Abstract
The Stewart approach-the application of basic physical-chemical principles of aqueous solutions to blood-is an appealing method for analyzing acid-base disorders. These principles mainly dictate that pH is determined by three independent variables, which change primarily and independently of one other. In blood plasma in vivo these variables are: (1) the PCO2; (2) the strong ion difference (SID)-the difference between the sums of all the strong (i.e., fully dissociated, chemically nonreacting) cations and all the strong anions; and (3) the nonvolatile weak acids (Atot). Accordingly, the pH and the bicarbonate levels (dependent variables) are only altered when one or more of the independent variables change. Moreover, the source of H+ is the dissociation of water to maintain electroneutrality when the independent variables are modified. The basic principles of the Stewart approach in blood, however, have been challenged in different ways. First, the presumed independent variables are actually interdependent as occurs in situations such as: (1) the Hamburger effect (a chloride shift when CO2 is added to venous blood from the tissues); (2) the loss of Donnan equilibrium (a chloride shift from the interstitium to the intravascular compartment to balance the decrease of Atot secondary to capillary leak; and (3) the compensatory response to a primary disturbance in either independent variable. Second, the concept of water dissociation in response to changes in SID is controversial and lacks experimental evidence. In addition, the Stewart approach is not better than the conventional method for understanding acid-base disorders such as hyperchloremic metabolic acidosis secondary to a chloride-rich-fluid load. Finally, several attempts were performed to demonstrate the clinical superiority of the Stewart approach. These studies, however, have severe methodological drawbacks. In contrast, the largest study on this issue indicated the interchangeability of the Stewart and conventional methods. Although the introduction of the Stewart approach was a new insight into acid-base physiology, the method has not significantly improved our ability to understand, diagnose, and treat acid-base alterations in critically ill patients.
Keywords: Acid-base metabolism, Stewart approach, Base excess, Bicarbonate, Anion gap, Strong ion difference, Strong ion gap
Core tip: In this article, we comprehensively reviewed the evidence that has been used to argue for the superiority of the Stewart approach over the traditional method for the analysis of acid-base metabolism in critically ill patients. The basic principles of the Stewart approach have severe weaknesses. In addition, the contribution of this method to the understanding of mechanisms is minor; furthermore, from a clinical standpoint, the Stewart approach has no advantage for diagnostic or prognostic purposes compared to the analysis based on bicarbonate, base excess, and albumin-corrected anion gap.
INTRODUCTION
Acid-base disorders are usually found in critically ill patients. Thus, the understanding and identification of these derangements is crucial to the practice of critical-care medicine. Without a doubt, the Stewart approach is an appealing method to analyze acid-base metabolism. The so-called quantitative physicochemical approach has triggered opposing opinions that seem to be related more to passion than to science. The Stewart approach was conceived as a method to revolutionize our ability to understand, predict, and control what happens to hydrogen ions in living systems[1], whereas the method has instead been characterized as absurd and anachronistic[2].
The goal of this review is to comprehensively discuss the evidence supporting the conclusion that the Stewart approach, although innovative and attractive, does not significantly contribute to the diagnosis of acid-base abnormalities in critically ill patients.
APPROACHES TO ACID-BASE METABOLISM: THE TRADITIONAL AND THE STEWART APPROACHES
Acid-base disorders can conceivably be described by different methods: First, by a traditional approach, in which the metabolic component of acid-base physiology is based on the analysis of plasma concentrations of bicarbonate (HCO3-)[3]. This basis be further completed with the use of base excess (BE)[4]. Despite considerable argument over which parameter is better[5-9], both are usually employed in clinical practice, and all blood-gas analyzers include both calculations. Anion gap (AG) constitutes an additional diagnostic contribution[10], though hypoalbuminemia might preclude its usefulness. For this reason, many researchers have recommended to adjust AG to the albumin level (AGcorrected)[11-16].
An alternative approach is the application of basic physical-chemical principles of aqueous solutions to blood[1]. Some of the bases of this so-called Stewart approach are: (1) the protons of medium come from dissociation of the water to maintain electroneutrality; (2) the pH is determined by three parameters called “independent variables” because they change primarily and independently of each other (Figure 1). In blood plasma in vivo these variables are: (a) the PCO2; (b) the “strong ion difference” (SID), i.e., the difference between the sums of all the strong (fully dissociated, chemically nonreacting) cations (Na+, K+, Ca2+, Mg2+) and all the strong anions (Cl- plus other strong anions such as ketones and lactate); (c) the concentrations of nonvolatile weak acids (Atot), that is, the sum of their dissociated and undissociated forms. Accordingly, neither the pH nor the bicarbonate (dependent variables) can be altered unless one or more of the independent variables change; and (3) The assessment of the metabolic component of acid-base physiology relies on the analysis of plasma SID and Atot.
Figure 1.
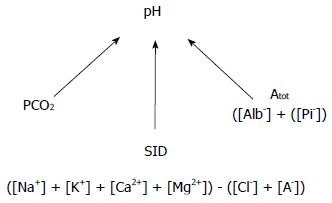
Independent determinants of pH according to the Stewart approach.
