Abstract
Cardiac arrest remains a leading cause of death and permanent disability worldwide. Although many victims are initially resuscitated, they often succumb to the extensive ischemia-reperfusion injury inflicted on the internal organs, especially the brain. Cardiac arrest initiates a complex cellular injury cascade encompassing reactive oxygen and nitrogen species, Ca2+ overload, ATP depletion, pro- and anti-apoptotic proteins, mitochondrial dysfunction, and neuronal glutamate excitotoxity, which injures and kills cells, compromises function of internal organs and ignites a destructive systemic inflammatory response. The sheer complexity and scope of this cascade challenges the development of experimental models of and effective treatments for cardiac arrest. Many experimental animal preparations have been developed to decipher the mechanisms of damage to vital internal organs following cardiac arrest and cardiopulmonary resuscitation (CPR), and to develop treatments to interrupt the lethal injury cascades. Porcine models of cardiac arrest and resuscitation offer several important advantages over other species, and outcomes in this large animal are readily translated to the clinical setting. This review summarizes porcine cardiac arrest-CPR models reported in the literature, describes clinically relevant phenomena observed during cardiac arrest and resuscitation in pigs, and discusses numerous methodological considerations in modeling cardiac arrest/CPR. Collectively, published reports show the domestic pig to be a suitable large animal model of cardiac arrest which is responsive to CPR, defibrillatory countershocks and medications, and yields extensive information to foster advances in clinical treatment of cardiac arrest.
Keywords: Acidemia, Asphyxia, Cardiopulmonary resuscitation, Countershocks, Hyperoxia, Vasopressin, Ventricular fibrillation
Core tip: Cardiac arrest remains a leading cause of death worldwide, despite tremendous improvements in emergency medical care and increased public delivery of bystander cardiopulmonary resuscitation (CPR). But progress is being achieved, thanks to the joint efforts of biomedical scientists, physicians and emergency medical personnel to translate laboratory discoveries to the ambulance and hospital. The domestic pig has proven to be a superb preclinical model of cardiac arrest, yielding a wealth of mechanistic insights and practical strategies to refine the delivery of CPR and to test promising treatments. This review examines pivotal factors in modeling cardiac arrest and CPR in the pig.
INTRODUCTION
Prior to 1960 cardiac resuscitation was administered by direct cardiac massage following thoracotomy. Based on animal experimentation a method of external cardiac massage administered by rapid, forceful compressions and passive recoil of the sternum was developed by Kouwenhoven et al[1]. Although over fifty years have passed since the inception of closed chest cardiac massage, and despite many refinements of this approach in the intervening decades, cardiac arrest remains a leading cause of death and persistent disability worldwide. All too often, victims who are initially resuscitated later succumb to extensive ischemia-reperfusion injury to their vital organs, especially the brain[2-5]. Further, many of the 10% of cardiac arrest patients who survive to hospital discharge experience persistent neurocognitive impairment which profoundly impacts their quality of life[3,6].
Although public health data and anecdotal evidence inform the refinement of cardiopulmonary resuscitation (CPR) protocols[7], knowledge of the complex mechanisms of internal organ damage, essential to foster development of effective pharmacological interventions, is incomplete. In the brain, ATP depletion, intracellular Ca2+ overload, excessive formation of reactive oxygen and nitrogen derivatives, inflammation and glutamate-induced excitotoxicity conspire to kill neurons and other cells and disrupt the blood-brain barrier. Currently there are no clinically effective pharmacological treatments to protect the brain during cardiac arrest and CPR[2], and therapeutic hypothermia is the only approved treatment in the United States[8]. Reliable preclinical models of cardiac arrest and resuscitation are essential to decipher the injury mechanisms and develop treatments to increase survival and improve quality of life after cardiac arrest.
Ischemia-reperfusion damage in the central nervous system is the result of a multifaceted injury cascade[9,10]. The structural complexity of the brain, which consists of integrated networks of different cell types including neurons, astrocytes, oligodendrocytes, microglia and vascular endothelium, presents fundamental challenges to developing neuroprotective treatments. The brain contains many functional regions which differ in their vulnerabilities to ischemia-reperfusion injury. Potential pharmacotherapeutic agents must first traverse the blood brain barrier, a significant permeability impediment to all but small, non-polar compounds, and act on multiple injury mechanisms, without producing untoward side effects.
Sophisticated animal models are required to model the composite structure and integrated function of the central nervous system and to evaluate the benefits and potential side effects of prospective treatments for ischemia and other brain disorders. Extensive research has established the domestic pig as an excellent animal model to study the impact of cardiac arrest, resuscitation, and therapeutic interventions on the brain and other internal organs. An impressive variety of swine cardiac arrest models are reported in the literature. By examining the features that distinguish these models, this article aims to assist the reader in evaluating the literature and in designing porcine cardiac arrest models appropriate to address specific research objectives.
ATTRIBUTES OF SWINE FOR MODELING CARDIAC ARREST AND RESUSCITATION
Several attributes make the domestic pig an ideal model for cardiac arrest research[11,12]: (1) a large mammal, the pig accommodates extensive instrumentation for blood sampling, monitoring of intravascular and intracardiac pressures, electrocardiography and intravenous administration of medications and experimental treatments; (2) pigs tolerate invasive surgical procedures and rapidly regain consciousness post-anesthesia; (3) resting heart rate, blood pressure, and serum chemistries of pigs and humans are very similar[13-15]; (4) pigs have sufficient blood volume to permit collection of multiple arterial and venous samples for analyses of blood gases and serum chemistry; (5) neurological examinations have been developed to evaluate neurobehavioral function in pigs[16,17]; (6) the pig’s large chest accommodates forceful precordial chest compressions and application of transthoracic defibrillatory countershocks of electrical energies similar to those used clinically; and (7) pigs have the largest brains among the commonly studied laboratory animals, which provides ample tissue for extensive biochemical and histological analyses of specific brain subregions. Porcine models are especially well-suited to study cardiac arrest and CPR, because they are easily tailored to address specific research objectives.
FACTORS TO CONSIDER WHEN MODELING CARDIAC ARREST AND RESUSCITATION IN PIGS
The pathophysiological complexities of sudden cardiac death and cardiopulmonary resuscitation challenge the development of animal models that accurately replicate the clinical situation. The primary factors in developing suitable animal models are the study end points and objectives. However, the myriad variables in model design and experimental protocol, which mirror the complexity of cardiac arrest and its treatment, challenge the direct comparison of results obtained in different studies. This section summarizes several factors that must be considered in developing and reporting cardiac arrest-resuscitation protocols in pigs, including the anesthetic regimen, method of inducing ventricular fibrillation, the depth, frequency and duration of chest compressions, whether or not to ventilate during resuscitation and the fraction of inspired O2 (FIO2), the pattern and intensity of defibrillatory countershocks, the criteria taken to indicate recovery of spontaneous circulation (ROSC), the use of inotropic and/or vasoconstrictor support during ROSC, and strategies to correct post-arrest systemic acidemia. With multiple options for each component, it is critical that cardiac arrest-resuscitation protocols be designed carefully to address the study’s specific objectives. Table 1 summarizes and compares key features of representative cardiac arrest-resuscitation protocols in domestic swine.
