Key Points
This study is the first to assess prognostic factors in patients with AHA treated according to a uniform immunosuppressive regimen.
Residual factor VIII activity and inhibitor concentration at baseline are potentially useful predictors of remission.
Abstract
Acquired hemophilia A (AHA) is caused by autoantibodies against factor VIII (FVIII). Immunosuppressive treatment (IST) results in remission of disease in 60% to 80% of patients over a period of days to months. IST is associated with frequent adverse events, including infections as a leading cause of death. Predictors of time to remission could help guide IST intensity but have not been established. We analyzed prognostic factors in 102 prospectively enrolled patients treated with a uniform IST protocol. Partial remission (PR; defined as no active bleeding, FVIII restored >50 IU/dL, hemostatic treatment stopped >24 hours) was achieved by 83% of patients after a median of 31 days (range 7-362). Patients with baseline FVIII <1 IU/dL achieved PR less often and later (77%, 43 days) than patients with ≥1 IU/dL (89%, 24 days). After adjustment for other baseline characteristics, low FVIII remained associated with a lower rate of PR (hazard ratio 0.52, 95% confidence interval 0.33-0.81, P < .01). In contrast, PR achieved on steroids alone within ≤21 days was more common in patients with FVIII ≥1 IU/dL and inhibitor concentration <20 BU/mL (odds ratio 11.2, P < .0001). Low FVIII was also associated with a lower rate of complete remission and decreased survival. In conclusion, presenting FVIII and inhibitor concentration are potentially useful to tailor IST in AHA.
Introduction
Acquired hemophilia A (AHA) is a serious condition with high morbidity and mortality that can occur in previously healthy men and women of every age.1,2 Neutralizing autoantibodies, called inhibitors, are formed against the factor VIII (FVIII) coagulant protein. The resulting lack of FVIII activity may cause significant spontaneous or trauma-induced bleeding.3,4 Risk factors for the occurrence of AHA include advanced age and underlying conditions such as malignancy, autoimmune disorders, pregnancy, and the postpartum period.3,5 Other risk factors, such as certain drugs, have also been suggested.3,6,7
Immunosuppressive treatment (IST) with steroids, alone or in combination with cyclophosphamide, rituximab, or other immunosuppressants, results in remission of the disease in 60% to 90%.7-11 With current IST regimens, the time needed to achieve remission varies from a few days to several months.12 During this time, patients are at high risk of severe, sometimes fatal bleeding.8 Given the high cost of hemostatic treatment of AHA, the long time needed to achieve remission is also economically important. Side effects of IST, in particular infections, contribute to an overall high morbidity and mortality. Recent observational studies reported mortality rates of 28% to 42%.3,8,10
Given the variable prognosis of AHA, we aimed to establish clinically useful predictors of remission. This is best done in a large unselected population of patients treated according to a uniform protocol. Previous registries collected patients treated with a variety of regimens. Data indicated that patient baseline characteristics influenced the choice of treatment8 and that in turn different regimens may have different outcomes.9 We designed a prospective observational study of patients treated according to a well-defined, uniform IST protocol that was developed by the Acquired Hemophilia Working Group of the German, Austrian and Swiss Thrombosis and Hemostasis Society (GTH). Study sites in Germany and Austria have used this protocol since 2010. It was designed based on recommendations published in 2009 and 201013,14 and additional information from a GTH survey among German, Austrian, and Swiss hemophilia centers.15
Methods
GTH AH 01/2010 was a multicenter prospective observational study of patients with AHA who were treated according to the GTH consensus protocol by 29 registered sites in Austria and Germany. The research protocol was approved by the ethic committees of participating institutions. Patients had to be enrolled within 7 days of starting IST to ensure unbiased prospective observation.
Study population
AHA was defined by the presence of a neutralizing FVIII inhibitor ≥ 0.6 Bethesda units (BU)/mL (lower limit of detection) and a FVIII activity < 50 IU/dL (lower limit of normal). Patients were eligible if they had AHA, gave informed consent, and were enrolled ≤7 days after starting IST. Patients developing AHA while on steroids for a concomitant disorder before the occurrence of AHA could be enrolled if IST according to the treatment protocol was initiated ≤7 days before enrolment. We excluded patients with congenital hemophilia A (with or without FVIII inhibitors) and patients planned to be enrolled in studies with investigational drugs.
