigma-Aldrich), W7 (Calcium-Dependent Protein Kinases inhibitor, Sigma-Aldrich), and K252a (Ser/Thr protein kinases inhibitor, LC Laboratories), using the same concentrations previously indicated for in vivo experiments by Chen et al. (2009).
Grana membranes have been prepared according to Morosinotto et al. (2010), and stroma lamellae have been prepared according to Sirpiö et al., 2007) and Barbato et al. (2000). PSII supercomplexes have been prepared from grana membranes according to Caffarri et al. (2009). Sodium fluoride (10 mm) was added to all the buffers used for preparation of grana membranes, stroma lamellae, and PSII supercomplexes.
Gel Electrophoresis and Immunoblotting
SDS-PAGE analysis was performed with the Tris-Gly buffer system (Laemmli, 1970) and 15% (w/v) acrylamide concentration, with the addition of 3 m urea to the running gel to separate phosphorylated and unphosphorylated CP29 polypeptides.
For western-blot analysis, chloroplast or thylakoid samples were loaded on SDS-PAGE and electroblotted on nitrocellulose membranes, and proteins were detected with homemade anti-CP29 (Bergantino et al., 1995), anti-STN7 (Lemeille et al., 2009), or anti-Rubisco serum, using alkaline phosphatase-conjugated secondary antibody (Sigma-Aldrich). For detection of phosphoproteins, antiphospho-Thr polyclonal antibody (Cell Signaling) was used. Signal amplitude was quantified using GelPro version 3.2 software (Bio-Rad).
Phosphopeptide Detection
For MS analysis, rice thylakoids have been isolated upon dark adaption or HL treatment (1,000 µmol m–2 s–1 white light for 30 min), and then monomeric antenna complexes, containing CP29, have been isolated upon thylakoid solubilization with n-Dodecyl β-d-maltoside and Suc gradient centrifugation (Betterle et al., 2009). Polypeptides have been separated through Tris-Gly SDS-PAGE, bands of interest, corresponding to CP29 and P-CP29, were cut from the gel, and proteins were digested with trypsin. Peptides were separated on an Acquity UPLC System (Waters) equipped with a 200-mm × 180-μm fused silica trap column packed with 5 μm of Symmetry C18 material (Waters) as well as a 200-mm × 75-μm fused silica separation column packed with 1.8 μm of BEH130 C18 material (Waters). After injection, peptides were trapped for 5 min at 1% B (A, 0.1% [v/v] trifluoroacetic acid in water; B, 0.1% [v/v] formic acid in acetonitrile) and subsequently separated at a flow rate of 300 nL min–1 with a linear gradient developed from 3% to 35% B (A, 0.1% [v/v] formic acid in water; B, 0.1% [v/v] formic acid in acetonitrile) within 120 min. Nanoliquid chromatography-high definition MS coupled with ion mobility separation data were acquired in three technical replicates. Eluted peptides were ionized at 2.1 kV and 80°C in a Waters nanoelectrospray ionization source using a precut PicoTip Emitter with the cone voltage set to 40 V. Intact peptide mass spectra and fragmentation spectra were acquired on a SYNAPT G2-S mass spectrometer (Waters) in resolution mode with positive ionization. Glu-1-fibrinopeptide B (250 fmol μL–1 and 0.5 μL min–1) was used as lock mass (mass-to-charge ratio = 785.8426 and ion = +2), and mass correction was applied to the spectra during data processing. Data analysis was carried out with ProteinLynx Global Server (PLGS 2.5.3, Apex3D algorithm version 2.128.5.0, 64 bit, Waters) with automated determination of chromatographic peak width as well as MS-time-of-flight resolution. Low and high energy thresholds were set to 200 and 20 counts, respectively. Elution start time was 5 min, and intensity threshold was set to 750 counts. In databank searches, peptide and fragment tolerances were set to automatic. We set carbamidomethyl on Cys as fixed and oxidation on Met and phosphorylation on Ser, Thr, and Tyr as variable modifications. MSE data were searched against the rice database (ftp://ftp.plantbiology.msu.edu/pub/data/Eukaryotic_Projects/o_sativa/annotation_dbs/) containing common contaminants such as keratin (ftp://ftp.thegpm.org/fasta/cRAP/crap.fasta).
In Vivo Fluorescence and NPQ Measurements
State transition experiments were performed on leaves according to established protocols (Jensen et al., 2000). Preferential PSII excitation was provided by illumination with blue light (40 μmol photons m–2 s–1), and excitation of PSI was achieved using far-red light from a light-emitting diode light source (Heinz-Walz, 102-FR) applied for 15 min simultaneously with blue light.