Table 1.
Details of representative cardiac arrest protocols in pigs
| Ref. | Lurie et al[93], 2002 | Mayr et al[38], 2004 | Tang et al[25], 2006 | Li et al[94], 2008 | Indik et al[95], 2009 | Hang et al[96], 2014 |
| Pre-anesthetic, induction anesthetic | Ketamine 20 mg/kg im Propofol 2.3 mg/kg iv | Ketamine 20 mg/kg im Propofol 1-2 mg/kg iv Piritramide 30 mg iv | Ketamine 20 mg/kg im Pentobarbital 30 mg/kg iv | Ketamine 20 mg/kg im Pentobarbital 30 mg/kg iv | 5% isoflurane | Ketamine 15 mg/kg im Midazolam 0.5 mg/kg im Atropine 0.05 mg/kg im Propofol 1 mg/kg iv |
| Maintenance anesthesia | Propofol 10 mg/kg per hour iv | Isoflurane (1%-2%) in 65% nitrous oxide | Pentobarbital 8 mg/kg per hour iv | Pentobarbital 8 mg/kg per hour iv | 1.5%-3% isoflurane | Propofol 9 mg/kg per hour iv Fentanyl 1 μg/kg per hour iv |
| Method of arrest | Electrical: 60 Hz, 140-160 V | Pharmacological: 5 mg/kg bupivacaine | Electrical: 1-2 mA | LAD balloon occluder | Steel plug in LAD | Asphyxiation: endotracheal clamping |
| Pre-CPR arrest | 6 min | 6 min | 7 min | 5 min | 8 min | 8 min |
| Precordial compressions (% of chest diameter) | Mechanical: 80/min (25%) | Manual: 100/min | Mechanical: 100/min (25%) | Mechanical: 100/min (Group 1 25%, Group 2 17.5%) | Manual: 100/min (c. 33%) | Manual: 100/min (c. 33%) |
| CPR duration | 6 min | 2 min | 1 min | 3 min | 2 min | 4 min |
| Ventilation during CPR? | FIO2 = 1.0; 5:1 compression: ventilation | FIO2 = 1.0 | FIO2 = 1.0; 15:2 compression: ventilation | FIO2 = 1.0; 15:2 compression: ventilation | None | FIO2 = 1.0; 12 cycles/min; 10 mL/kg per cycle |
| Countershocks | 1-3 x 200 J | 3, 4, 6 J/kg | 150-360 J | 150 J | 150 J | 4 J/kg |
| CPR between countershocks | 90 s | None | 1 min/shock | 3 min | 2 min | 2 min |
| Vasopressors to enhance CPR | EPI 0.045 mg/kg | AVP 0.4 or 0.8 U/kg ± EPI 45 or 200 μg/kg | None | None | EPI 0.02 μg/kg; 1-3 doses | EPI 0.02 μg/kg |
| Definition of ROSC | Systolic BP > 70 mmHg | Systolic BP ≥ 80 mmHg for ≥ 5 min | Mean aortic BP > 60 mmHg for ≥ 5 min | Mean aortic BP ≥ 60 mmHg for ≥ 5 min | Systolic BP > 50 mmHg for > 1 min | Systolic BP > 50 mmHg for > 10 min |
| ROSC duration | 24 h | 1 h | 3 d | 72 h | 24 h | 6 h |
| Pigs completing protocol | 11/20 | Placebo: 0/7; AVP: 5/7; EPI: 4/7; AVP + EPI: 7/7 | 36/44 | Group 1: 6/6 Group 2: 0/6 | 11/15 | ROSC: 8/16; Complete protocol: 3/16 |
AVP: Vasopressin; BP: Systemic arterial blood pressure; CPR: Cardiopulmonary resuscitation; EPI: Epinephrine; LAD: Left anterior descending coronary artery; ROSC: Recovery of spontaneous circulation.
Induction and maintenance of anesthesia
Invasive surgical procedures and ethical constraints require the induction and maintenance of an appropriate anesthetic plane. Anesthetics are infused intravenously or, in the case of volatile anesthetics, inhaled. The temporary or persistent effects of the anesthetic on study endpoints, e.g., cardiac function, cell death, inflammation or neurobehavioral recovery must be taken into account. For example, the cardiodepressant effects of some volatile anesthetics, e.g., halothane and isoflurane may produce hypotension[18-21], yet these anesthetics also exert cardioprotection[22,23]; thus, the anesthetic plane must be controlled carefully. Signs of inadequate anesthesia include increased jaw tension, limb withdrawal when the soft tissue between the hooves is pinched, wink reflex in response to delicate contact of the ocular canthus, spontaneous limb movements, and/or unexpected increases in heart rate and systemic arterial pressure.
Methods of inducing cardiac arrest
The major causes of clinical cardiac arrest are asphyxiation, electric shock and, most commonly, coronary artery occlusion and reperfusion. There are different methods of inducing cardiac arrest which model these clinical situations. The first and most common method of inducing ventricular fibrillation is the application of electrical current to the epicardium or, in closed-chest preparations, the left or right ventricular endocardium. Typically, a pacing wire is introduced into the external jugular vein and advanced into the right ventricle (Figure 1)[24-27]. While the wire is in contact with the right ventricular endocardium, a rapid train of impulses is transmitted which, within seconds, initiates ventricular fibrillation. The characteristic “torsades de pointes” pattern on electrocardiogram (cf. Figure 2), monophasic decline in aortic pressure and the absence of an arterial pulse confirm ventricular fibrillation. Aside from modeling electrocution-induced cardiac arrest, an important advantage of this method is the well-defined and reproducible time of ventricular fibrillation onset. Electrical induction of ventricular fibrillation does not impart substantial myocardial injury, which is advantageous if the study is examining other internal organs in which persistent cardiac insufficiency might be a confounding factor.
Figure 1.
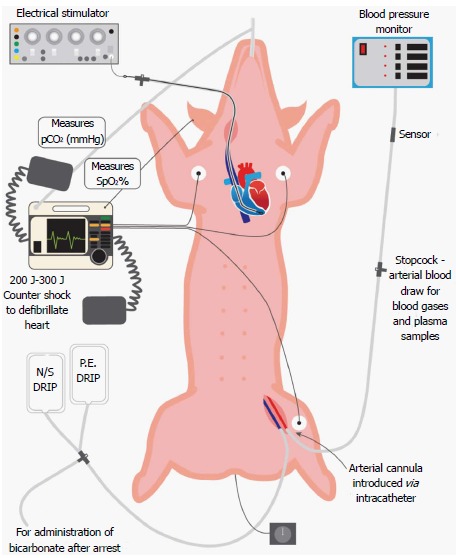
Porcine preparation for cardiac arrest-cardiopulmonary resuscitation studies. The pig is placed in supine recumbency and mechanically ventilated via an endotracheal tube, through which isoflurane anesthesia is administered. Hemodynamic function is monitored by a femoral arterial catheter connected to a pressure transducer, and electrocardiographic activity is monitored by standard limb lead II electrocardiogram. Cardiac arrest is induced by a train of electrical impulses conducted by an intrajugular pacing wire from an electrical stimulator to the right ventricular endocardium. Body temperature is measured with a rectal probe, and end-tidal pCO2 by a sensor placed in the endotracheal tube. Defibrillatory countershocks (200-300 J) are administered with external paddles. Intravenous treatments include normal saline (N/S), phenylephrine (PE), sodium bicarbonate and experimental resuscitative fluids. spO2: Percentage oxyhemoglobin saturation.