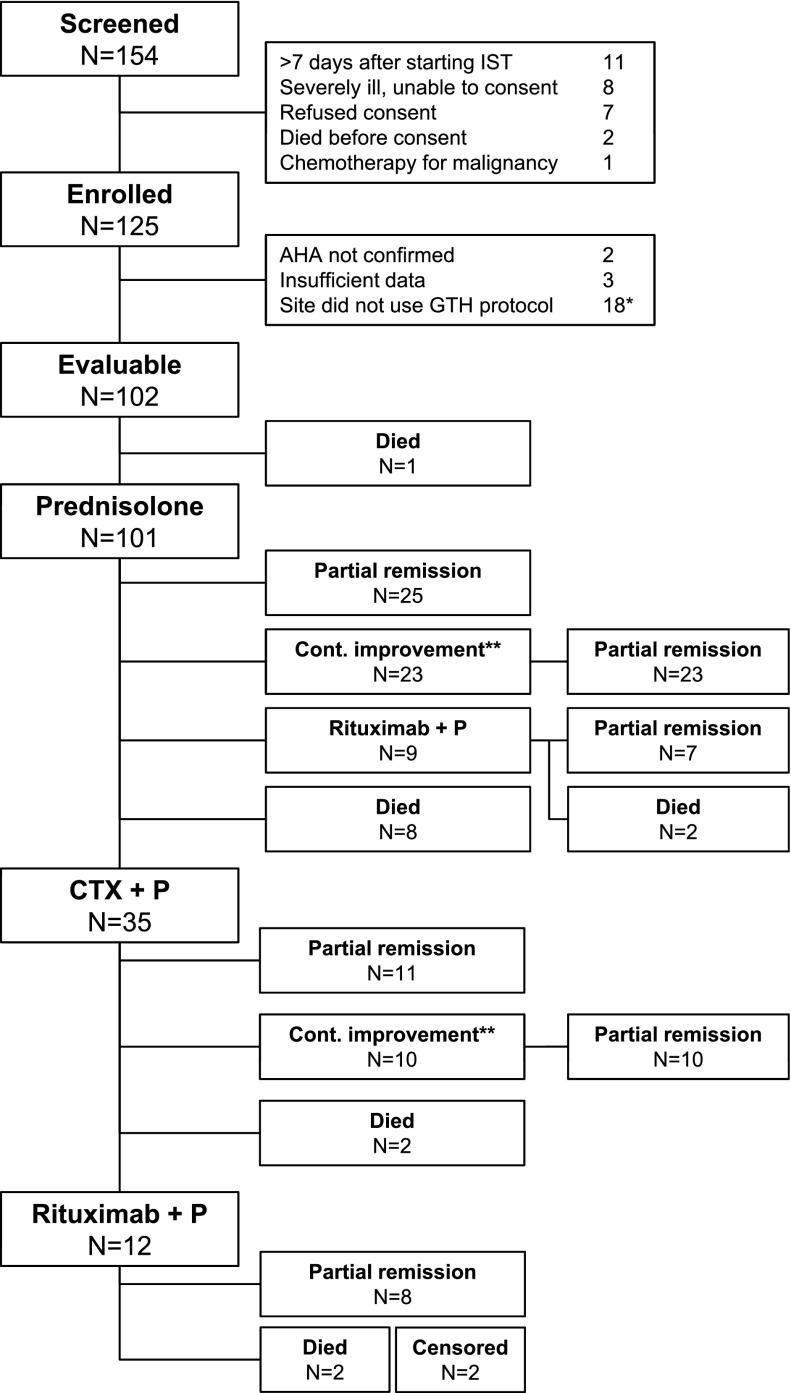
One hundred and fifty-four patients were screened by the study centers between April 2010 and April 2013 (36 months). The number of patients per center varied between 1 and 17. Twenty-nine patients were not enrolled for various reasons (Figure 1). Of the 125 initial study patients, 5 were excluded because they did not meet the inclusion criteria (diagnosis of AHA not confirmed) or had insufficient baseline and follow-up information. Two study centers did not follow the GTH treatment protocol, and it was decided in November 2010 to collect their data but to exclude all patients from these centers from analysis (n = 18). The resulting 102 patients were included into the primary and all secondary analyses.
Figure 1.
Course and outcome of immunosuppressive therapy. *All 18 patients from the 2 sites not following the IST protocol were excluded regardless of their actual therapy. **Patients not yet achieving PR but continuously improving their FVIII activity until day 21 (while on prednisolone) or day 42 (while on cyclophosphamide and prednisolone) remained on the current treatment as per protocol. CTX, cyclophosphamide; P, prednisolone.
End points
The primary end point was the time to achieve partial remission (PR), defined as FVIII activity restored to >50 IU/dL and no active bleeding after stopping any hemostatic drug for >24 hours. Secondary end points were time to complete remission (CR), defined as PR plus negative inhibitor test, prednisolone tapered to <15 mg/day, and any other IST stopped; overall survival (OS); adverse events (AEs); and causes of death.
Treatment
Consensus statements published at the time of protocol development recommended starting IST with either steroid alone or steroids plus cyclophosphamide and to add either cyclophosphamide or rituximab once first-line treatment was unsuccessful.13,14 The GTH AH 01/2010 protocol used here was developed based on that statement.