According to the Stewart approach, metabolic acidosis only occurs if the SID decreases or the Atot increases. On the contrary, metabolic alkalosis develops only if the SID increases or the Atot decreases.
In addition, the Stewart method allows the quantification of the magnitude of each acid-base disorder comparing actual values of the SID and the Atot with normal reference values. Moreover, the approach also allows the computation of the effect of each individual component (Na+, K+, Ca2+, Mg2+, Cl-, strong ion gap (SIG), albumin, and phosphate) on the SID and Atot. The Stewart-approach supporters argue that the strength of the method lies in being essentially quantitative because the technique not only measures the magnitude of the deviation of all variables from the normal range but is also mechanistic, as it provides a clear idea of the causes of the acid-base disorders[1].
DRAWBACKS OF THE PRINCIPLES UNDERLYING THE STEWART APPROACH
Although the Stewart method constitutes an interesting analysis of acid-base metabolism, some of the underlying principles have been questioned:(1) Stewart stated that the protons of the environment come from the dissociation of the water. For example, low SID increases H+ secondary to water dissociation. This concept, however, is controversial and lacks of experimental evidence; and (2) are the variables SID, PCO2 and Atot really independent of one another? An independent variable is defined as one that influences the system but is not influenced by the system. The term “system” here, refers to any single aqueous compartment (plasma, the interstitium, the intercellular, or cerebrospinal fluids). Within this scenario, PCO2, Atot, and SID fulfill the criteria for independent variables because those parameters directly influence the dissociation reactions that generate weak electrolytes, while they themselves are determined by distinctly separate control mechanisms[1]. The Stewart analysis, however, involves a single-compartment model and therefore does not take into account exchanges with red blood cells or with the interstitium as occurs when dealing with whole blood; the latter being considered as a tricompartmental model (intersititium, plasma, erythrocytes). In such a setting, PCO2, SID and Atot are not completely independent from each other[17-20], where this lack of independence is exemplified by the following situations: (1) PCO2/SID interaction: The Hamburger effect or “chloride shift” is defined as the exchange between Cl- and HCO3- caused by the addition to the venous plasma of CO2 produced by the cellular metabolism[17-20]. In this condition, the increase in plasma PCO2 and HCO3- is associated with the entrance of chloride in red blood cells, with the ensuing reduction in the plasma Cl-. As a consequence of this process, the blood Cl- becomes lower in the venous than in the arterial blood; (2) Atot/SID: The loss of Donnan equilibrium describes the shift of chloride from the interstitium to the intravascular compartment. This change is produced in order to balance the decrease in Atot secondary to an albumin transudation from the intravascular space in patients with capillary damage and thus an increased permeability[21]; and (3) the compensatory response to a primary disturbance in either independent variable: In these situations, an adjustment in other variables occurs. For example, hypercapnia causes an increased H+, which is compensated by a decrease in Cl-[22] along with an increase in the SID. On the other hand, the compensatory response to reduction in Atot (hypoproteinemia) is a decrease in SID, secondary to an increase in Cl-[23]. The net result of these complementary changes in these theoretically independent variables is an amelioration of the effect of the primary disorder on H+.
In summary, contrary to the principles of Stewart approach, SID, PCO2 and Atot can be considered not completely independent from each other within certain particular settings (e.g., blood plasma in vivo).
UNDERSTANDING THE MECHANISMS OF ACID-BASE ALTERATIONS
A relevant question has to do with an understanding of the mechanisms that underlie the development of hyperchloremic metabolic acidosis after fluid resuscitation with chloride-rich solutions. The traditional approach states that acidosis is caused by a dilution of plasma HCO3-[24-26]. This classical dilution concept regarding bicarbonate is rejected by the proponents of Stewart’s approach, who highlight the mechanistic insight into acid-base physiology as the method’s main strength and principal advantage over the traditional model. Therefore, the Stewart approach provides a “strong-ion”-based explanation for the mechanism of dilutional acidosis. They argue
that dilutional acidosis is explained by a decrease in the SID[1,27-31].
This issue has been comprehensively studied by other researchers[32,33]. Based on simulations of dilution studies along with in vitro experiments, they tried to clarify the chemical mechanism responsible for dilutional acidosis. Consequently, they examined the effects of diluting normal extracellular fluid with different solutions both in a closed system (i.e., a system not exchanging matter with the environment, such as venous blood before reaching the lung) and in a system open to gases (i.e., one capable of equilibrating with the PCO2). They observed that dilution of extracellular fluid did not lead to any detectable change in the H+ when the system was closed. The explanation was that all the determinants of the H+, SID, PCO2 and Atot were equally diluted so that their relative proportions did not change. In actuality, the decrease in the SID (leading to acidosis) was exactly balanced by the decrease in the CO2 content and noncarbonic buffers (leading to alkalosis). As a consequence, the pH did not change. On the contrary, acidosis was only found when the system was open to the gases with normal PCO2 (40 mmHg). In this situation, the CO2 entered into the system because of the differing tensions between the gas phase and the diluted solution until the PCO2 was equilibrated. Therefore, the excess of protons observed in this dilutional acidosis came from CO2 hydration to carbonic acid (Figure 2). In other words, the chemical mechanism of the dilutional acidosis in blood plasma involves the dilution of an open CO2/HCO3- buffer system, where the buffer base (i.e., the HCO3-) is diluted but not the buffer acid (the CO2).