Figure 2.
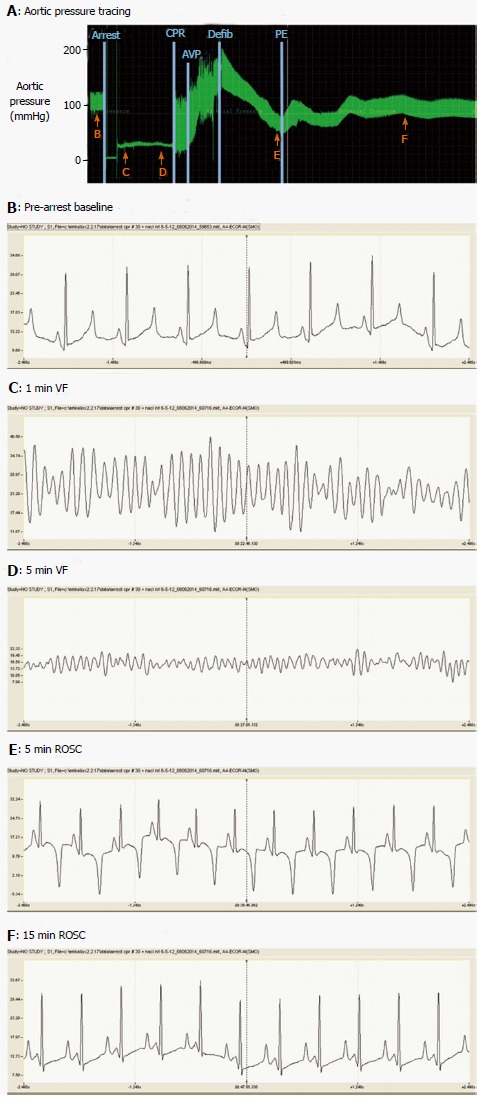
Aortic pressures and lead IIelectrocardiogram during cardiac arrest, cardiopulmonary resuscitation and recovery of spontaneous circulation. A: Phasic aortic pressure tracing during the period from pre-arrest baseline to 20 min ROSC. Lettered arrows indicate times at which the electrocardiograms shown in panels B-F were obtained. Vertical lines indicate: (1) induction of ventricular fibrillation cardiac arrest; (2) commencement of precordial compressions (CPR); (3) injection of vasopressin (AVP); (4) defibrillation (Defib) by 200 J countershock; and (5) initiation of intravenous phenylephrine (PE; c. 2 μg/kg per minute) to stabilize systemic arterial pressure during ROSC. Mechanical ventilation was suspended during cardiac arrest and CPR. Panels B-F show 5 s electrocardiographic recordings. CPR: Cardiopulmonary resuscitation; ROSC: Recovery of spontaneous circulation; AVP: Vasopressin; PE: Phenylephrine.
Myocardial ischemia imposed by coronary stenosis or occlusion is the leading cause of cardiac arrest. Porcine models of ischemia-induced ventricular fibrillation are available that accurately reproduce the pathophysiology of cardiac arrest. Porcine myocardium lacks significant coronary collateral vessels, so the ischemia imposed by occlusion of a major coronary artery, e.g., the left anterior descending coronary artery, is sufficiently severe to initiate ventricular fibrillation within several minutes of occlusion. Coronary occlusions may be imposed by introducing a balloon occluder (one used for percutaneous transluminal coronary angioplasty) and routing it, with the aid of fluoroscopy[28], into the target vessel before inflating it. An alternative approach is the use of an ameroid occluder around a coronary artery; however, this procedure requires invasive thoracotomy and pericardiotomy to permit placement of the occluder, necessitating post-surgical recovery of the animal before the cardiac arrest experiment. In either case, occlusion may be confirmed by arteriography[28,29]. A third method is advancement of a Teflon[30] or steel[31] plug into the coronary artery. Ventricular fibrillation typically ensues within 5-10 min of coronary occlusion[29,31]. The coronary occlusion may be released, e.g., release of the balloon or ameroid occluder upon defibrillation[28,29] or the intracoronary plug may be permanently installed, resulting in a myocardial infarct[30]. Because the onset of cardiac arrest is delayed to a variable extent while the artery is occluded, the severity of myocardial injury may vary considerably among experiments. The number and intensity of the countershocks required to restore sinus rhythm is greater in porcine models of ischemically-induced vs electrically-induced arrest, as is the incidence of post-resuscitation ventricular premature beats and recurrence of ventricular fibrillation[29,32,33]. Nevertheless, ischemia-induced cardiac arrest replicates the most common cause of cardiac arrest, affording ready translation of results to clinical settings.
Asphyxiation is the second most common cause of cardiac arrest and the leading cause in children. A facile method of producing asphyxia in anesthetized swine is to block the endotracheal tube while monitoring the electrocardiogram and arterial blood pressure[34-36]. Hypoxemia and hypercapnia progressively intensify until cardiac arrest ensues, typically within 10-15 min after blocking ventilation[37]. The principal advantages of asphyxia are its accurate modeling of a major cause of pediatric cardiac arrest and mortality, including the changes in blood gases and pH, and the noninvasive approach which obviates the introduction of pacing wires or occluders into the vasculature. Depending on the study endpoints and objectives, disadvantages may include the changes in blood gas chemistry[37] and the variable duration of asphyxiation before ventricular fibrillation, which imposes hypoxemia on the brain and other internal organs even before onset of cardiac arrest.
High dosages of certain chemicals, e.g., bupivacaine[38], may be injected into the right atrium to arrest the heart, modeling cardiac arrest secondary to drug overdose. In such models the potential systemic side effects of the chemicals must be taken into account.
Duration of pre-CPR arrest
The duration of pre-intervention arrest is crucial; as this interval is prolonged, cardioversion, survival and good neurological recovery become progressively less achievable. The three-phase model of cardiac arrest[39] subdivides the pre-intervention period into three phases. The first 4-5 min constitute the electrical phase, during which countershocks are likely to achieve cardioversion even without pre-shock CPR. During the next 5-10 min, the circulatory phase, interventions to effect circulation, e.g., chest compressions, are essential to ensure countershocks produce cardioversion. After 10-15 min arrest, the victim enters the metabolic phase, in which increasingly intense metabolic derangements result in protracted or permanent organ damage and severe neurological impairment even if cardioversion is achieved. If the study requires a high survival rate, the period of pre-intervention cardiac arrest may be limited to assure a high rate of defibrillation and ROSC.