Treatment started with prednisolone alone (daily dose 75 mg for patients <60 kg, 100 mg for 60-100 kg, 150 mg for >100 kg) in weeks 1 to 3. Unless PR was achieved, treatment was escalated to prednisolone (same dose) plus cyclophosphamide (100 mg for <60 kg, 150 mg for 60-100 kg, 200 mg for >100 kg) in weeks 4 to 6, and to prednisolone (same dose) plus rituximab (4 weekly doses of 375 mg/m2) starting in week 7. Treatment escalation was withheld if a steady increase of FVIII activity was observed during 7 days before the planned escalation, but it had to be started whenever no further improvement was noted during a 7-day period. As soon as PR was achieved, cyclophosphamide and rituximab were stopped, if applicable, and prednisolone was tapered over 6 weeks (descending daily doses of 50, 25, 20, 15, 10, 5 mg for 1 week each).
If AHA was first diagnosed while on prednisolone >15 mg/day, patients were immediately escalated to treatment according to week 7 (prednisolone/rituximab). If cyclophosphamide was contraindicated, patients started on prednisolone (weeks 1-3) and were escalated in week 4 to prednisolone/rituximab (according to week 7). If prednisolone was contraindicated, patients started on rituximab alone from week 1. Cyclophosphamide was to be interrupted in case of neutropenia (<1000/µL) or thrombocytopenia (<100/nL).
Data collection
Data were collected using standardized case report forms. We collected demographic and baseline data (age, gender, underlying conditions, concomitant disorders, World Health Organization performance status [WHO-PS], local laboratory baseline FVIII activity, and Bethesda inhibitor titer) at study entry of each patient. FVIII activity was determined by 1-stage or chromogenic substrate assay; inhibitor concentration was measured using the Bethesda assay according to standard procedures at the local laboratory. Until CR was achieved, follow-up data were collected weekly, including treatment information (calendar dates of start and stop, dose, frequency and route of administration for any IST, hemostatic drug, and transfusion), laboratory data (FVIII activity and inhibitor concentration), and outcome data (calendar day of PR and CR; number, duration, and severity of bleeds and AEs including serious adverse events [SAEs]; and calendar day and cause of death). After achieving CR, data were collected in months 1, 3, 6, 12, 18, and 24 after achieving CR. Individual patient data as entered into the database were provided back to the local study coordinator for source-data verification.
Statistical analysis
The day of starting prednisolone was defined as day 1 for all analyses. Medians and ranges or interquartile ranges (IQR), or patient/event numbers and frequencies, were used to describe data as appropriate. Age, baseline FVIII activity, inhibitor concentration, and WHO-PS were dichotomized by a clinically useful divider near the median as indicated. Frequencies were compared using odds ratios (ORs) or relative risk with 95% confidence intervals (CIs) and compared using Fisher’s exact test.
Univariate and multivariate Cox regression analysis was performed for the time to PR, CR, and OS. Patients were censored if they were alive but had not reached the end point at the end of study. In those who died before reaching PR or CR, the time to the PR/CR end point was set to infinity because these patients no longer had a chance to achieve this end point. Predefined categorical and dichotomized baseline variables were entered as independent factors as indicated. Hazard ratios (HRs) were calculated for each factor together with CI.
Logistic regression and receiver-operator characteristic (ROC) analysis were performed to assess baseline characteristics of patients who achieved PR within 21 days with steroids alone. For logistic regression, dichotomized factors entered into the analysis were age, gender, underlying disorder, WHO-PS, FVIII activity, and inhibitor concentration. Stepwise backward selection was performed to eliminate nonsignificant factors (P > .05). For ROC analysis, FVIII activity and inhibitor concentration were entered as test variables. ROC curves and the respective areas under the curves (AUC) with 95% CI intervals were determined, and sensitivity, specificity, and positive and negative predictive values were calculated.
For all analyses, a P value <.05 was considered statistically significant.
Results
Baseline characteristics
Demographics and baseline characteristics of the 102 patients are summarized in Table 1. The median age was 74 years. Malignancy as an underlying disorder was more often reported in male patients (19%) than in female patients (5%, P = .04). In contrast, autoimmunity as an underlying disorder tended to be more frequent in females (28%) than in males (14%, P = .08). The distribution of factor activity and inhibitor concentration is presented in supplemental Figure 1 (available on the Blood Web site). The median FVIII activity at baseline was 1.4 IU/dL (range <1 to 31), and the median inhibitor concentration was 19 BU/mL (range 1.1 to 1449). More clinically useful thresholds of FVIII activity <1 IU/dL and inhibitor concentration >20 BU/mL were used for subsequent analyses. Patients with a high inhibitor concentration (>20 BU/mL) more often had a low FVIII (<1 IU/dL, 60% of patients) compared with other patients (33%, P = .009). There was no significant association of inhibitor concentration or FVIII activity with underlying disorder, age, gender, or other baseline characteristics.