NPQ was measured through chlorophyll fluorescence on whole leaves or functional chloroplasts at room temperature with a PAM 101 fluorimeter (Heinz-Walz; Andersson et al., 2001), a saturating light pulse of 4,500 μmol photons m–2 s–1 for 0.8 s, and white actinic light of 1,000 μmol photons m–2 s–1 supplied by a KL1500 halogen lamp (Schott). For NPQ measurements, intact chloroplasts were dissolved immediately before analysis in an optimized hypotonic buffer containing 100 mm sorbitol, 5 mm MgCl2, 10 mm NaCl, 20 mm KCl, 30 mm HEPES, and 0.03% (w/v) agarose (40 μg mL–1 chloroplasts final chlorophylls concentration, supplemented with cofactors as previously discussed below). NPQ was calculated according to the following equations (Baker, 2008): NPQ = (Fm/Fm′)/Fm′, where Fm/Fm′ is the maximal fluorescence from dark-adapted/light-adapted leaves measured after the application of a saturating flash. For NPQ measurements on functional chloroplasts, final 0.05% (w/v) agarose was added to avoid chloroplasts sedimentation. NPQ kinetics during three consecutive periods of illumination were measured using Fluorcam FC-800 (PSI) fluorescence imaging system (PSI), allowing contemporary analysis of multiple samples and preventing chloroplasts deterioration during such long analyses.
For measurements of the PSII photoinhibition, functional chloroplasts were illuminated with white light of 1,000 μmol photons m–2 s–1, and then Fv/Fm ratios were subsequently followed at irradiances of 20 μmol photons m–2 s–1 at 48°C (Aro et al., 1994), using Fluorcam FC-800 fluorescence imaging system.
Singlet Oxygen Production
Singlet oxygen production in isolated functional chloroplasts was measured using Singlet Oxygen Sensor Green dye (Flors et al., 2006), as described (Betterle et al., 2010), illuminating the samples with red light of 1,500 μmol photons m–2 s–1.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtSTN7 (AT1G68830), AtSTN8 (AT5G01920), AtLHCB4.1 (AT5G01530), AtLHCB4.2 (AT3G08940), and AtLHCB4.3 (AT2G40100).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Identification of the HL-dependent phosphorylated residues of P-CP29.
Supplemental Figure S2. Analysis of anti-CP29 antibody sensitivity compared with Coomassie Blue gel staining.
Supplemental Figure S3. Transfer DNA insertion mutagenesis sites in stn7 and pph1 mutants.
Supplemental Figure S4. Inhibition of CP29 phosphorylation and NPQ components upon treatment with different concentrations of kinase inhibitor k252a.
Supplemental Figure S5. Analysis of rice thylakoid phosphoproteins upon NPQ induction in HL.
Supplemental Figure S6. NPQ kinetics of wild-type, stn7, and stn8 Arabidopsis chloroplasts during three consecutive periods of illumination.
Supplemental Figure S7. Analysis of Arabidopsis thylakoid phosphoproteins upon NPQ induction.
Supplemental Figure S8. Time course of CP29 phosphorylation and zeaxanthin production in the presence or absence of inhibitor k252a.
Supplemental Figure S9. Sequence comparison of STN7, STN8, and CP29 (LHCB4) of Arabidopsis and rice.
Supplemental Table S1. Identification of the phosphorylation site in LOC_Os07g37240.1.
Supplementary Material
Acknowledgments
We thank Drs. Paolo Pesaresi and Michela Osnato for helpful information on rice growth procedures, Luca Dall’Osto for help with NPQ measurements on isolated chloroplasts, Dr. Alessandro Alboresi for helpful suggestions during mutant isolation, and Dr. Stefan Helm for MS analysis.
Glossary
- NPQ
nonphotochemical quenching
- HL
high light
- cyt
cytochrome
- DCMU
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- Fv/Fm
maximum photochemical efficiency of PSII in the dark-adapted state
- RT
reverse transcription
Footnotes
This work was supported by the Italian Ministry of Agriculture (BioMassVal, grant no. 2/01/140), the Marie Curie Actions Initial Training Networks ACCLIPHOT (PITN–GA–2012–316427), the Land Sachsen-Anhalt (grant no. W21004490), and a Valeria and Vincenzo Landi 2014 grant issued by the Accademia dei Lincei.