Cardiopulmonary resuscitation: force, frequency and duration
By affording modest delivery of O2 and metabolic fuels to the myocardium, precordial compressions may support enough myocardial ATP production to sustain ion transport and repolarize cardiomyocytes, enabling defibrillatory countershocks to restore spontaneous electrical rhythm. Indeed, in a canine cardiac arrest model, effective CPR afforded partial recovery of myocardial Gibbs free energy of ATP hydrolysis[40], the immediate energy source for cardiac electromechanical activity. Cardiopulmonary resuscitation protocols are readily customized to address the study end points. The frequency and depth of precordial compressions can profoundly influence outcome[41-44]. In some studies, CPR is administered by a pneumatic, piston-driven device (e.g., Thumper®), which can be adjusted to deliver forceful compressions at a predetermined frequency and depth, ensuring consistency of frequency and depth of compressions across experiments[25]. Alternatively, precordial compressions can be administered manually, modeling the CPR given by a bystander responding to an out-of-hospital cardiac arrest. Current American Heart Association guidelines[45] recommend manual mid-sternal chest compressions be sufficiently forceful to compress the chest by one-fourth of its antero-posterior diameter, followed by release and recoil, at a rate of 100 cycles/min.
The duration of CPR before defibrillatory countershocks is an important factor. Longer intervals model the protracted CPR given by bystanders before arrival of the ambulance team, but also lower the likelihood of post-arrest survival with good neurological outcome. Another important consideration is whether or not the animal will be ventilated during CPR and, if so, at what compression:ventilation ratio and FIO2. Extensive clinical evidence demonstrates that assisted ventilation during CPR following witnessed cardiac arrest offers little or no neurological or survival benefit[26,46-49]. In accordance with current recommendations for bystander CPR[50], mechanical ventilation may be suspended for the duration of cardiac arrest and CPR, then resumed after confirming defibrillation to a productive sinus rhythm.
A systemic vasoconstrictor may be administered intravenously to increase the arterial pressures produced by the chest compressions, thereby increasing perfusion of brain and myocardium at the expense of peripheral organs and tissues. The most widely used vasoconstrictors include epinephrine, a physiological adrenergic agonist, and vasopressin, a non-adrenergic vasoconstrictor which may afford greater survival to hospital discharge than epinephrine, especially in patients with asystole[51,52].
Although epinephrine has been used in this manner for decades, its potentially detrimental effects, including increased physiological shunt compromising pulmonary gas exchange[53,54], intensified myocardial ATP consumption and oxygen demand[55], and the resultant post-resuscitation myocardial dysfunction[56] and ventricular arrhythmias[57,58] have raised concerns regarding its clinical application for CPR. Preclinical and clinical evidence has shown the non-adrenergic vasoconstrictor vasopressin to be at least as effective as epinephrine at augmenting arterial pressure during precordial compressions, but without epinephrine’s untoward effects. In a porcine cardiac arrest model, vasopressin vs epinephrine produced greater myocardial and brain blood flows and mean arterial pressures during CPR[59]. Thus, vasopressin was associated with higher incidence of conversion to productive sinus rhythm[60], increased post-arrest cardiac function and decreased morbidity and mortality vs epinephrine. We have found[61] that vasopressin (c. 0.3 U/kg) injected into the right jugular vein at 60 s CPR improved markedly the quality of CPR, increasing the mean arterial pressures from 25-30 to c. 60 mmHg within 3 min (cf. Figure 1). Although epinephrine produced a more abrupt increase in arterial pressure following its injection, within 2 min vasopressin increased mean arterial pressure to a similar extent; during the first 15 min ROSC, the vasopressin-treated swine had less intense tachycardia and more moderate heart rate x arterial pressure product, a measure of myocardial energy expenditure, than their epinephrine-treated counterparts[61].
Defibrillation and cardioversion
The defibrillation protocol presents the investigator several options for model design. One choice is the sequence of defibrillatory countershocks, i.e., whether the shocks will be administered singly, or in a sequence of multiple (often three) countershocks, before checking for cardioversion. The electrical energies of the countershocks must be considered, including that of the initial countershock, and, if the initial shock fails to achieve cardioversion, whether or at what progression the intensity will be increased for subsequent countershocks. It must be determined if and for how long CPR will be administered during the interval between an unsuccessful cardioversion and the next attempt. When pre-CPR arrest exceeds the electrical phase, bouts of CPR, including a minimum of 20-25 s of chest compressions following unsuccessful countershocks, are essential to ensure effective countershocks. A similar protocol of single shocks with intervening chest compressions increased post-arrest survival vs a conventional 3-shock protocol in a porcine model of ventricular fibrillation cardiac arrest[25].
The cardiocirculatory values that constitute ROSC, including the presence of an organized electrical rhythm and maintenance of arterial blood pressure above a predetermined target value for a minimum duration (cf. Table 1) must be specified. Core body temperature has a marked effect on post-arrest and neurological injury and mortality; indeed, moderate hypothermia is the only currently approved intervention consistently shown to produce significant clinical benefit[62-64]. Pigs do not thermoregulate effectively while under anesthesia, so typically the animal must be maintained on a heating pad during the cardiac arrest-resuscitation protocol to avoid the impact of hypothermia on study endpoints. Finally, the criteria for abandoning futile resuscitation efforts must be defined.
Post-resuscitation management
Because cardiac arrest imposes ischemia on the heart itself, cardiac mechanical function may be depressed for several hours of ROSC, a manifestation of reversible myocardial injury termed cardiac “stunning”[65]. As the period of ROSC progresses, interventions may be necessary to maintain adequate arterial pressure. Intravenous saline solutions may be infused to expand extracellular fluid volume. Vasopressor agents, e.g., phenylephrine, may be administered, but it should be recognized that vasopressors may lose their efficacy over time due to desensitization of their membrane receptors[66] and, thus, may be unsuitable for long-term maintenance of arterial pressure. Accordingly, the vasoconstrictor infusion can be tapered and ultimately discontinued as cardiac function recovers. It may be necessary to adjust tidal volume and frequency of ventilations or administer bicarbonate to compensate for post-arrest hypercapnia and/or acidemia. Isotonic saline (0.9% NaCl) may be infused iv to maintain extracellular fluid volume over the course of the protocol.
