Abstract
AIM: To explore whether serum bicarbonate at admission to intensive care unit (ICU) predicted development of acute kidney injury (AKI).
METHODS: We studied all patients admitted to our ICU over a 2 year period (February 2010 to 2012). The ICU has a case mix of medical and surgical patients excluding cardiac surgical, trauma and neurosurgical patients. We analysed 2035 consecutive patients admitted to ICU during the study period. Data were collected by two investigators independently and in duplicate using a standardised spread sheet to ensure accuracy. Ambiguous data were checked for accuracy where indicated. AKI was defined using the Kidney Disease Improving Global Outcomes criteria. Patients were divided into two groups; patients who developed AKI or those who did not, in order to compare the baseline characteristics, and laboratory and physiologic data of the two cohorts. Regression analysis was used to identify if serum bicarbonate on admission predicted the development of AKI.
RESULTS: Of 2036 patients 152 (7.5%) were excluded due to missing data. AKI developed in 43.1% of the patients. The AKI group, compared to the non-AKI group, was sicker based on their lower systolic, diastolic and mean arterial pressures and a higher acute physiology and chronic health evaluation (APACHE) III and SAPS II scores. Moreover, patients who developed AKI had more co-morbidities and a higher proportion of patients who developed AKI required mechanical ventilation. The multi-regression analysis of independent variables showed that serum bicarbonate on admission (OR = 0.821; 95%CI: 0.796-0.846; P < 0.0001), APACHE III (OR = 1.011; 95%CI: 1.007-1.015; P < 0.0001), age (OR = 1.016; 95%CI: 1.008-1.024; P < 0.0001) and presence of sepsis at ICU admission (OR = 2.819; 95%CI: 2.122-23.744; P = 0.004) were each significant independent predictors of AKI. The area under the ROC curve was 0.8 (95%CI: 0.78-0.83), thereby demonstrating that the predictive model has relatively good discriminating power for predicting AKI.
CONCLUSION: Serum bicarbonate on admission may independently be used to make a diagnosis of AKI.
Keywords: Acute kidney injury, Bicarbonate, Mortality, Sepsis
Core tip: Metabolic acidosis is often associated with acute kidney injury (AKI) and can result in multiple complications, including cardiac dysfunction, hypotension and mortality. There is however, a paucity of data regarding the value of metabolic acidosis, especially serum bicarbonate, in making an early diagnosis of AKI in an intensive care unit (ICU) setting. We demonstrated that serum bicarbonate on admission may independently be used to make a diagnosis of AKI, in a mixed ICU setting. Our results are relevant since serum bicarbonate is inexpensive and easily available, which will enable initiate prompt treatment of AKI, for better outcomes.
INTRODUCTION
Acute kidney injury (AKI) is defined as an abrupt decline in renal function, resulting in the inability to excrete metabolic wastes and maintain proper fluid, electrolyte and acid base balance. It results in multiple complications including hyperkalaemia, acidosis, volume overload, encephalopathy and anaemia[1]. Patients who develop AKI have worse long-term outcomes, especially in the immediate post-intensive care unit (ICU) period[2].
Metabolic acidosis, which is often associated with AKI, can result in cardiac dysfunction, hypotension, increased risk of infection and mortality. Hence clinical practice guidelines recommend initiation of alkali therapy when serum bicarbonate level is ≤ 22 mmol/L[1] although a recent Cochrane review demonstrated the benefit of sodium bicarbonate in AKI management as equivocal[3].
A more thorough understanding of the impact and association of different risk factors with AKI is very important for designing predictive models of patients at high risk of developing this lethal condition, in order that preventative strategies may be created to benefit such a group. Predictive models for development of AKI already exist in cardiac-surgery critically ill patients[4-6]. There is however a lack of meaningful predictive models in mixed and medical ICUs. Most of the prediction models in these context have focused on the impact on mortality of AKI in ICU patients[7,8].
Multiple biomarkers including serum and urinary CysC, neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-18 (IL-18) have been used to predict AKI[9]. The usefulness of these serum biomarkers in predicting the development of AKI appears to be evolving. Yet assay to assays to identify these biomarkers are expensive and not widely available.