Table 1.
Baseline characteristics
| Characteristic | All patients (n = 102) |
|---|---|
| Gender | |
| Female | 43 (42) |
| Male | 59 (58) |
| Underlying disorder | |
| None/idiopathic | 68 (67) |
| Autoimmunity | 20 (20) |
| Malignancy | 13 (13) |
| Pregnancy | 5 (5) |
| Concomitant disorders | |
| Coronary artery disease | 28 (27) |
| Heart failure | 30 (29) |
| Renal failure | 37 (36) |
| Arterial hypertension | 59 (58) |
| Diabetes mellitus type 2 | 28 (27) |
| WHO-PS | |
| 0 | 15 (15) |
| 1 | 26 (25) |
| 2 | 23 (23) |
| 3 | 22 (22) |
| 4 | 15 (15) |
| 5 | 1 (1) |
| Age in y, median (range) | 74 (26-97) |
| FVIII activity in IU/dL, median (range) | 1.4 (<1-31) |
| Inhibitor concentration in BU/mL, median (range) | 19 (1-1449) |
Values are n (%) unless otherwise noted.
IST
The patient flow along the course of IST is outlined in Figure 1. All but 1 patient, who died on day 1, started on prednisolone. Forty-eight (47%) patients achieved a PR with prednisolone alone, 25 (52%) of them within 21 days. The other 23 patients, who achieved PR with prednisolone alone, had not yet achieved PR on day 21 but had shown a continuous increase of their FVIII activity. As determined by the protocol, therapy was not escalated as long as FVIII was still improving. These patients achieved PR on median day 29 (IQR 26-43, ie, 3-22 days after day 21). Of the 44 patients requiring second-line therapy, 35 (80%) received cyclophosphamide, and 9 (20%) patients received rituximab because of contraindications for cyclophosphamide. Of the 35 patients on cyclophosphamide, 21 (60%) achieved PR with this treatment. Twelve patients (34%) received third-line therapy with rituximab.
Outcomes
PR and CR
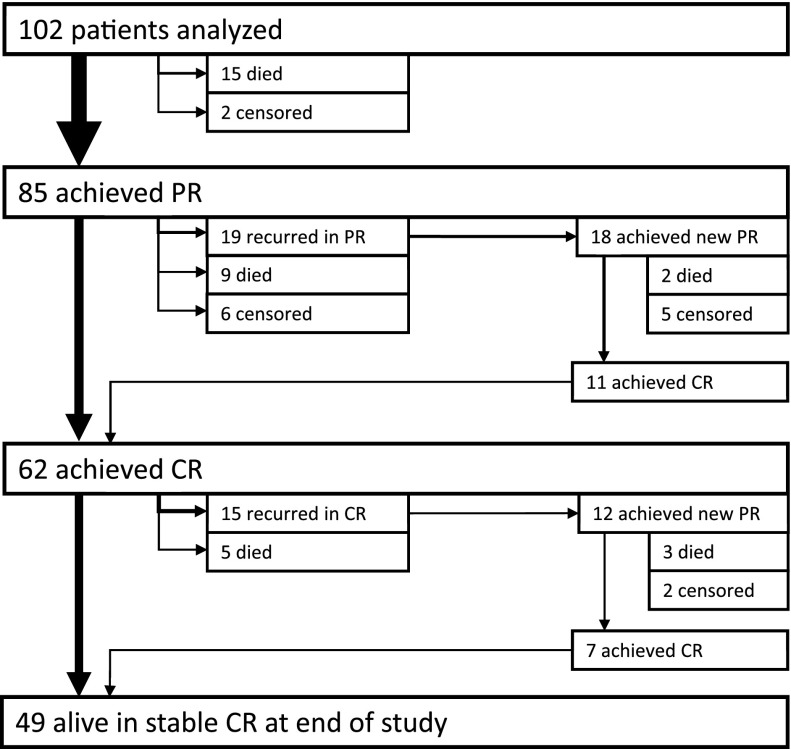
Figure 2 shows the patient flow through different remission states. Median, IQR, and range for the time to remission are provided in Table 2. A total of 85 (83%) patients achieved the primary end point, PR, after a median of 31 days, and 62 (61%) patients achieved CR after a median of 79 days.
Figure 2.
Pathways to complete remission. Arrows are proportional to patient numbers.
Table 2.