Figure 2.
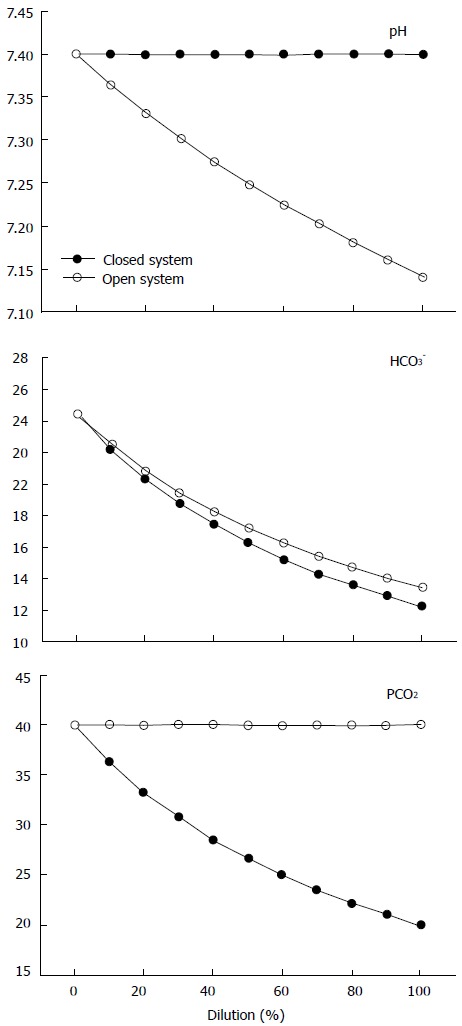
Behavior of pH (top), HCO3- (middle) and PCO2 (bottom) in a closed system (black dots) and in an open system with a PCO2 of 40 mmHg (withe dots), during stepwise dilution with 0.9% NaCl, as modified from Gattinoni et al[32].
Stated in brief, the Stewart and the traditional approaches may account for these results[32,33]: (1) according to Stewart’s approach, since the SID and the Atot remain unchanged after opening the system to gases, the only determinant of the decrease in the pH is the increase in PCO2 or, more precisely, the increase in CO2 content. Therefore, the change in the SID - it being merely a mathematical construct - is not the cause of dilutional acidosis, but rather a marker for the dilutional process. In addition, the increase in water dissociation is not the chemical mechanism of dilutional acidosis, and consequently the Stewart approach does not provide any mechanistic insight into acid-base disorders[33]; and (2) according to the traditional model, the acidosis is explained by the increase in the PCO2 in the face of a dilution of the buffer’s base.
CLINICAL USEFULNESS OF THE STEWART APPROACH IN CRITICALLY ILL PATIENTS
Although the principles of the Stewart approach have weaknesses and this method does not offer clear advantages for explaining mechanisms, several attempts have been made to show the superiority of that approach over conventional analysis in the diagnosis of acid-base alterations in critically ill patients.
The first question is if the SID is really different from the buffer base concentration (BB). The SID is actually equal to the buffer base described more than half a century ago. Consequently, the BE becomes the deviation of SID from its normal value. The SID and the BB are mirror images of each other[34].
Nevertheless, Fencl et al[35] studied a series of 152 critically ill patients and concluded that the Stewart approach allowed a detection and quantification of all the various individual components of even the most complex acid-base disturbances seen in critically ill patients[35]. In that study, the Stewart approach was able to detect metabolic acidosis in 20 patients with normal HCO3- and in 22 patients with normal BE. Low SID was unnoticed by changes in BE, because the low SID acidosis was masked by the alkalinizing effect of hypoalbuminemia. The AGcorrected, however, adequately identified all patients with elevated unmeasured anions. For this reason, a correct use of the traditional approach would have allowed a similar diagnosis. In addition, in normal volunteers, the SIG, the variable from the Stewart approach that quantifies unmeasured anions, was 8 mEq/L, which is an extremely high value. The expected values should have been close to zero. This finding suggests the presence of some methodological error.