Inspired oxygen concentration
The oxygen concentration of medical gases used during resuscitation is an important consideration when designing a model of cardiac arrest-resuscitation. For decades, it has been recommended that patients be ventilated with 100% oxygen during resuscitation to increase oxygen delivery to ischemic tissues[67,68]. Recently, however, hyperoxic ventilation during resuscitation has been shown to intensify formation of reactive oxygen and nitrogen intermediates within tissues and, thus, exacerbate ischemia-reperfusion injury[69-75]. A recent meta-analysis of clinical trial data showed hyperoxia (PaO2 > 300 mmHg) to be associated with increased in-hospital mortality following cardiac arrest[76]. Oxygen toxicity has been studied for years in a perioperative setting, but only recently has there been sufficient clinical evidence for the European Resuscitation Council to recommend that patients not be ventilated with 100% oxygen after cardiac arrest, but rather with room air supplemented with enough O2 to maintain an oxyhemoglobin saturation (spO2) of 94%-98%[77,78]. Thus, when designing a cardiac arrest model, the oxygen concentration used during resuscitation may be adjusted depending on whether the study aims to mimic the conventional approach of ventilation with 100% oxygen, or newly recommended strategies such as titration of oxygen administration to maintain a desired spO2.
CHALLENGES TO MODELING CARDIAC ARREST IN PIGS
Pulseless electrical activity
Pulseless electrical activity (PEA) is a “non-shockable” cardiac electrical rhythm that does not produce ventricular contraction or forward movement of blood. Approximately 60% of out-of-hospital resuscitation attempts result in the development of PEA as the presenting rhythm[79]. Only 2%-5% of patients who present with PEA as their initial rhythm survive to hospital discharge[80-82], well below the 15%-40% survival rate of those presenting with ventricular fibrillation[83-85]. Even fewer patients in whom ventricular fibrillation converted to PEA following countershocks survive to hospital discharge[79,86]. In our porcine cardiac arrest model, PEA is an ominous finding; typically, even heroic efforts fail to convert PEA to a productive sinus rhythm. None of the 9 pigs developing PEA during resuscitative efforts survived for 4 h ROSC. This situation replicates the clinical setting of out-of-hospital cardiac arrest, where a much lower rate of survival to hospital discharge is achieved in cardiac arrest victims in which PEA is the initial rhythm vs patients with an initial electrocardiographic substrate of ventricular fibrillation[87].
Malignant hyperthermia
A small minority of pigs harbor a genetic lesion in the skeletal muscle sarcoplasmic reticular Ca2+ release channels[88,89] that predisposes them to develop malignant hyperthermia (aka porcine stress syndrome), often triggered by exposure to volatile anesthetics[90]. Malignant hyperthermia has no overt clinical phenotype detectable by routine screening. As post-arrest survival and neurobehavioral recovery are negatively correlated with body temperature, an episode of malignant hyperthermia, during which core body temperature may rise above 42 °C, can have disastrous consequences, including systemic hypotension, acidemia, hypercapnia and hyperkalemia that are refractory to conventional interventions. Indeed, in our studies none of the five anesthetized pigs (4% of the total) that developed acute malignant hyperthermia survived to 4 h ROSC, despite aggressive measures including intravenous infusion of ice-cold saline and the K+ chelator calcium gluconate.
Limitations of porcine models
An important limitation of many porcine cardiac arrest models is that juvenile, disease-free pigs are generally used. In clinical settings, patients who experience cardiac arrest typically are elderly and suffer from chronic disorders such as hypertension, atherosclerosis, congestive heart failure, diabetes, emphysema or end-stage renal disease. The Ossabaw swine, which is predisposed to develop metabolic syndrome when consuming a high fat diet[91,92], provides a unique, clinically relevant experimental model suitable for studying cardiac arrest and resuscitation superimposed on metabolic syndrome. Indeed, under anesthesia these swine develop severe arrhythmias, responsive to amiodarone, that may deteriorate into cardiac arrest (Johnathan D. Tune, personal communication).
Unlike most porcine preparations, human victims of out-of-hospital cardiac arrest are not anesthetized when they are stricken. Cardiac arrest is an unanticipated event, and when it occurs outside the hospital, the delays to effective treatments are variable, poorly defined and all too often lethal. Most preclinical cardiac arrest studies employ well defined protocols, such as those reviewed herein. The fundamental differences between these protocols and the highly variable and exceedingly challenging clinical situation must be acknowledged.
CONCLUSION
Over the last few decades the collective efforts of many investigators have fostered the development of sophisticated porcine models of cardiac arrest, CPR and ROSC. The domestic pig provides an excellent large animal model of the human cardiovascular system and yields ample tissue for extensive analyses of mechanisms of injury and cytoprotection in the internal organs, such that each experiment generates a wealth of information. Although there is much to consider when constructing an experimental design, the swine model of cardiac arrest-resuscitation is easily tailored to accommodate the desired study end points. The swine model provides unparalleled translational value among current mammalian models of cardiac arrest and CPR, permitting an integrative approach to bridge the gap from bench to bedside.
ACKNOWLEDGMENTS
The authors thank Egeenee Q. Daniels, DVM, Arthur G. Williams, Jr., BS, Shirley R. Nelson, RLAT, and Besim Hoxha, MD for their expert advice and assistance in helping develop and refine their porcine cardiac arrest-resuscitation preparation.
Footnotes
Supported by Grants from The United States National Institute of Neurological Disorders and Stroke, No. R01 NS076975-03; a predoctoral fellowship from the United States National Institute of Aging, Training in the Neurobiology of Aging, No. T31 AG020494; and a predoctoral fellowship from the University of North Texas Health Science Center’s Physician Scientist Program.