Primary and secondary end points for the entire study population
| End point | End point achieved n (%) | Time to end point in days | ||
|---|---|---|---|---|
| Median | IQR | Range | ||
| Primary end point | ||||
| PR | 85 (83) | 31 | 19-51 | 7-362 |
| Secondary end points | ||||
| CR | 62 (61) | 79 | 48-102 | 26-856 |
| Mortality | 34 (33) | 66 | 23-235 | 1-599 |
Recurrence after PR and CR
After achieving PR, 19 (22%) patients recurred before achieving CR, ie, FVIII activity dropped to <50 IU/dL during the 6-week steroid tapering phase (Figure 2); 18 of them (95%) achieved a second PR after increasing steroids according to protocol, and 11 (58%) achieved a CR. Recurrence after achieving CR was noted in 15 of 62 patients (24%). Twelve (80%) of these patients achieved a new PR, and 7 (47%) achieved a new CR. At the end of study, 49 patients (48% of the entire cohort) were in stable CR for a median time of 403 days.
Survival and causes of death
The median follow-up time for all subjects was 262 days (IQR 87-612). At final follow-up, 68 (77%) patients were alive. The 1-year survival rate was 68% (CI 58% to 77%). Frequent causes of death among the 34 patients who died were infections (n = 16), cardiovascular disorders (n = 6), the underlying disorder (n = 3), and bleeding (n = 3; supplemental Table 1). Fourteen deaths were reported as being possibly, probably, or definitely related to complications of IST. Of the 6 deaths from cardiovascular events, 3 events (2 patients with ischemic stroke and 1 patient with myocardial infarction) occurred >50 days after achieving PR and were unrelated to hemostatic treatment. Three deaths from cardiovascular cause were attributed to hemostatic treatments: 1 death was due acute portal vein thrombosis occurring on day 6 (while on recombinant factor VIIa [rFVIIa] for 3 days) and 2 deaths were due to ischemic stroke occurring on days 5 and 35 (both while on rFVIIa and tranexamic acid for 7 and 11 days, respectively). Taking into account the total number of patients exposed to rFVIIa (n = 63), tranexamic acid (n = 32), or both (n = 21), the crude rate of fatal vascular events was 5% for rFVIIa and 10% for the combination of rFVIIa and tranexamic acid.
AEs
In total, 169 AEs, including 92 SAEs and 38 fatal AEs, were recorded in 67 of the 102 patients (supplemental Table 2). Of these, 51 (30%) were probably or definitely related to IST, including infection, worsened or newly diagnosed diabetes mellitus, mucous membrane ulcers, and psychiatric disorders, all being typical complications of prolonged corticosteroid therapy. Infections were significantly associated with mortality: 20 of 37 (54%) patients with infections died, compared with 14 of 65 (22%) patients without infections (P = .001). Cardiovascular events were the second most frequent type of AEs, including 7 fatal events in 6 patients (supplemental Tables 1 and 2). Eight cardiovascular events were of thromboembolic nature, 4 of which occurred after achieving PR with no hemostatic treatment involved. Four events were related to hemostatic treatments (3 fatal events reported above and 1 nonfatal myocardial infarction occurring on day 21 while on a combination of rFVIIa and tranexamic acid since day 1).