In another study, Boniatti et al[36] concluded that their main result was the demonstration of a greater sensitivity on the part of physicochemical evaluation in identifying acid-base disorders in critically ill patients[36]. An evaluation according to the Stewart method allowed an additional diagnosis of a metabolic disorder in 34% of the cases, because of the greater sensitivity of the SID compared to BE. These results, however, might have been expected because of the methodological limitations of the study. The authors considered as normal BE values from -5 to 5 mmol/L, while the normal SID was arbitrarily defined as values from 38 to 42 mmol/L. Consequently, the diagnosis of metabolic acidosis required a decrease in the BB of 5 mmol/L, when the BE was used as the criterion. In contrast, a reduction of only 2 mmol/L in BB identified the presence of metabolic acidosis when the SID was used. Therefore, the use of a more sensitive threshold for the diagnosis of metabolic acidosis by means of SID completely explained the results. This study has several other limitations - such as not measuring the arterial blood gases and electrolytes simultaneously, the negative values of SIG that were frequently found, the arbitrary choice of normal ranges, and the failure to evaluate the agreement between the acid-base variables of both approaches. Finally, the authors presented two cases to illustrate the diagnosis of metabolic acidosis by means of the Stewart approach (Table 1), but unfortunately those two were misinterpreted. Actually, the authors mistakenly chose patients with respiratory alkalosis instead of metabolic acidosis. The presence of the low SID was the result of the renal compensation for a respiratory alkalosis, which condition was the primary diagnosis of their cases, as indicated by the high pH and low PCO2 values. As previously shown, the use of the Stewart approach, without consideration of the metabolic response to a primary respiratory disorder can lead to an incorrect diagnosis in 15% of the cases[37]. Indeed, the examples cited here adequately and definitively illustrate the very drawbacks of the Stewart approach, instead of its advantages.
Table 1.
Examples of acid-base disorders
| Patient 1 | Patient 2 | |
| Measured variables | ||
| Sodium (mmol/L) | 151 | 146 |
| Potassium (mmol/L) | 3.4 | 3.8 |
| Calcium (mg/dL) | 7.0 | 7.2 |
| Magnesium (mmol/L) | 2.0 | 1.8 |
| Phosphate (mmol/L) | 1.0 | 2.0 |
| Albumin (g/L) | 27.0 | 27.0 |
| Chloride (mmol/L) | 121 | 124 |
| pH | 7.48 | 7.43 |
| PaCO2 (mmHg) | 29.0 | 30.2 |
| Lactate (mmol/L) | 2.0 | 1.3 |
| Derived variables | ||
| HCO3- (mmol/L) | 21.5 | 20 |
| BE (mmol/L) | -0.7 | -3.8 |
| AG (mmol/L) | 12.4 | 6.3 |
| SID (mmol/L) | 29.9 | 28.8 |
| SIG (mmol/L) | 4.5 | -1.4 |
BE: Base excess; AG: Anion gap; SID: Strong ion difference; SIG: Strong ion gap. Modified from Boniatti et al[36].
Kaplan et al[38] performed a controlled study to show that the clinical use of the Stewart approach improved the accuracy of acid-base diagnosis and reduced the possibility of an inappropriate fluid loading. For this purpose, one-hundred consecutive trauma patients admitted to a surgical ICU were prospectively allocated to care by either one physician who was to use the Stewart approach or four other practitioners who would employ the conventional method. The diagnoses and interventions made by the “conventional physicians” were reviewed by the “Stewart physician”. The results showed that the conventional approach missed a lot of diagnoses. Moreover, the acid-base balance normalized sooner (3.3 ± 3.4 d vs 8.3 ± 7.4 d) and fewer volume expansions were given through the use of Stewart approach. The ostensible conclusion was that the physician who used the Stewart approach more correctly diagnosed and treated the patients compared to the other four physicians who only utilized the conventional method. Nevertheless, the criteria used by these physicians were not presented in the study, and the Stewart physician himself determined what was right or wrong without any established definition. For example, 43 of the 50 patients treated by the physicians who used the traditional analysis were unnecessarily volume-expanded because of the presence of hyperchloremic metabolic acidosis. The Stewart approach, however, would not have been needed for that diagnosis. Consequently, the absence of a well-defined methodology precludes any conclusion from this trial.
Some studies have assessed SIG as a potential tool, not only for the measurement of anions but also as a surrogate of tissue hypoperfusion and a predictor of outcome. A theoretical advantage of SIG over AG is that the parameter remains stable and reliable, even in cases of extreme variations in the PCO2 and pH[39].
Kaplan et al[40] studied the acid-base determinants of the outcome in trauma patients with major vascular injuries and concluded that SIG was a better predictor of mortality than AG[40]. Regrettably, these conclusions were not supported by the findings. The area under the ROC curve and the confidence intervals for SIG, BE and AG were quite similar. Therefore, these acid-base variables showed the same prognostic ability.
Other studies performed in pediatric critically ill patients came to similar conclusions[41,42]. The SIG was more strongly associated with mortality than traditional variables such as BE, AG, or lactate levels. Nevertheless, a proper evaluation of the unmeasured anions by the traditional method was not performed because the AG values were not corrected with respect to albumin levels.
A study by Funk et al[43] investigated the association between the SIG and the long-term outcome after cardiac arrest, in patients treated with therapeutic hypothermia[43]. The authors concluded that the SIG, measured 12 h after the return of spontaneous circulation, was an independent predictor of outcome. The AG and the SIG were strongly correlated, but the predictive capacity of the AG was not tested. Surprisingly, an editorial entitled “Another Nail in the Coffin of Traditional Acid-base Quantification” was published along with this same study[44].