References
- Ahn TK, Avenson TJ, Ballottari M, Cheng YC, Niyogi KK, Bassi R, Fleming GR (2008) Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320: 794–797 [DOI] [PubMed] [Google Scholar]
- Allorent G, Tokutsu R, Roach T, Peers G, Cardol P, Girard-Bascou J, Seigneurin-Berny D, Petroutsos D, Kuntz M, Breyton C, et al. (2013) A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Horton P, Kim EH, Chow WS (2012) Towards elucidation of dynamic structural changes of plant thylakoid architecture. Philos Trans R Soc Lond B Biol Sci 367: 3515–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Walters RG, Horton P, Jansson S (2001) Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation. Plant Cell 13: 1193–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E, Mccaffery S, Anderson JM (1994) Recovery from photoinhibition in peas (Pisum sativum L.) acclimated to varying growth irradiances. Plant Physiol 104: 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Rokka A, Vener AV (2004) Determination of phosphoproteins in higher plant thylakoids. Methods Mol Biol 274: 271–285 [DOI] [PubMed] [Google Scholar]
- Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR (2008) Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 283: 3550–3558 [DOI] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Barbato R, Bergo E, Szabò I, Dalla Vecchia F, Giacometti GM (2000) Ultraviolet B exposure of whole leaves of barley affects structure and functional organization of photosystem II. J Biol Chem 275: 10976–10982 [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Bergantino E, Dainese P, Cerovic Z, Sechi S, Bassi R (1995) A post-translational modification of the photosystem II subunit CP29 protects maize from cold stress. J Biol Chem 270: 8474–8481 [DOI] [PubMed] [Google Scholar]
- Bergantino E, Sandonà D, Cugini D, Bassi R (1998) The photosystem II subunit CP29 can be phosphorylated in both C3 and C4 plants as suggested by sequence analysis. Plant Mol Biol 36: 11–22 [DOI] [PubMed] [Google Scholar]
- Betterle N, Ballottari M, Hienerwadel R, Dall’Osto L, Bassi R (2010) Dynamics of zeaxanthin binding to the photosystem II monomeric antenna protein Lhcb6 (CP24) and modulation of its photoprotection properties. Arch Biochem Biophys 504: 67–77 [DOI] [PubMed] [Google Scholar]
- Betterle N, Ballottari M, Zorzan S, de Bianchi S, Cazzaniga S, Dall’osto L, Morosinotto T, Bassi R (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284: 15255–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi FC, Zhang QF, Liu Z, Fang C, Li J, Su JB, Greenberg JT, Wang HB, Yao N (2011) A conserved cysteine motif is critical for rice ceramide kinase activity and function. PLoS ONE 6: e18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437: 1179–1182 [DOI] [PubMed] [Google Scholar]
- Caffarri S, Broess K, Croce R, van Amerongen H (2011) Excitation energy transfer and trapping in higher plant photosystem II complexes with different antenna sizes. Biophys J 100: 2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarri S, Kouril R, Kereïche S, Boekema EJ, Croce R (2009) Functional architecture of higher plant photosystem II supercomplexes. EMBO J 28: 3052–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza AP, Tarantino D, Soave C (2001) Preparation and functional characterization of thylakoids from Arabidopsis thaliana. Photosynth Res 68: 175–80 [DOI] [PubMed] [Google Scholar]
- Chen YE, Yuan S, Du JB, Xu MY, Zhang ZW, Lin HH (2009) Phosphorylation of photosynthetic antenna protein CP29 and photosystem II structure changes in monocotyledonous plants under environmental stresses. Biochemistry 48: 9757–9763 [DOI] [PubMed] [Google Scholar]
- Chen YE, Zhao ZY, Zhang HY, Zeng XY, Yuan S (2013) The significance of CP29 reversible phosphorylation in thylakoids of higher plants under environmental stresses. J Exp Bot 64: 1167–1178 [DOI] [PubMed] [Google Scholar]
- Crimi M, Dorra D, Bösinger CS, Giuffra E, Holzwarth AR, Bassi R (2001) Time-resolved fluorescence analysis of the recombinant photosystem II antenna complex CP29. Effects of zeaxanthin, pH and phosphorylation. Eur J Biochem 268: 260–267 [DOI] [PubMed] [Google Scholar]
- Croce R, Breton J, Bassi R (1996) Conformational changes induced by phosphorylation in the CP29 subunit of photosystem II. Biochemistry 35: 11142–11148 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L, Caffarri S, Bassi R (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17: 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Osto L, Cazzaniga S, Havaux M, Bassi R (2010) Enhanced photoprotection by protein-bound vs. free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol Plant 3: 576–593 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L, Cazzaniga S, North H, Marion-Poll A, Bassi R (2007) The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photooxidative stress. Plant Cell 19: 1048–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bianchi S, Ballottari M, Dall’osto L, Bassi R (2010) Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans 38: 651–660 [DOI] [PubMed] [Google Scholar]