Prognostic factors
PR
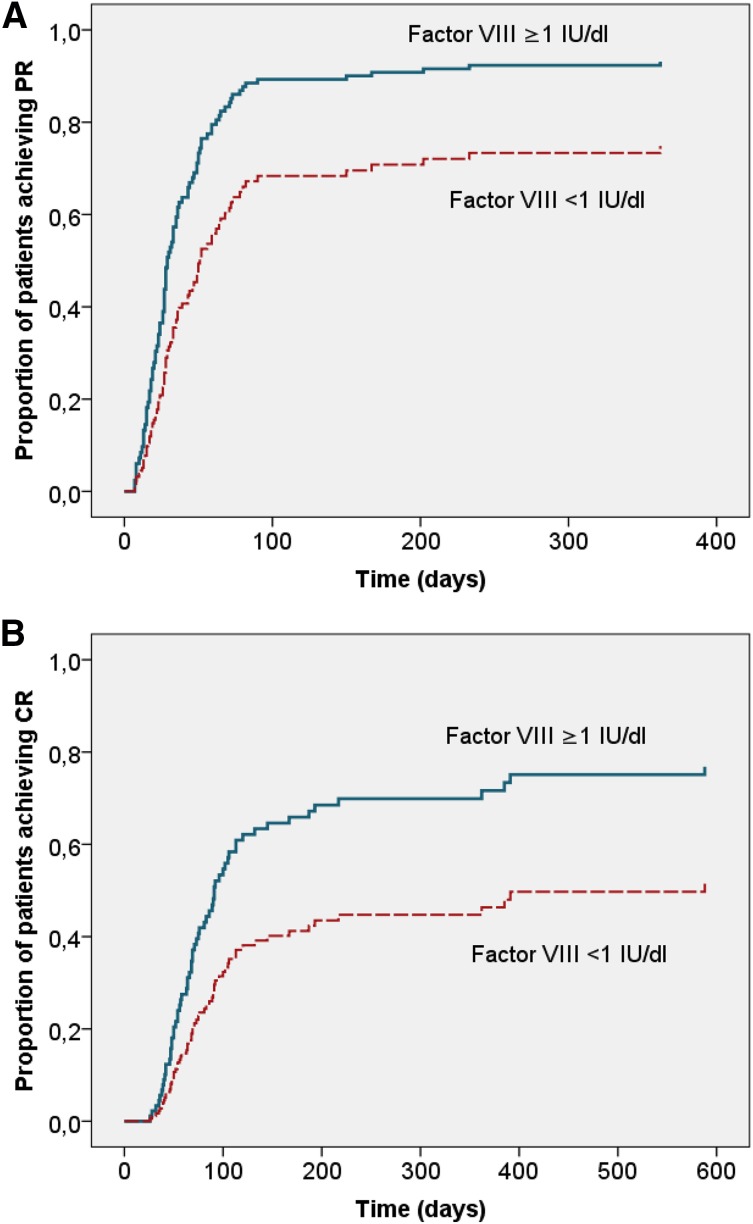
The probability of achieving PR and the time needed to achieve PR are outlined according to baseline characteristics in Table 3. PR was achieved less frequently, after a longer time, and with a lower rate (as expressed by a HR below 1) in patients with a baseline FVIII activity <1 IU/dL. There was a trend toward a lower PR rate with higher baseline inhibitor titer that did not reach statistical significance (Table 3). Age, gender, underlying disorder, and WHO-PS were not significantly associated with time to PR. When these factors were entered into a multivariate Cox proportional hazard model, baseline FVIII activity <1 IU/dL remained significantly associated with time to PR (Table 4 and Figure 3A).
Table 3.
PR according to baseline characteristics: univariate analysis
| Baseline variable | PR achieved n (%) | Time to PR in days median (IQR) | Unadjusted HR (CI) |
|---|---|---|---|
| FVIII activity | |||
| <1 IU/dL (n = 47) | 36 (77) | 43 (28-64) | 0.54 (0.35-0.83)* |
| ≥1 IU/dL (n = 55) | 49 (89) | 24 (15-44) | |
| Inhibitor concentration | |||
| ≤20 BU/mL (n = 54) | 45 (83) | 26 (15-46) | 0.76 (0.50-1.16) |
| >20 BU/mL (n = 48) | 40 (83) | 35 (28-59) | |
| Gender | |||
| Female (n = 43) | 40 (93) | 35 (23-62) | 1.23 (0.80-1.89) |
| Male (n = 59) | 45 (76) | 28 (18-49) | |
| Age | |||
| ≤74 y (n = 53) | 48 (91) | 41 (23-64) | 0.83 (0.54-1.28) |
| >74 y (n = 49) | 37 (76) | 28 (18-36) | |
| WHO-PS | |||
| Good (≤2, n = 64) | 55 (86) | 32 (18-52) | 0.80 (0.51-1.25) |
| Poor (>2, n = 38) | 30 (79) | 30 (21-47) | |
| Underlying disorder | |||
| Autoimmunity (n = 20) | 19 (95) | 32 (23-51) | 1.31 (0.78-2.19) |
| Malignancy (n = 13) | 9 (69) | 21 (17-28) | 0.74 (0.37-1.48) |
| Pregnancy (n = 5) | 5 (100) | 50 (43-82) | 0.92 (0.38-1.28) |
P < .05 (statistical significance from univariate Cox regression analysis).
Table 4.
Predictors of remission and survival: multivariate analysis
| Baseline variable | PR | CR | OS |
|---|---|---|---|
| FVIII activity <1 IU/dL | 0.52 (0.33-0.81)** | 0.49 (0.29-0.85)* | 2.40 (1.10-5.22)* |
| Inhibitor concentration >20 BU/mL | 0.77 (0.49-1.21) | 0.75 (0.43-1.29) | 1.20 (0.54-2.67) |
| Female gender | 1.22 (0.77-1.91) | 1.30 (0.76-2.24) | 0.58 (0.26-1.31) |
| Age >74 y | 0.94 (0.58-1.50) | 0.76 (0.43-1.32) | 1.76 (0.82-3.78) |
| Underlying disorder | |||
| Autoimmunity | 1.32 (0.77-2.28) | 0.88 (0.45-1.72) | 1.02 (0.36-2.84) |
| Malignancy | 0.58 (0.28-1.21) | 0.62 (0.27-1.44) | 2.91 (1.12-7.52)* |
| Pregnancy | 0.61 (0.23-1.65) | 0.74 (0.27-2.04) | — |
| WHO-PS >2 | 0.76 (0.48-1.21) | 0.39 (0.21-0.72)** | 3.38 (1.55-7.37)** |
Data are presented as adjusted HR (CI).