We compared the traditional and Stewart approaches in a series of 935 critically ill patients and in 7 healthy volunteers in order to demonstrate that the Stewart approach does not offer any diagnostic or prognostic advantage[37]. With the use of an analysis based on HCO3-, BE and AGcorrected, only 1% of the patients with low SID acidosis were left undiagnosed. In contrast, diagnosis by the Stewart approach was normal in 2% of the patients in whom metabolic acidosis was identified by the criteria of decreased HCO3- and BE, and increased AGcorrected. Moreover, in normal volunteers, BE and SID, and AGcorrected and SIG were strongly correlated, exhibiting narrow limits of agreement (Figure 3). Something similar occurred in the critically ill patients (Figure 4). In addition, the prognostic ability of the different acid-base parameters was similar. The results from this study suggest that the approaches are rather similar in terms of diagnostic and prognostic performance.
Figure 3.
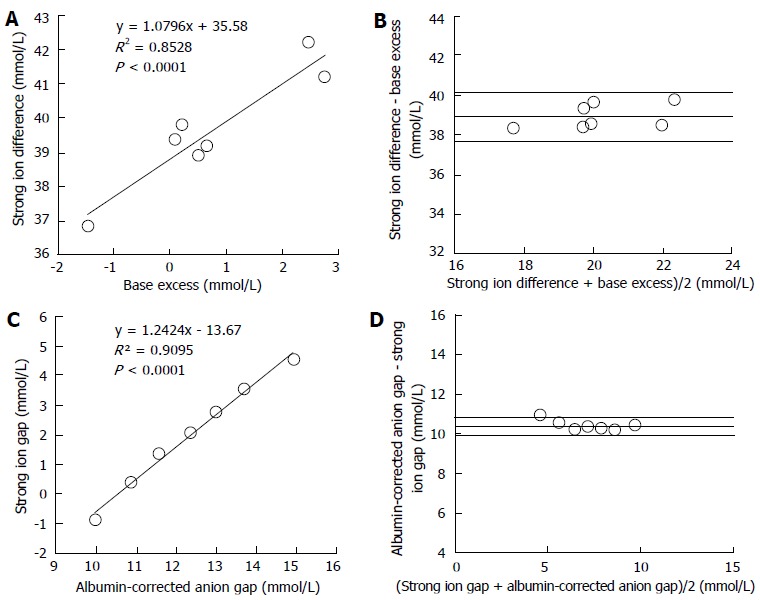
Regression and Bland and Altman analysis between metabolic parameters of different approaches in seven normal volunteers. A: Lineal regression between base excess and strong ion difference; B: Agreement between base excess and strong ion difference; C: Lineal regression between albumin-corrected anion gap and strong ion gap; D: Agreement between albumin-corrected anion gap and strong ion gap; Panel B and D display the relationship between the mean value and the difference of both measurements. The lines indicate the mean difference between both parameters (bias) ± 2 SD (95% limits of agreement). Modified from Dubin et al[37].
Figure 4.
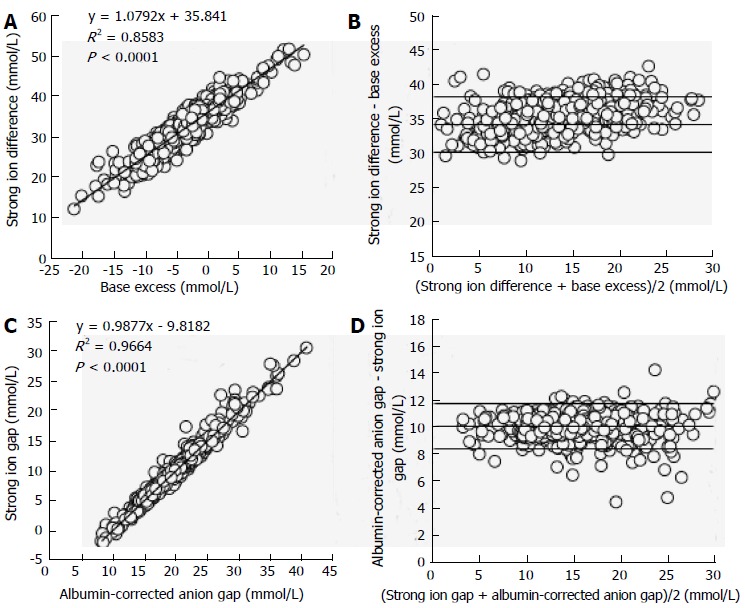
Regression and Bland and Altman analysis between metabolic parameters of different approaches in 935 critically ill patients. A: Lineal regression between base excess and strong ion difference; B: Agreement between base excess and strong ion difference; C: Lineal regression between albumin-corrected anion gap and strong ion gap; D: Agreement between albumin-corrected anion gap and strong ion gap. Panel B and D display the relationship between the mean value and the difference between both measurements. The lines indicate the mean difference between both parameters (bias) ± 2 SD (95% limits of agreement). Modified from Dubin et al[37].
Another relevant issue with the Stewart approach is the poor reproducibility with respect to the determination of its variables. A study analyzed 179 routine blood samples from consecutive patients over a 3-mo period. The determinations were performed by two automated blood-chemistry analyzers. An analysis of the agreement obtained indicated a lack of reproducibility among the simultaneous measurements as illustrated by the wide 95% limits of agreement: 10 mmol/L for SID and 12 mmol/L for SIG[45].