Conflict-of-interest: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 8, 2014
First decision: October 31, 2014
Article in press: November 27, 2014
P- Reviewer: Lin J, Yao YM S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Kouwenhoven WB, Milnor WR, Knickerbocker GG, Chesnut WR. Closed chest defibrillation of the heart. Surgery. 1957;42:550–561. [PubMed] [Google Scholar]
- 2.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 4.Heron M. Deaths: leading causes for 2009. Natl Vital Stat Rep. 2012;61:1–94. [PubMed] [Google Scholar]
- 5.Nolan JP, Lyon RM, Sasson C, Rossetti AO, Lansky AJ, Fox KA, Meier P. Advances in the hospital management of patients following an out of hospital cardiac arrest. Heart. 2012;98:1201–1206. doi: 10.1136/heartjnl-2011-301293. [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 7.Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–1435. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 8.Corry JJ. Use of hypothermia in the intensive care unit. World J Crit Care Med. 2012;1:106–122. doi: 10.5492/wjccm.v1.i4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen AQ, Cherry BH, Scott GF, Ryou MG, Mallet RT. Erythropoietin: powerful protection of ischemic and post-ischemic brain. Exp Biol Med (Maywood) 2014;239:1461–1475. doi: 10.1177/1535370214523703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xanthos T, Lelovas P, Vlachos I, Tsirikos-Karapanos N, Kouskouni E, Perrea D, Dontas I. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: a research model. Lab Anim. 2007;41:353–362. doi: 10.1258/002367707781282820. [DOI] [PubMed] [Google Scholar]
- 12.Walters EM, Agca Y, Ganjam V, Evans T. Animal models got you puzzled?: think pig. Ann N Y Acad Sci. 2011;1245:63–64. doi: 10.1111/j.1749-6632.2011.06345.x. [DOI] [PubMed] [Google Scholar]
- 13.Breecher MM, Dworken AM. The Merck Manual. Med Herit. 1986;2:229–231. [PubMed] [Google Scholar]
- 14.American Association for Laboratory Animal Science. Normative biological data for common laboratory animal species. In: ALAT training manual., editor. Memphis, TN: American Association for Laboratory Animal Science; 2009. pp. 242–243. [Google Scholar]
- 15.Bildfell RJ. Collection and submission of laboratory samples. The Merck veterinary manual. 10th ed. Whitehouse Station, NJ: Merck & Co., Inc; 2010. pp. 1463–1469. [Google Scholar]
- 16.Bircher N, Safar P. Cerebral preservation during cardiopulmonary resuscitation. Crit Care Med. 1985;13:185–190. doi: 10.1097/00003246-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Benson DM, O’Neil B, Kakish E, Erpelding J, Alousi S, Mason R, Piper D, Rafols J. Open-chest CPR improves survival and neurologic outcome following cardiac arrest. Resuscitation. 2005;64:209–217. doi: 10.1016/j.resuscitation.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Newman B, Gelb AW, Lam AM. The effect of isoflurane-induced hypotension on cerebral blood flow and cerebral metabolic rate for oxygen in humans. Anesthesiology. 1986;64:307–310. doi: 10.1097/00000542-198603000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Lessard MR, Trépanier CA. Renal function and hemodynamics during prolonged isoflurane-induced hypotension in humans. Anesthesiology. 1991;74:860–865. doi: 10.1097/00000542-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Matta BF, Lam AM, Mayberg TS, Eng CC, Strebel S. Cerebrovascular response to carbon dioxide during sodium nitroprusside- and isoflurane-induced hypotension. Br J Anaesth. 1995;74:296–300. doi: 10.1093/bja/74.3.296. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman WE, Edelman G, Ripper R, Koenig HM. Sodium nitroprusside compared with isoflurane-induced hypotension: the effects on brain oxygenation and arteriovenous shunting. Anesth Analg. 2001;93:166–170. doi: 10.1097/00000539-200107000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Kato R, Foëx P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002;49:777–791. doi: 10.1007/BF03017409. [DOI] [PubMed] [Google Scholar]
- 23.Landoni G, Fochi O, Torri G. Cardiac protection by volatile anaesthetics: a review. Curr Vasc Pharmacol. 2008;6:108–111. doi: 10.2174/157016108783955284. [DOI] [PubMed] [Google Scholar]
- 24.Geddes LA, Roeder RA, Rundell AE, Otlewski MP, Kemeny AE, Lottes AE. The natural biochemical changes during ventricular fibrillation with cardiopulmonary resuscitation and the onset of postdefibrillation pulseless electrical activity. Am J Emerg Med. 2006;24:577–581. doi: 10.1016/j.ajem.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Tang W, Snyder D, Wang J, Huang L, Chang YT, Sun S, Weil MH. One-shock versus three-shock defibrillation protocol significantly improves outcome in a porcine model of prolonged ventricular fibrillation cardiac arrest. Circulation. 2006;113:2683–2689. doi: 10.1161/CIRCULATIONAHA.105.592121. [DOI] [PubMed] [Google Scholar]
- 26.Ewy GA, Zuercher M, Hilwig RW, Sanders AB, Berg RA, Otto CW, Hayes MM, Kern KB. Improved neurological outcome with continuous chest compressions compared with 30: 2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation. 2007;116:2525–2530. doi: 10.1161/CIRCULATIONAHA.107.711820. [DOI] [PubMed] [Google Scholar]
- 27.Wu CJ, Li CS, Zhang Y, Yang J, Yin Q, Hang CC. Differences of postresuscitation myocardial dysfunction in ventricular fibrillation versus asphyxiation. Am J Emerg Med. 2013;31:1690–1696. doi: 10.1016/j.ajem.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Niemann JT, Rosborough JP, Youngquist ST, Shah AP. Transthoracic defibrillation potential gradients in a closed chest porcine model of prolonged spontaneous and electrically induced ventricular fibrillation. Resuscitation. 2010;81:477–480. doi: 10.1016/j.resuscitation.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Weil MH, Tang W, Chang YT, Huang L. A comparison of electrically induced cardiac arrest with cardiac arrest produced by coronary occlusion. Resuscitation. 2007;72:477–483. doi: 10.1016/j.resuscitation.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 30.Lancaster LD, Kern KB, Morrison DA, Olajos M, Goldman S. Changes in right ventricular relaxation during acute anterior myocardial infarction in pigs. Cardiovasc Res. 1989;23:46–52. doi: 10.1093/cvr/23.1.46. [DOI] [PubMed] [Google Scholar]
- 31.Indik JH, Allen D, Shanmugasundaram M, Zuercher M, Hilwig RW, Berg RA, Kern KB. Predictors of resuscitation in a swine model of ischemic and nonischemic ventricular fibrillation cardiac arrest: superiority of amplitude spectral area and slope to predict a return of spontaneous circulation when resuscitation efforts are prolonged. Crit Care Med. 2010;38:2352–2357. doi: 10.1097/CCM.0b013e3181fa01ee. [DOI] [PubMed] [Google Scholar]
- 32.Qin H, Walcott GP, Killingsworth CR, Rollins DL, Smith WM, Ideker RE. Impact of myocardial ischemia and reperfusion on ventricular defibrillation patterns, energy requirements, and detection of recovery. Circulation. 2002;105:2537–2542. doi: 10.1161/01.cir.0000016702.86180.f6. [DOI] [PubMed] [Google Scholar]
- 33.Walcott GP, Killingsworth CR, Smith WM, Ideker RE. Biphasic waveform external defibrillation thresholds for spontaneous ventricular fibrillation secondary to acute ischemia. J Am Coll Cardiol. 2002;39:359–365. doi: 10.1016/s0735-1097(01)01723-5. [DOI] [PubMed] [Google Scholar]