P < .05.
P < .01.
Figure 3.
Adjusted time to PR and CR according to baseline FVIII activity. Cox proportional hazard model of time to end point with age, gender, underlying disorder, WHO-PS, inhibitor concentration, and baseline FVIII as categorical covariates. (A) PR (log rank P < .01). (B) Complete remission (log rank P < .05). Closed blue line, FVIII activity ≥1 IU/dL (n = 55); dashed red line, FVIII activity <1 IU/dL (n = 47).
We also assessed characteristics of the 25 patients who achieved PR by day 21 with steroids alone. This subset of patients had a particularly good prognosis: 88% survived until the end of the study, and 85% achieved a CR after a median of 50 days (IQR 42-75). These patients were best predicted by a multivariate logistic regression model containing baseline FVIII activity ≥1 IU/dL (adjusted OR 5.01, CI 1.52-16.9, P < .01) and inhibitor concentration ≤20 BU/mL (5.40, CI 1.63-17.9, P < .01). Age, gender, underlying disorder, and WHO-PS did not significantly contribute to the model. Patients with FVIII activity ≥1 IU/dL and inhibitor ≤20 BU/mL at baseline comprised 30% of the study population. Their chance to achieve PR with steroid alone by day 21 was 53%, compared with 9% in the other patients (OR 11.2; CI 3.86-32.4; P < .0001). ROC analysis was performed to assess the full range of FVIII activity and inhibitor concentrations to predict early PR (Table 5). Particularly useful negative predictive values (>0.9) were found for low FVIII activity and high inhibitor concentration. Moderate positive predictive values of about 0.6 were found for higher FVIII activity thresholds and lower inhibitor concentrations.
Table 5.
ROC analysis to FVIII activity and inhibitor concentrations to predict PR within 21 days with steroids alone
| Threshold | AUC (CI) | Sensitivity (CI) | Specificity (CI) | LR | PPV | NPV |
|---|---|---|---|---|---|---|
| FVIII activity, IU/dL | 0.75 (0.64-0.86) | |||||
| ≥1 | 0.84 (0.64-0.95) | 0.56 (0.44-0.67) | 1.9 | 0.38 | 0.91 | |
| >2 | 0.72 (0.51-0.88) | 0.73 (0.61-0.82) | 2.6 | 0.46 | 0.89 | |
| >6 | 0.32 (0.15-0.54) | 0.92 (0.84-0.97) | 4.1 | 0.57 | 0.81 | |
| >18 | 0.08 (0.01-0.26) | 0.99 (0.93-1.00) | 6.2 | 0.67 | 0.77 | |
| Inhibitor concentration, BU/mL | 0.78 (0.67-0.89) | |||||
| <2 | 0.20 (0.07-0.41) | 0.97 (0.91-1.00) | 7.7 | 0.80 | 0.78 | |
| <5 | 0.52 (0.31-0.72) | 0.90 (0.81-0.95) | 5.0 | 0.62 | 0.85 | |
| <20 | 0.84 (0.64-0.95) | 0.57 (0.45-0.68) | 2.0 | 0.40 | 0.88 | |
| <100 | 0.92 (0.74-0.99) | 0.26 (0.17-0.37) | 1.2 | 0.29 | 0.91 |
LR, likelihood ratio; NPV negative predictive value; PPV, positive predictive value.
CR
CR was achieved with a lower rate in patients with baseline FVIII <1 IU/dL and WHO-PS >2 according to univariate analysis (data not shown) and multivariate analysis (Table 4 and Figure 3B). The impact of WHO-PS on the time to CR was primarily due to the fact that patients with a WHO-PS >2 were more likely to die before achieving CR (relative risk 2.37; CI 1.50-3.74, P < .001). In patients who actually achieved CR, there was no difference in the median time to CR comparing patients with WHO-PS ≤2 (71 days) and WHO-PS >2 (68 days).