Finally, we demonstrated a similar diagnostic performance for the two approaches in a complex metabolic disorder[46]. Of the patients admitted to the ICU with severe hyperlactatemia (lactate levels ≥ 4 mmol/L), some 20% had normal pH, HCO3-, and BE - but also normal SID. This finding was explained by the simultaneous presence of hypochloremic metabolic alkalosis. Equimolar changes had occurred in the variables of the two approaches that had allowed the identification of the mixed metabolic alteration. The Stewart approach showed normal SID values together with a low chloride level, while the traditional analysis indicated an increase in the difference between the changes in AG and HCO3- (Figures 5 and 6). Consequently, the Stewart and conventional approaches were able to describe this complex acid-base disorder in a similar way.
Figure 5.
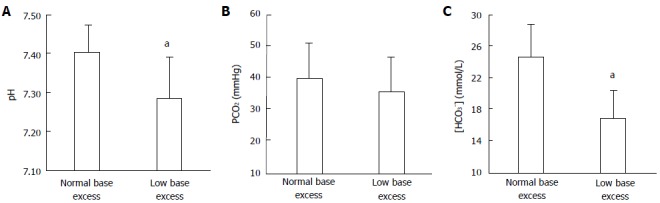
Arterial pH, and bicarbonate levels in patients with severe hyperlactatemia. Values for (A) arterial pH, (B) PCO2, and (C) bicarbonate ([HCO3-]) in patients with severe hyperlactatemia, with normal or low base excess. aP < 0.05 vs the other group.
Figure 6.
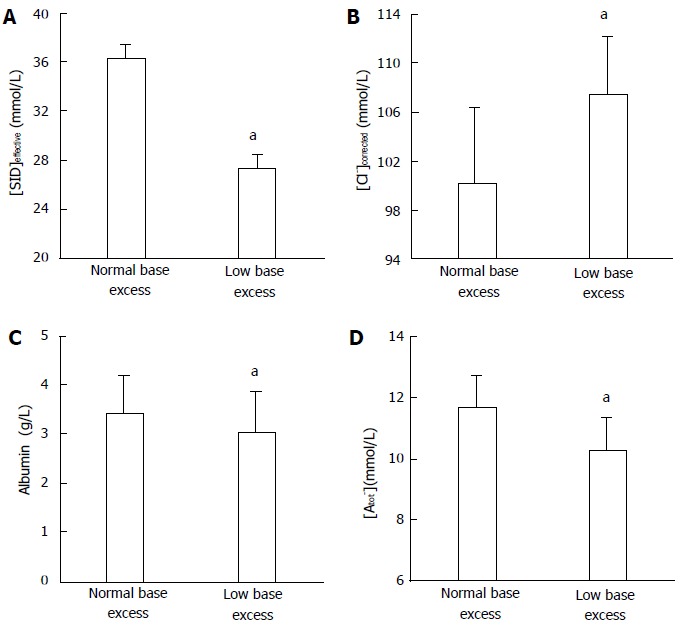
Strong-ion difference, sodium-corrected chloride, albumin, and nonvolatile weak acid levels in severe hyperlactatemia patients. Values for (A) the effective strong-ion difference (SIDeffective), (B) sodium-corrected chloride levels (Cl-corrected), (C) the albumin concentration, and (D) nonvolatile weak acid (Atot) levels in patients with severe hyperlactatemia, with normal or low base excess. aP < 0.05 vs the other group. SIDeffective: Effective strong-ion difference.
CONCLUSION
The Stewart approach has allowed a new insight into acid-base physiology. Unfortunately, the introduction of that method did not result in relevant advantages, compared to the judicious use of HCO3-, BE, and AGcorrected either for an understanding of the mechanisms of acid-base alterations or for diagnosis or prognosis. Furthermore, the Stewart approach is cumbersome, requires more determinations and calculations, and is more time-consuming and expensive. On the basis of the compelling evidence that we have discussed here, in order to improve acid-base evaluation we only need to continue with the proper use of the old tools.