There is however, a paucity of data regarding the value of metabolic acidosis, especially serum bicarbonate, in making an early diagnosis of AKI in an ICU setting. Measurement of serum bicarbonate is possible in most hospital settings and is not expensive. Hence we aimed to primarily assess the role of serum bicarbonate, measured during the first 24 h of ICU admission, in diagnosing AKI and identify other independent predictors of AKI.
MATERIALS AND METHODS
Ethics
The Human Research Ethics Committee of Peninsula Health reviewed the study protocol and waived the requirement for full ethics committee application, as the study was seen as a retrospective audit of data routinely collected for patient care and not experimental research.
Study design and setting
We studied all patients admitted to our ICU over a 2 year period (February 2010 to 2012). The ICU has a case mix of medical and surgical patients excluding cardiac surgical, trauma and neurosurgical patients. The study was undertaken at Frankston Hospital, the acute care hospital for Peninsula Health. The hospital is a tertiary referral centre that is affiliated with Monash University.
Clinical and laboratory features at admission and during ICU stay were collected from our ICU database (called STATIC), our hospital’s pathology database and the case records of the patients included in the study. Data were collected by two investigators independently and in duplicate using a standardised spread sheet to ensure accuracy. Ambiguous data were checked for accuracy where indicated.
Definition of parameters
Clinical and laboratory features at admission and during ICU stay were studied. AKI was defined using the Kidney Disease Improving Global Outcomes (KDIGO) Practice Guidelines[10]. As per the guidelines, a patient was considered to have AKI if there was an increase in serum creatinine by 26.5 μmol/L or more within 48 h, or an increase in serum creatinine to 1.5 times baseline or more within the last 7 d. Baseline renal function was defined as the lowest known serum creatinine during the preceding 3 mo prior to hospital admission. Patients with unknown baseline serum creatinine were excluded from the study. Patients were considered as having new AKI if they did not have AKI on ICU admission but subsequently met the KDIGO Guidelines during the first 48 h of ICU presentation. Metabolic acidosis was defined as pH < 7.35 and an arterial bicarbonate < 20 mmol/L.
Patient population and data collection
The patients were divided into two groups, the AKI and non-AKI groups, in order to investigate if there were differences in relation to all the studied parameters. Thereafter, the proportion of patients with metabolic acidosis in the AKI group was determined. The presence of hypertension, diabetes and peripheral vascular disease, as well as the length of ICU and hospital stays were analysed in all patients included in the study. The acute physiology and chronic health evaluation (APACHE) III score[11] and simplified acute physiology score (SAPS) II[12] were calculated for the first 24 h of admission. Physiological parameters during the first 24 h of ICU admission, including vital signs and partial pressures of oxygen and carbon dioxide were recorded. Serum urea and creatinine were recorded during the first 48 h of ICU admission, at 24 h-intervals, in all patients included in the study. The laboratory parameters consisted of serum bicarbonate, pH, lactate, albumin, urea, potassium, white cell count (WCC) and glucose levels.
Statistical analysis
Statistical analysis was performed by a biomedical statistician. Categorical data were assessed using Fisher’s exact test. Student’s t tests (for parametric data) or Mann-Whitney U (Non parametric) tests was used for continuously-scaled data.
Logistic regression analysis was used to distinguish independent predictors of hospital mortality. In regression analysis models data variables were entered using “Enter” method. The first model contained data variables including age, mean BP, serum lactate, pH, APACHE III score, serum bicarbonate on admission, presence of sepsis at admission and the need for mechanical ventilation. Further models were constructed aiming for a parsimonious model. Every model constructed was assessed by Cox and Snell and Nagelkerke R square and Hosmer-Lemeshow goodness-of-fit statistic. Regression models were constructed using Wald statistic. The final model contained four variables including age, sepsis on admission, serum bicarbonate and APACHE III score. A P value < 0.05 was considered to be statistical significant. Data analyses were performed using IBM SPSS statistics version 22.0 (SPSS Inc, Chicago, IL).