- de Bianchi S, Betterle N, Kouril R, Cazzaniga S, Boekema E, Bassi R, Dall’Osto L (2011) Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 23: 2659–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depège N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572–1575 [DOI] [PubMed] [Google Scholar]
- Drop B, Yadav KN S, Boekema EJ, Croce R (2014) Consequences of state transitions on the structural and functional organization of photosystem I in the green alga Chlamydomonas reinhardtii. Plant J 78: 181–191 [DOI] [PubMed] [Google Scholar]
- Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR (2006) Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green. J Exp Bot 57: 1725–1734 [DOI] [PubMed] [Google Scholar]
- Friso G, Majeran W, Huang M, Sun Q, van Wijk KJ (2010) Reconstruction of metabolic pathways, protein expression, and homeostasis machineries across maize bundle sheath and mesophyll chloroplasts: large-scale quantitative proteomics using the first maize genome assembly. Plant Physiol 152: 1219–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R, Vener AV (2011) High light-induced disassembly of photosystem II supercomplexes in Arabidopsis requires STN7-dependent phosphorylation of CP29. PLoS ONE 6: e24565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galka P, Santabarbara S, Khuong TTH, Degand H, Morsomme P, Jennings RC, Boekema EJ, Caffarri S (2012) Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 24: 2963–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY (1991) Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol 96: 635–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobets B, van Grondelle R (2001) Energy transfer and trapping in photosystem I. Biochim Biophys Acta 1507: 80–99 [DOI] [PubMed] [Google Scholar]
- Hansson M, Vener AV (2003) Identification of three previously unknown in vivo protein phosphorylation sites in thylakoid membranes of Arabidopsis thaliana. Mol Cell Proteomics 2: 550–559 [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall’osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145: 1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li XP, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307: 433–436 [DOI] [PubMed] [Google Scholar]
- Horton P, Johnson MP, Perez-Bueno ML, Kiss AZ, Ruban AV (2008) Photosynthetic acclimation: does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J 275: 1069–1079 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban A (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56: 365–373 [DOI] [PubMed] [Google Scholar]
- Hwang H, Xu C, Moon B, Lee C (2003) Recovery from low-temperature photoinhibition is related to dephosphorylation of phosphorylated CP29 rather than zeaxanthin epoxidation in rice leaves. J Plant Biol 46: 122–129 [Google Scholar]
- Jensen PE, Gilpin M, Knoetzel J, Scheller HV (2000) The PSI-K subunit of photosystem I is involved in the interaction between light-harvesting complex I and the photosystem I reaction center core. J Biol Chem 275: 24701–24708 [DOI] [PubMed] [Google Scholar]
- Kargul J, Turkina MV, Nield J, Benson S, Vener AV, Barber J (2005) Light-harvesting complex II protein CP29 binds to photosystem I of Chlamydomonas reinhardtii under State 2 conditions. FEBS J 272: 4797–4806 [DOI] [PubMed] [Google Scholar]
- Külheim C, Agren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Larmande P, Gay C, Lorieux M, Périn C, Bouniol M, Droc G, Sallaud C, Perez P, Barnola I, Biderre-Petit C, et al. (2008) Oryza Tag Line, a phenotypic mutant database for the Genoplante rice insertion line library. Nucleic Acids Res 36: D1022–D1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeille S, Willig A, Depège-Fargeix N, Delessert C, Bassi R, Rochaix JD (2009) Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni C, Pietrzykowska M, Kiss AZ, Suorsa M, Ceci LR, Aro EM, Jansson S (2013) Very rapid phosphorylation kinetics suggest a unique role for Lhcb2 during state transitions in Arabidopsis. Plant J 76: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Liu WJ, Chen YE, Tian WJ, Du JB, Zhang ZW, Xu F, Zhang F, Yuan S, Lin HH (2009) Dephosphorylation of photosystem II proteins and phosphorylation of CP29 in barley photosynthetic membranes as a response to water stress. Biochim Biophys Acta 1787: 1238–1245 [DOI] [PubMed] [Google Scholar]