Footnotes
Conflict-of-interest: The authors have no conflict of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 28, 2014
First decision: December 17, 2014
Article in press: Janurary 19, 2015
P- Reviewer: Abdel-Salam OME, Jeschke MG, Saniabadi AR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 2.Siggaard-Andersen O, Fogh-Andersen N. Base excess or buffer base (strong ion difference) as measure of a non-respiratory acid-base disturbance. Acta Anaesthesiol Scand Suppl. 1995;107:123–128. doi: 10.1111/j.1399-6576.1995.tb04346.x. [DOI] [PubMed] [Google Scholar]
- 3.Narins RG. (Editor). Maxwell and Kleeman’s Clinical Disorders of Fluid and Electrolyte Metabolism. 5th ed. New York: McGraw-Hill; 1994. [Google Scholar]
- 4.Narins RG, Emmett M. Simple and mixed acid-base disorders: a practical approach. Medicine (Baltimore) 1980;59:161–187. doi: 10.1097/00005792-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Siggaard-Andersen O. The Acid-Base Status of the Blood. 4th ed. Baltimore: Williams & Wilkins; 1974. [Google Scholar]
- 6.Schwartz WB, Relman AS. A critique of the parameters used in the evaluation of acid-base disorders. “Whole-blood buffer base” and “standard bicarbonate” compared with blood pH and plasma bicarbonate concentration. N Engl J Med. 1963;268:1382–1388. doi: 10.1056/NEJM196306202682503. [DOI] [PubMed] [Google Scholar]
- 7.Bunker JP. The great trans-atlantic acid-base debate. Anesthesiology. 1965;26:591–594. [PubMed] [Google Scholar]
- 8.Severinghaus JW. Acid-base balance nomogram--a Boston-Copenhagen detente. Anesthesiology. 1976;45:539–541. [PubMed] [Google Scholar]
- 9.Severinghaus JW. Siggaard-Andersen and the “Great Trans-Atlantic Acid-Base Debate”. Scand J Clin Lab Invest Suppl. 1993;214:99–104. [PubMed] [Google Scholar]
- 10.Emmet M, Narins RG. Clinical use of anion gap. Medicine (Baltimore) 1977;56:38–54. [PubMed] [Google Scholar]
- 11.Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26:1807–1810. doi: 10.1097/00003246-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Durward A, Mayer A, Skellett S, Taylor D, Hanna S, Tibby SM, Murdoch IA. Hypoalbuminaemia in critically ill children: incidence, prognosis, and influence on the anion gap. Arch Dis Child. 2003;88:419–422. doi: 10.1136/adc.88.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatherill M, Waggie Z, Purves L, Reynolds L, Argent A. Correction of the anion gap for albumin in order to detect occult tissue anions in shock. Arch Dis Child. 2002;87:526–529. doi: 10.1136/adc.87.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvounis CP, Feinfeld DA. A simple estimate of the effect of the serum albumin level on the anion Gap. Am J Nephrol. 2000;20:369–372. doi: 10.1159/000013618. [DOI] [PubMed] [Google Scholar]
- 15.Taylor D, Durward A, Tibby SM, Thorburn K, Holton F, Johnstone IC, Murdoch IA. The influence of hyperchloraemia on acid base interpretation in diabetic ketoacidosis. Intensive Care Med. 2006;32:295–301. doi: 10.1007/s00134-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 16.Corey HE. The anion gap (AG): studies in the nephrotic syndrome and diabetic ketoacidosis (DKA) J Lab Clin Med. 2006;147:121–125. doi: 10.1016/j.lab.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Hamburger HJ. Anionenwanderungen in Serum und Blut unter dem Einfluss von CO2, Saeure und Alkali. Biochem Z. 1918;86:309–324. [Google Scholar]
- 18.Westen EA, Prange HD. A reexamination of the mechanisms underlying the arteriovenous chloride shift. Physiol Biochem Zool. 2003;76:603–614. doi: 10.1086/380208. [DOI] [PubMed] [Google Scholar]
- 19.Langer T, Zani L, Carlesso E, Protti A, Caironi P, Chierichetti M, Caspani ML, Gattinoni L. Contribution of red blood cells to the compensation for hypocapnic alkalosis through plasmatic strong ion difference variations. Critical Care. 2011;15(Suppl 1):P134. [Google Scholar]
- 20.Langer T, Carlesso E, Gattinoni L. The Hamburger Effect: Beyond Chloride Shift. Am J Respir Crit Care Med. 2012;185:A3168. [Google Scholar]
- 21.Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–368. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Polak A, Haynie GD, Hays RM, Schwartz WB. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961;40:1223–1237. doi: 10.1172/JCI104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkes P. Hypoproteinemia, strong-ion difference, and acid-base status in critically ill patients. J Appl Physiol (1985) 1998;84:1740–1748. doi: 10.1152/jappl.1998.84.5.1740. [DOI] [PubMed] [Google Scholar]
- 24.Shires GT, Holman J. Dilution acidosis. Ann Intern Med. 1948;28:557–559. doi: 10.7326/0003-4819-28-3-557. [DOI] [PubMed] [Google Scholar]
- 25.Asano S, Kato E, Yamauchi M, Ozawa Y, Iwasa M. The mechanism of acidosis caused by infusion of saline solution. Lancet. 1966;1:1245–1246. doi: 10.1016/s0140-6736(66)90248-0. [DOI] [PubMed] [Google Scholar]