RESULTS
Over the two year study period 2035 patients were admitted to our ICU. We excluded 152 (7.5%) patients due to missing data on serum creatinine. 877 patients (43.1%) of the cohort developed AKI compared to 1006 patients (49.4%) who did not develop AKI in the first 48 h. Patient demographics and clinical parameters at the time of admission are shown in Table 1.
Table 1.
Comparison of demographical and clinical characteristics at the time of admission to intensive care
| NO AKI (n = 1006) | AKI (n = 877) | P value | |
| Age (yr) | 61.4 (18.8) | 66.2 (16.6) | < 0.0001 |
| Sex, M:F | 1.1:1 (726:662) | 1.1:1 (242:220) | 0.978 |
| Requirement for mechanical ventilation | 46% | 51.5% | 0.01 |
| Severity of illness | |||
| APACHE III | 47.8 (25.3) | 63 (35.7) | < 0.0001 |
| SAPS II | 33.7 (13.8) | 45.9 (17.6) | < 0.0001 |
| Vital signs | |||
| Heart rate (/min) | 99 (22) | 105 (23) | < 0.001 |
| Respiratory rate (/min) | 24 (6.6) | 26 (7.5) | < 0.001 |
| Temperature1 (°C) | 35.3 (0.9) | 35.2 (1.2) | 0.004 |
| Systolic BP1 (mmHg) | 102.9 (16.8) | 96.5 (169) | < 0.001 |
| Diastolic BP1 (mmHg) | 55.8 (12.2) | 52.3 (12.7) | < 0.001 |
| MAP (mmHg) | 71.3 (12.7) | 67.0 (12.8) | < 0.001 |
Lowest. Data are presented in mean ± SD; number of patients where data were available for analyses. SAPS II: Simplified acute physiology score II; APACHE III: Acute physiology age and chronic health evaluation III; BP: Blood pressure; MAP: Mean arterial pressure.
The AKI group was older than the non-AKI groups, and had a significantly higher proportion of hypertensive and diabetic patients (P = 0.003 and < 0.001 respectively). Other comorbidities such as peripheral vascular disease, ischemic heart disease and chronic obstructive airway disease were not significantly different (P = 0.58, 0.24 and 0.07 respectively). The AKI group, compared to the non-AKI group, was sicker based on the lower systolic, diastolic and mean arterial pressures and a higher APACHE III and SAPS II scores. Moreover, patients who developed AKI were more likely to require mechanical ventilation (51.5% vs 46.0%, P = 0.01). Patients who developed AKI were more acidotic with lower serum bicarbonate (20.1 mmol/L vs 23.5 mmol/L, P < 0.001) and higher lactate (3.4 mmol/L vs 2.1 mmol/L, P < 0.001) (Table 2).
Table 2.
Comparison of laboratory characteristics at the time of admission to intensive care
| NO AKI (n = 1006) | AKI (n = 877) | P value | |
| pH | 7.4 (0.08) | 7.3 (0.12) | < 0.001 |
| PaCO2 (mmHg) | 41 (11.3) | 40 (13.7) | 0.005 |
| PaO2, (mmHg) | 116 (85.4) | 113 (79.7) | 0.5 |
| HCO3 (mmol/L) | 23.5 (3.7) | 20.1 (5.3) | < 0.001 |
| Sodium (mmol/L) | 141 (4.2) | 141 (5.3) | 0.7 |
| Potassium (mmol/L) | 4.5 (0.6) | 4.7 (0.8) | < 0.001 |
| Urea (μmol/L) | 6.2 (3.9) | 14.4 (10.6) | < 0.0001 |
| Baseline Creatinine (μmol/L) | 72 (36.8) | 180 (173.6) | < 0.001 |
| Peak creatinine (μmol/L)1 | 76 (36.6) | 196 (175.3) | < 0.001 |
| Serum albumin (g/L) | 35 (6.1) | 34 (6.1) | 0.001 |
| Blood glucose (mmol/L) | 8.9 (3.4) | 11.1 (5.7) | < 0.001 |
| Lactate (mmol/L) | 2.1 (2.0) | 3.4 (3.1) | < 0.001 |
| White cell count (× 109/L) | 12.9 (9.6) | 15.6 (10.8) | < 0.001 |
| Hematocrit (%) | 0.36 (0.057) | 0.34 (0.061) | < 0.001 |
During first 48 h of ICU admission. Data are presented in mean ± SD. ICU: Intensive care unit.