- Mauro S, Dainese P, Lannoye R, Bassi R (1997) Cold-resistant and cold-sensitive maize lines differ in the phosphorylation of the photosystem II subunit, CP29. Plant Physiol 115: 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosinotto T, Baronio R, Bassi R (2002) Dynamics of chromophore binding to Lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J Biol Chem 277: 36913–36920 [DOI] [PubMed] [Google Scholar]
- Morosinotto T, Segalla A, Giacometti GM, Bassi R (2010) Purification of structurally intact grana from plants thylakoids membranes. J Bioenerg Biomembr 42: 37–45 [DOI] [PubMed] [Google Scholar]
- Nath K, Poudyal RS, Eom JS, Park YS, Zulfugarov IS, Mishra SR, Tovuu A, Ryoo N, Yoon HS, Nam HG, et al. (2013) Loss-of-function of OsSTN8 suppresses the photosystem II core protein phosphorylation and interferes with the photosystem II repair mechanism in rice (Oryza sativa). Plant J 76: 675–686 [DOI] [PubMed] [Google Scholar]
- Nelson N, Ben-Shem A (2004) The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol 5: 971–982 [DOI] [PubMed] [Google Scholar]
- Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797: 466–475 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16: 307–314 [DOI] [PubMed] [Google Scholar]
- Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D (2010) Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol 8: e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiheimo S, Martinsuo P, Rintamäki E, Aro E (2003) Photosystem II protein phosphorylation follows four distinctly different regulatory patterns induced by environmental cues. Plant Cell Environ 26: 1995–2003 [Google Scholar]
- Rintamäki E, Martinsuo P, Pursiheimo S, Aro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97: 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Salonen M, Suoranta UM, Carlberg I, Andersson B, Aro EM (1997) Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo. Application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J Biol Chem 272: 30476–30482 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP (2010) Xanthophylls as modulators of membrane protein function. Arch Biochem Biophys 504: 78–85 [DOI] [PubMed] [Google Scholar]
- Ruegg UT, Burgess GM (1989) Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci 10: 218–220 [DOI] [PubMed] [Google Scholar]
- Savitch LV, Ivanov AG, Gudynaite-Savitch L, Huner NP, Simmonds J (2011) Cold stress effects on PSI photochemistry in Zea mays: differential increase of FQR-dependent cyclic electron flow and functional implications. Plant Cell Physiol 52: 1042–1054 [DOI] [PubMed] [Google Scholar]
- Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix JD, Vener AV, Goldschmidt-Clermont M (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci USA 107: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirpiö S, Allahverdiyeva Y, Suorsa M, Paakkarinen V, Vainonen J, Battchikova N, Aro EM (2007) TLP18.3, a novel thylakoid lumen protein regulating photosystem II repair cycle. Biochem J 406: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Regel RE, Paakkarinen V, Battchikova N, Herrmann RG, Aro EM (2004) Protein assembly of photosystem II and accumulation of subcomplexes in the absence of low molecular mass subunits PsbL and PsbJ. Eur J Biochem 271: 96–107 [DOI] [PubMed] [Google Scholar]
- Testi MG, Croce R, Polverino-De Laureto P, Bassi R (1996) A CK2 site is reversibly phosphorylated in the photosystem II subunit CP29. FEBS Lett 399: 245–250 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Grieco M, Aro EM (2011) Novel insights into plant light-harvesting complex II phosphorylation and ‘state transitions’. Trends Plant Sci 16: 126–131 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Nurmi M, Kangasjärvi S, Aro EM (2008) Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim Biophys Acta 1777: 1432–1437 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Piippo M, Suorsa M, Sirpiö S, Mulo P, Vainonen J, Vener AV, Allahverdiyeva Y, Aro EM, Paula M, et al. (2006) State transitions revisited: a buffering system for dynamic low light acclimation of Arabidopsis. Plant Mol Biol 62: 779–793 [DOI] [PubMed] [Google Scholar]
- Vainonen JP, Hansson M, Vener AV (2005) STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J Biol Chem 280: 33679–33686 [DOI] [PubMed] [Google Scholar]
- Wunder T, Liu Q, Aseeva E, Bonardi V, Leister D, Pribil M (2013) Control of STN7 transcript abundance and transient STN7 dimerisation are involved in the regulation of STN7 activity. Planta 237: 541–558 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, Wang S (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res 34: D745–D748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J 18: 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.