- 26.Garella S, Chang BS, Kahn SI. Dilution acidosis and contraction alkalosis: review of a concept. Kidney Int. 1975;8:279–283. doi: 10.1038/ki.1975.114. [DOI] [PubMed] [Google Scholar]
- 27.Kellum JA. Saline-induced hyperchloremic metabolic acidosis. Crit Care Med. 2002;30:259–261. doi: 10.1097/00003246-200201000-00046. [DOI] [PubMed] [Google Scholar]
- 28.Constable PD. Hyperchloremic acidosis: the classic example of strong ion acidosis. Anesth Analg. 2003;96:919–922. doi: 10.1213/01.ANE.0000053256.77500.9D. [DOI] [PubMed] [Google Scholar]
- 29.Mathes DD, Morell RC, Rohr MS. Dilutional acidosis: is it a real clinical entity? Anesthesiology. 1997;86:501–503. doi: 10.1097/00000542-199702000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Prough DS, White RT. Acidosis associated with perioperative saline administration: dilution or delusion? Anesthesiology. 2000;93:1167–1169. doi: 10.1097/00000542-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Prough DS, Bidani A. Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9% saline. Anesthesiology. 1999;90:1247–1249. doi: 10.1097/00000542-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Gattinoni L, Carlesso E, Maiocchi G, Polli F, Cadringher P. Dilutional acidosis: where do the protons come from? Intensive Care Med. 2009;35:2033–2043. doi: 10.1007/s00134-009-1653-7. [DOI] [PubMed] [Google Scholar]
- 33.Doberer D, Funk GC, Kirchner K, Schneeweiss B. A critique of Stewart’s approach: the chemical mechanism of dilutional acidosis. Intensive Care Med. 2009;35:2173–2180. doi: 10.1007/s00134-009-1528-y. [DOI] [PubMed] [Google Scholar]
- 34.Kellum JA. Determinants of blood pH in health and disease. Crit Care. 2000;4:6–14. doi: 10.1186/cc644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fencl V, Jabor A, Kazda A, Figge J. Diagnosis of metabolic acid-base disturbances in critically ill patients. Am J Respir Crit Care Med. 2000;162:2246–2251. doi: 10.1164/ajrccm.162.6.9904099. [DOI] [PubMed] [Google Scholar]
- 36.Boniatti MM, Cardoso PR, Castilho RK, Vieira SR. Acid-base disorders evaluation in critically ill patients: we can improve our diagnostic ability. Intensive Care Med. 2009;35:1377–1382. doi: 10.1007/s00134-009-1496-2. [DOI] [PubMed] [Google Scholar]
- 37.Dubin A, Menises MM, Masevicius FD, Moseinco MC, Kutscherauer DO, Ventrice E, Laffaire E, Estenssoro E. Comparison of three different methods of evaluation of metabolic acid-base disorders. Crit Care Med. 2007;35:1264–1270. doi: 10.1097/01.CCM.0000259536.11943.90. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan LJ, Cheung NH, Maerz L, Lui F, Schuster K, Luckianow G, Davis K. A physicochemical approach to acid-base balance in critically ill trauma patients minimizes errors and reduces inappropriate plasma volume expansion. J Trauma. 2009;66:1045–1051. doi: 10.1097/TA.0b013e31819a04be. [DOI] [PubMed] [Google Scholar]
- 39.Morgan TJ, Cowley DM, Weier SL, Venkatesh B. Stability of the strong ion gap versus the anion gap over extremes of PCO2 and pH. Anaesth Intensive Care. 2007;35:370–373. doi: 10.1177/0310057X0703500308. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004;32:1120–1124. doi: 10.1097/01.ccm.0000125517.28517.74. [DOI] [PubMed] [Google Scholar]
- 41.Durward A, Tibby SM, Skellett S, Austin C, Anderson D, Murdoch IA. The strong ion gap predicts mortality in children following cardiopulmonary bypass surgery. Pediatr Crit Care Med. 2005;6:281–285. doi: 10.1097/01.PCC.0000163979.33774.89. [DOI] [PubMed] [Google Scholar]
- 42.Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl-Stewart method predict mortality better than base excess, anion gap, and lactate in patients in the pediatric intensive care unit. Crit Care Med. 1999;27:1577–1581. doi: 10.1097/00003246-199908000-00030. [DOI] [PubMed] [Google Scholar]
- 43.Funk GC, Doberer D, Sterz F, Richling N, Kneidinger N, Lindner G, Schneeweiss B, Eisenburger P. The strong ion gap and outcome after cardiac arrest in patients treated with therapeutic hypothermia: a retrospective study. Intensive Care Med. 2009;35:232–239. doi: 10.1007/s00134-008-1315-1. [DOI] [PubMed] [Google Scholar]
- 44.Honore PM, Joannes-Boyau O, Boer W. Strong ion gap and outcome after cardiac arrest: another nail in the coffin of traditional acid-base quantification. Intensive Care Med. 2009;35:189–191. doi: 10.1007/s00134-008-1316-0. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen BV, Vincent JL, Hamm JB, Abalain JH, Carre JL, Nowak E, Ahmed MO, Arvieux CC, Gueret G. The reproducibility of Stewart parameters for acid-base diagnosis using two central laboratory analyzers. Anesth Analg. 2009;109:1517–1523. doi: 10.1213/ANE.0b013e3181b62664. [DOI] [PubMed] [Google Scholar]
- 46.Tuhay G, Pein MC, Masevicius FD, Kutscherauer DO, Dubin A. Severe hyperlactatemia with normal base excess: a quantitative analysis using conventional and Stewart approaches. Crit Care. 2008;12:R66. doi: 10.1186/cc6896. [DOI] [PMC free article] [PubMed] [Google Scholar]