The AKI group was sub-classified into 3 categories as per the grade of the renal impairment. Stage 1, 2 and 3 respectively had a serum bicarbonate of 20.8 ± 5.1, 18.2 ± 5.0 and 17.2 ± 6.3 mmol/L. There were however no significant differences in the death rates in ICU across the 3 groups.
In terms of morbidity and mortality, the AKI group had longer ICU and hospital duration and a higher ICU and hospital mortality (Table 3). The multi-regression analysis of independent variables showed that serum bicarbonate on admission (OR = 0.821; 95%CI: 0.796-0.846; P < 0.0001), APACHE III (Odds ratio 1.011; 95% CI 1.007-1.015; P < 0.0001), age (OR = 1.016; 95%CI: 1.008-1.024; P < 0.0001) and presence of sepsis at ICU admission (OR = 2.819; 95%CI: 2.122-23.744; P = 0.004) were each significant independent predictors of AKI. The area under the ROC curve was 0.8 (Figure 1) confirming the discriminatory power of the model for predicting AKI.
Table 3.
Comparison of outcomes
| NO AKI (n = 1006) | AKI (n = 877) | P value | |
| Died in hospital (%) | 30.9 | 69.1 | < 0.001 |
| Death in ICU (%) | 57.0 | 43.0 | < 0.001 |
| Hospital length of stay (d) | 12.6 (20.1) | 14.5 (16.3) | 0.02 |
| ICU length of stay (d) | 2.8 (4.1) | 4.4 (5.7) | < 0.001 |
Data are presented in mean ± SD. ICU: Intensive care unit.
Figure 1.
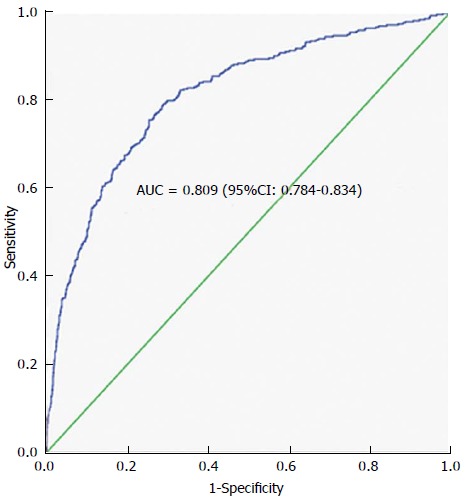
Receiver operating characteristic curve of the final model (including age, sepsis, serum bicarbonate and APACHE III score) predicting acute kidney injury. AUC: Area under the curve.
DISCUSSION
Our study is amongst the first studies investigating whether serum bicarbonate predicts the development of AKI in unselected critically ill ICU patients, in whom AKI aetiology and timing are often unclear. We demonstrated that patients who developed AKI were more acidotic with a lower serum bicarbonate. Hence our study proved that in an ICU setting, serum bicarbonate on admission can be used to make an early diagnosis of AKI. Patients with more severe AKI were more acidotic, although the mortality across the sub-groups of AKI severity did not significantly differ. Also, serum lactate levels on ICU admission did not predict the onset of AKI. We also found that AKI significantly increased morbidity and mortality, hence highlighting the need for an early diagnostic tool.