- 34.Voelckel WG, Lurie KG, McKnite S, Zielinski T, Lindstrom P, Peterson C, Krismer AC, Lindner KH, Wenzel V. Comparison of epinephrine and vasopressin in a pediatric porcine model of asphyxial cardiac arrest. Crit Care Med. 2000;28:3777–3783. doi: 10.1097/00003246-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Varvarousi G, Goulas S, Agrogiannis G, Valsamakis N, Iliopoulos D, Perrea D, Stefanadis C, Papadimitriou L, Xanthos T. Epinephrine, vasopressin, and nitroglycerin improve neurologic outcome in porcine asphyxial cardiac arrest. Am J Emerg Med. 2012;30:1549–1554. doi: 10.1016/j.ajem.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Sutton RM, Friess SH, Bhalala U, Maltese MR, Naim MY, Bratinov G, Niles D, Nadkarni VM, Becker LB, Berg RA. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López-Herce J, Fernández B, Urbano J, Mencía S, Solana MJ, Rodríguez-Núñez A, Bellón JM, Carrillo A. Hemodynamic, respiratory, and perfusion parameters during asphyxia, resuscitation, and post-resuscitation in a pediatric model of cardiac arrest. Intensive Care Med. 2011;37:147–155. doi: 10.1007/s00134-010-2006-2. [DOI] [PubMed] [Google Scholar]
- 38.Mayr VD, Raedler C, Wenzel V, Lindner KH, Strohmenger HU. A comparison of epinephrine and vasopressin in a porcine model of cardiac arrest after rapid intravenous injection of bupivacaine. Anesth Analg. 2004;98:1426–1431, table of contents. doi: 10.1213/01.ane.0000108488.05900.a8. [DOI] [PubMed] [Google Scholar]
- 39.Gilmore CM, Rea TD, Becker LJ, Eisenberg MS. Three-phase model of cardiac arrest: time-dependent benefit of bystander cardiopulmonary resuscitation. Am J Cardiol. 2006;98:497–499. doi: 10.1016/j.amjcard.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 40.Sharma AB, Knott EM, Bi J, Martinez RR, Sun J, Mallet RT. Pyruvate improves cardiac electromechanical and metabolic recovery from cardiopulmonary arrest and resuscitation. Resuscitation. 2005;66:71–81. doi: 10.1016/j.resuscitation.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Abella BS, Sandbo N, Vassilatos P, Alvarado JP, O’Hearn N, Wigder HN, Hoffman P, Tynus K, Vanden Hoek TL, Becker LB. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111:428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 42.Ristagno G, Tang W, Chang YT, Jorgenson DB, Russell JK, Huang L, Wang T, Sun S, Weil MH. The quality of chest compressions during cardiopulmonary resuscitation overrides importance of timing of defibrillation. Chest. 2007;132:70–75. doi: 10.1378/chest.06-3065. [DOI] [PubMed] [Google Scholar]
- 43.Idris AH, Guffey D, Aufderheide TP, Brown S, Morrison LJ, Nichols P, Powell J, Daya M, Bigham BL, Atkins DL, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125:3004–3012. doi: 10.1161/CIRCULATIONAHA.111.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace SK, Abella BS, Becker LB. Quantifying the effect of cardiopulmonary resuscitation quality on cardiac arrest outcome: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2013;6:148–156. doi: 10.1161/CIRCOUTCOMES.111.000041. [DOI] [PubMed] [Google Scholar]
- 45.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 46.Bohm K, Rosenqvist M, Herlitz J, Hollenberg J, Svensson L. Survival is similar after standard treatment and chest compression only in out-of-hospital bystander cardiopulmonary resuscitation. Circulation. 2007;116:2908–2912. doi: 10.1161/CIRCULATIONAHA.107.710194. [DOI] [PubMed] [Google Scholar]
- 47.Iwami T, Kawamura T, Hiraide A, Berg RA, Hayashi Y, Nishiuchi T, Kajino K, Yonemoto N, Yukioka H, Sugimoto H, et al. Effectiveness of bystander-initiated cardiac-only resuscitation for patients with out-of-hospital cardiac arrest. Circulation. 2007;116:2900–2907. doi: 10.1161/CIRCULATIONAHA.107.723411. [DOI] [PubMed] [Google Scholar]
- 48.Berger S. Gasping, survival, and the science of resuscitation. Circulation. 2008;118:2495–2497. doi: 10.1161/CIRCULATIONAHA.108.823203. [DOI] [PubMed] [Google Scholar]
- 49.Nagao K. Chest compression-only cardiocerebral resuscitation. Curr Opin Crit Care. 2009;15:189–197. doi: 10.1097/MCC.0b013e3283295f2c. [DOI] [PubMed] [Google Scholar]
- 50.Rea TD, Fahrenbruch C, Culley L, Donohoe RT, Hambly C, Innes J, Bloomingdale M, Subido C, Romines S, Eisenberg MS. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010;363:423–433. doi: 10.1056/NEJMoa0908993. [DOI] [PubMed] [Google Scholar]
- 51.Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- 52.Mentzelopoulos SD, Zakynthinos SG, Siempos I, Malachias S, Ulmer H, Wenzel V. Vasopressin for cardiac arrest: meta-analysis of randomized controlled trials. Resuscitation. 2012;83:32–39. doi: 10.1016/j.resuscitation.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Tang W, Weil MH, Gazmuri RJ, Sun S, Duggal C, Bisera J. Pulmonary ventilation/perfusion defects induced by epinephrine during cardiopulmonary resuscitation. Circulation. 1991;84:2101–2107. doi: 10.1161/01.cir.84.5.2101. [DOI] [PubMed] [Google Scholar]
- 54.Thrush DN, Downs JB, Smith RA. Is epinephrine contraindicated during cardiopulmonary resuscitation? Circulation. 1997;96:2709–2714. doi: 10.1161/01.cir.96.8.2709. [DOI] [PubMed] [Google Scholar]
- 55.Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation. 1988;78:382–389. doi: 10.1161/01.cir.78.2.382. [DOI] [PubMed] [Google Scholar]
- 56.Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92:3089–3093. doi: 10.1161/01.cir.92.10.3089. [DOI] [PubMed] [Google Scholar]
- 57.Niemann JT, Haynes KS, Garner D, Rennie CJ, Jagels G, Stormo O. Postcountershock pulseless rhythms: response to CPR, artificial cardiac pacing, and adrenergic agonists. Ann Emerg Med. 1986;15:112–120. doi: 10.1016/s0196-0644(86)80003-8. [DOI] [PubMed] [Google Scholar]
- 58.Tovar OH, Jones JL. Epinephrine facilitates cardiac fibrillation by shortening action potential refractoriness. J Mol Cell Cardiol. 1997;29:1447–1455. doi: 10.1006/jmcc.1997.0387. [DOI] [PubMed] [Google Scholar]
- 59.Lindner KH, Brinkmann A, Pfenninger EG, Lurie KG, Goertz A, Lindner IM. Effect of vasopressin on hemodynamic variables, organ blood flow, and acid-base status in a pig model of cardiopulmonary resuscitation. Anesth Analg. 1993;77:427–435. doi: 10.1213/00000539-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Wenzel V, Lindner KH, Krismer AC, Miller EA, Voelckel WG, Lingnau W. Repeated administration of vasopressin but not epinephrine maintains coronary perfusion pressure after early and late administration during prolonged cardiopulmonary resuscitation in pigs. Circulation. 1999;99:1379–1384. doi: 10.1161/01.cir.99.10.1379. [DOI] [PubMed] [Google Scholar]
- 61.Cherry BH, Nguyen AQ, Williams AG Jr. Scott GF, Hollrah RA, Ryou MG, Hoxha B, Olivencia-Yurvati AH, Mallet RT. Vasopressin instead of epinephrine enhances efficacy of CPR without causing tachycardia. FASEB J. 2014;28:1150.5. [Google Scholar]
- 62.Dumas F, White L, Stubbs BA, Cariou A, Rea TD. Long-term prognosis following resuscitation from out of hospital cardiac arrest: role of percutaneous coronary intervention and therapeutic hypothermia. J Am Coll Cardiol. 2012;60:21–27. doi: 10.1016/j.jacc.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 63.Hörburger D, Testori C, Sterz F, Herkner H, Krizanac D, Uray T, Schober A, Stöckl M, Stratil P, Weiser C, et al. Mild therapeutic hypothermia improves outcomes compared with normothermia in cardiac-arrest patients--a retrospective chart review. Crit Care Med. 2012;40:2315–2319. doi: 10.1097/CCM.0b013e31825333cf. [DOI] [PubMed] [Google Scholar]