The rationale behind the association between AKI and low serum bicarbonate levels can be extrapolated from previous studies which have explored the benefits of sodium bicarbonate in reducing the risk of AKI[13-17]. Most of those have been performed in a cardiac surgery setting because of the ability to prospectively follow patients before and after a well-timed renal insult. Haase et al[15] designed a double-blind, randomized controlled trial in patients undergoing cardiac surgery, and found that sodium bicarbonate treatment was associated with an absolute risk reduction for AKI of 20% and with a significant attenuation in the postoperative increase of plasma urea, urinary NGAL and urinary NGAL/urinary creatinine ratio[15]. It is thought that sodium bicarbonate contributes to increasing oxygen delivery to the renal medulla, while reducing iron-mediated free radical formation due to neutralizing acidosis in this vulnerable region of the kidney[18]. Therefore, it can be argued that a lower serum bicarbonate level would increase the risk of ischemic injury to the kidneys, especially in a critical illness setting. This logic would support our findings and current model.
Of late, there has been a lot of interest in identifying novel serum and urinary biomarkers which would be more sensitive in predicting AKI. This is because serum creatinine has a poor predictive accuracy for renal injury, particularly in the early stages of AKI[19]. Our study supports the use of serum bicarbonate, an easily accessible parameter, usually readily available in all patients. Other markers which have been used as predictors of AKI are NGAL, kidney injury molecule-1, Cystatin C, IL-6, IL-8, IL-18, N-acetyl-glucosaminidase, glutathione transferases and liver fatty acid binding protein. However, there is still a lot of debate about their reliability. For example, a wide range of predictive value of NGAL has been only reported across observational cohort studies[20,21]. Also a clear cut off NGAL concentration for the detection of AKI has not yet been reported, whilst the predictive value of urinary Cystatin C should be interpreted with caution in pre-renal AKI[22]. More recently, a review emphasized on the cumbersome nature of these markers, especially in those settings where timing and aetiology of AKI are not well defined[23]. Hence, we support the use of serum bicarbonate as an in-expensive and potentially reliable predictor of AKI.
We do acknowledge the limitations of our study. It is a retrospective study with limitations on the selection of patients and the quality of the data. Nevertheless we aimed to include all patients admitted to ICU to reduce the selection bias and all data was collected by two investigators independently and in duplicate using a standardised spread sheet to ensure accuracy. Also, although 7.5% of our cohort had to be excluded from the study due to the unavailability of a baseline creatinine, they were not demographically different to the rest of the cohort.
This study showed that serum bicarbonate at admission may be a predictor of developing AKI in a mixed ICU setting. The current findings can allow timely patient management decisions, including withholding nephrotoxic agents, administration of putative therapeutic agents, and the initiation of RRT since a bicarbonate level is cheap and readily available.
COMMENTS
Background
Metabolic acidosis is often associated with acute kidney injury (AKI) and can result in multiple complications, including cardiac dysfunction, hypotension and mortality. There is however, a paucity of data regarding the value of metabolic acidosis, especially serum bicarbonate, in making an early diagnosis of AKI in an intensive care unit (ICU) setting.
Research frontiers
A more thorough understanding of the impact and association of different risk factors with AKI is very important for designing predictive models of patients at high risk of developing this lethal condition, in order that preventative strategies may be created to benefit such a group. Predictive models for development of AKI already exist in cardiac-surgery critically ill patients. There is however a lack of meaningful predictive models in mixed and medical ICUs.
Innovations and breakthroughs
Multiple biomarkers including serum and urinary CysC, NGAL and interleukin-18 have been used to predict AKI. The usefulness of these serum biomarkers in predicting the development of AKI appears to be evolving. Yet assay of these biomarkers are currently expensive and the facilities to assay these biomarkers are not widely available. There is however, a paucity of data regarding the value of metabolic acidosis, especially serum bicarbonate, in making an early diagnosis of AKI in an ICU setting.
Applications
The current study found that serum bicarbonate measured in the early phases of admission to ICU could be used to make an early diagnosis of AKI. Serum bicarbonate measurement is inexpensive and easily available, hence making it an easy test available to anticipate AKI, hence launch the necessary treatment promptly.
Peer-review
This study investigates the utility of serum bicarbonate as a marker of acute kidney injury.
Footnotes
Ethics approval: Human Ethics Review Committee of Peninsuln Health hace reviewed (Ref HREC/11/PH/63) and approved the study for publication. a copy of approval can be provided on request.
Informed consent: The Human Ethics Review Committee of Peninsuln Health consent from individual patients as the study was seen as a retrospective audit of data routinely collected for patient care and not experimental research.
Conflict-of-interest: None of the authors have commercial association or financial involvement that might pose a conflict of interest in connection with this article.
Data sharing: Data presented in the manuscript is anonymised and the risk of identifying individual patient is very low. No additional data is available other than stated in the manuscript for this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 10, 2014
First decision: June 6, 2014
Article in press: January 12, 2015
P- Reviewer: Olowu WA, Pickering JW S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EA, De Corte W. AKI patients have worse long-term outcomes, especially in the immediate post-ICU period. Crit Care. 2012;16:148. doi: 10.1186/cc11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewitt J, Uniacke M, Hansi NK, Venkat-Raman G, McCarthy K. Sodium bicarbonate supplements for treating acute kidney injury. Cochrane Database Syst Rev. 2012;6:CD009204. doi: 10.1002/14651858.CD009204.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortescue EB, Bates DW, Chertow GM. Predicting acute renal failure after coronary bypass surgery: cross-validation of two risk-stratification algorithms. Kidney Int. 2000;57:2594–2602. doi: 10.1046/j.1523-1755.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 5.Eriksen BO, Hoff KR, Solberg S. Prediction of acute renal failure after cardiac surgery: retrospective cross-validation of a clinical algorithm. Nephrol Dial Transplant. 2003;18:77–81. doi: 10.1093/ndt/18.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216; quiz 2208. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 7.Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46:1038–1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Kolhe NV, Stevens PE, Crowe AV, Lipkin GW, Harrison DA. Case mix, outcome and activity for patients with severe acute kidney injury during the first 24 hours after admission to an adult, general critical care unit: application of predictive models from a secondary analysis of the ICNARC Case Mix Programme database. Crit Care. 2008;12 Suppl 1:S2. doi: 10.1186/cc7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes KDIGO) - Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. 2012;2:1–138. [Google Scholar]
- 11.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 12.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 13.Calvert S, Shaw A. Perioperative acute kidney injury. Perioper Med (Lond) 2012;1:6. doi: 10.1186/2047-0525-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3:178–199. doi: 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase M, Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Reade MC, Bagshaw SM, Seevanayagam N, Seevanayagam S, et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med. 2009;37:39–47. doi: 10.1097/CCM.0b013e318193216f. [DOI] [PubMed] [Google Scholar]
- 16.Haase M, Haase-Fielitz A, Plass M, Kuppe H, Hetzer R, Hannon C, Murray PT, Bailey MJ, Bellomo R, Bagshaw SM. Prophylactic perioperative sodium bicarbonate to prevent acute kidney injury following open heart surgery: a multicenter double-blinded randomized controlled trial. PLoS Med. 2013;10:e1001426. doi: 10.1371/journal.pmed.1001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heringlake M, Heinze H, Schubert M, Nowak Y, Guder J, Kleinebrahm M, Paarmann H, Hanke T, Schön J. A perioperative infusion of sodium bicarbonate does not improve renal function in cardiac surgery patients: a prospective observational cohort study. Crit Care. 2012;16:R156. doi: 10.1186/cc11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins JL. Effect of sodium bicarbonate preloading on ischemic renal failure. Nephron. 1986;44:70–74. doi: 10.1159/000183915. [DOI] [PubMed] [Google Scholar]
- 19.Drawz PE, Miller RT, Sehgal AR. Predicting hospital-acquired acute kidney injury--a case-controlled study. Ren Fail. 2008;30:848–855. doi: 10.1080/08860220802356515. [DOI] [PubMed] [Google Scholar]
- 20.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–3265. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 21.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 22.Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, Frei U, Dragun D, Haase M. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24:3349–3354. doi: 10.1093/ndt/gfp234. [DOI] [PubMed] [Google Scholar]
- 23.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28:254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]