- 64.Wang CJ, Yang SH, Lee CH, Lin RL, Peng MJ, Wu CL. Therapeutic hypothermia application vs standard support care in post resuscitated out-of-hospital cardiac arrest patients. Am J Emerg Med. 2013;31:319–325. doi: 10.1016/j.ajem.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 65.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 66.Chalothorn D, McCune DF, Edelmann SE, García-Cazarín ML, Tsujimoto G, Piascik MT. Differences in the cellular localization and agonist-mediated internalization properties of the alpha(1)-adrenoceptor subtypes. Mol Pharmacol. 2002;61:1008–1016. doi: 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- 67.Smith J, Penninckx JJ, Kampschulte S, Safar P. Need for oxygen enrichment in myocardial infarction, shock and following cardiac arrest. Acta Anaesthesiol Scand Suppl. 1968;29:127–145. doi: 10.1111/j.1399-6576.1968.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 68.O’Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63 Suppl 6:vi1–v68. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 69.Mantell LL, Lee PJ. Signal transduction pathways in hyperoxia-induced lung cell death. Mol Genet Metab. 2000;71:359–370. doi: 10.1006/mgme.2000.3046. [DOI] [PubMed] [Google Scholar]
- 70.Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007;38:1578–1584. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richards EM, Rosenthal RE, Kristian T, Fiskum G. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med. 2006;40:1960–1970. doi: 10.1016/j.freeradbiomed.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koch JD, Miles DK, Gilley JA, Yang CP, Kernie SG. Brief exposure to hyperoxia depletes the glial progenitor pool and impairs functional recovery after hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2008;28:1294–1306. doi: 10.1038/jcbfm.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 74.Pilcher J, Weatherall M, Shirtcliffe P, Bellomo R, Young P, Beasley R. The effect of hyperoxia following cardiac arrest - A systematic review and meta-analysis of animal trials. Resuscitation. 2012;83:417–422. doi: 10.1016/j.resuscitation.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 75.Solberg R, Longini M, Proietti F, Vezzosi P, Saugstad OD, Buonocore G. Resuscitation with supplementary oxygen induces oxidative injury in the cerebral cortex. Free Radic Biol Med. 2012;53:1061–1067. doi: 10.1016/j.freeradbiomed.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 76.Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014;85:1142–1148. doi: 10.1016/j.resuscitation.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 77.Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37:3008–3013. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, Perkins GD. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81:1305–1352. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 79.Niemann JT, Stratton SJ, Cruz B, Lewis RJ. Outcome of out-of-hospital postcountershock asystole and pulseless electrical activity versus primary asystole and pulseless electrical activity. Crit Care Med. 2001;29:2366–2370. doi: 10.1097/00003246-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 80.Rea TD, Eisenberg MS, Sinibaldi G, White RD. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004;63:17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 81.Atwood C, Eisenberg MS, Herlitz J, Rea TD. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation. 2005;67:75–80. doi: 10.1016/j.resuscitation.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Kajino K, Iwami T, Daya M, Nishiuchi T, Hayashi Y, Ikeuchi H, Tanaka H, Shimazu T, Sugimoto H. Subsequent ventricular fibrillation and survival in out-of-hospital cardiac arrests presenting with PEA or asystole. Resuscitation. 2008;79:34–40. doi: 10.1016/j.resuscitation.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 83.Rea TD, Helbock M, Perry S, Garcia M, Cloyd D, Becker L, Eisenberg M. Increasing use of cardiopulmonary resuscitation during out-of-hospital ventricular fibrillation arrest: survival implications of guideline changes. Circulation. 2006;114:2760–2765. doi: 10.1161/CIRCULATIONAHA.106.654715. [DOI] [PubMed] [Google Scholar]
- 84.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garza AG, Gratton MC, Salomone JA, Lindholm D, McElroy J, Archer R. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119:2597–2605. doi: 10.1161/CIRCULATIONAHA.108.815621. [DOI] [PubMed] [Google Scholar]
- 86.Warner LL, Hoffman JR, Baraff LJ. Prognostic significance of field response in out-of-hospital ventricular fibrillation. Chest. 1985;87:22–28. doi: 10.1378/chest.87.1.22. [DOI] [PubMed] [Google Scholar]
- 87.Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med. 2001;344:1304–1313. doi: 10.1056/NEJM200104263441707. [DOI] [PubMed] [Google Scholar]
- 88.Fujii J, Otsu K, Zorzato F, de Leon S, Khanna VK, Weiler JE, O’Brien PJ, MacLennan DH. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991;253:448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- 89.Mickelson JR, Louis CF. Malignant hyperthermia: excitation-contraction coupling, Ca2+ release channel, and cell Ca2+ regulation defects. Physiol Rev. 1996;76:537–592. doi: 10.1152/physrev.1996.76.2.537. [DOI] [PubMed] [Google Scholar]
- 90.Liou YM, Jiang MJ, Wu MC. Altered expression of cardiac myosin isozymes associated with the malignant hyperthermia genotype in swine. Anesthesiology. 2000;93:1312–1319. doi: 10.1097/00000542-200011000-00026. [DOI] [PubMed] [Google Scholar]
- 91.Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2010;298:H1182–H1189. doi: 10.1152/ajpheart.00888.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, et al. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol (1985) 2012;113:1128–1140. doi: 10.1152/japplphysiol.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lurie KG, Zielinski T, McKnite S, Aufderheide T, Voelckel W. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation. 2002;105:124–129. doi: 10.1161/hc0102.101391. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Ristagno G, Bisera J, Tang W, Deng Q, Weil MH. Electrocardiogram waveforms for monitoring effectiveness of chest compression during cardiopulmonary resuscitation. Crit Care Med. 2008;36:211–215. doi: 10.1097/01.CCM.0000295594.93345.A2. [DOI] [PubMed] [Google Scholar]
- 95.Indik JH, Shanmugasundaram M, Allen D, Valles A, Kern KB, Hilwig RW, Zuercher M, Berg RA. Predictors of resuscitation outcome in a swine model of VF cardiac arrest: A comparison of VF duration, presence of acute myocardial infarction and VF waveform. Resuscitation. 2009;80:1420–1423. doi: 10.1016/j.resuscitation.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hang CC, Li CS, Wu CJ, Yang J. Acute kidney injury after cardiac arrest of ventricular fibrillation and asphyxiation swine model. Am J Emerg Med. 2014;32:208–215. doi: 10.1016/j.ajem.2013.10.043. [DOI] [PubMed] [Google Scholar]