Survival
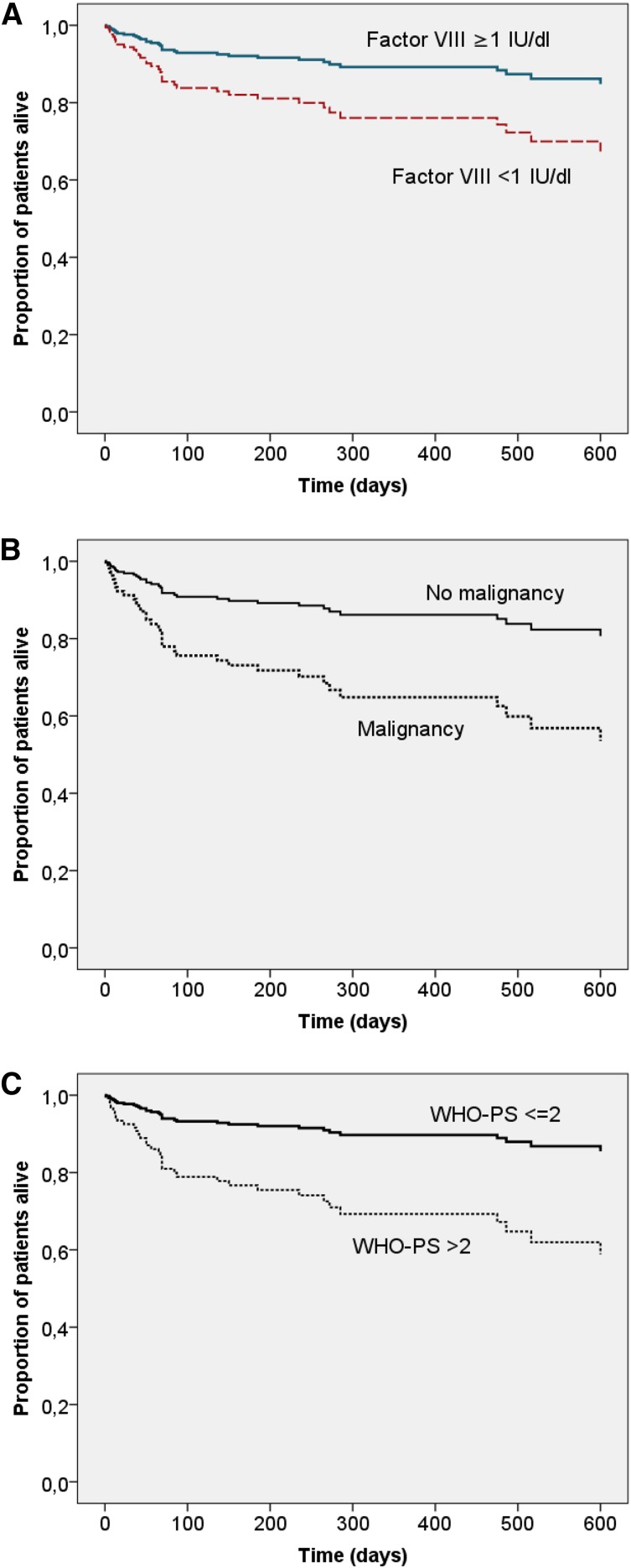
Univariate analysis indicated that baseline FVIII activity <1 IU/dL, inhibitor concentration >20 BU/mL, age >74 years, WHO-PS >2, male gender, malignancy, and renal failure were associated with poor OS (not shown). In multivariate analysis, 3 baseline characteristics remained independent predictors of poor OS: FVIII activity <1 IU/dL, WHO-PS >2, and malignancy (Table 4 and Figure 4).
Figure 4.
Adjusted OS according to baseline characteristics. Cox proportional hazard model of OS with age, gender, underlying disorder, WHO-PS, inhibitor concentration, and baseline FVIII activity as categorical covariates. Patterns were drawn according to (A) baseline FVIII (<1 IU/dL n = 47 vs ≥1 IU/dL n = 55, P < .05), (B) malignancy status (present n = 13 vs absent n = 89, P < .05), and (C) WHO-PS (≤2 n = 64 vs >2 n = 38, P < .01).
Discussion
This study is the first to establish baseline characteristics as predictors for outcome in AHA. We demonstrate that baseline FVIII activity has a strong impact on the time needed to achieve PR and CR. A baseline FVIII activity of <1 IU/dL was also associated with poor survival. In contrast, a higher FVIII activity and a lower inhibitor concentration at baseline increased the chance to achieve PR within 21 days while on treatment with steroid alone. We found a weak correlation between baseline FVIII activity and inhibitor concentration and, in fact, FVIII activity was a better predictor of outcomes than the Bethesda inhibitor titer. This may be due in part to the high variability of inhibitor measurement, in particular with type 2 inhibitors that are often seen in AHA.16 Nevertheless, inhibitor concentration appeared useful, similar to FVIII activity, to predict early PR within 21 days while on treatment with steroid alone. In particular, a low FVIII activity and a high inhibitor concentration at baseline provided a high negative predictive value (>0.9) to exclude early PR. This finding may provide a basis to treat those patients with more intensive IST up front, as their probability of obtaining remission with steroids only is very low.
Clinical management of AHA, in particular IST, has been mainly based on expert opinion and limited information from registries.2,13,17 A single controlled study has been performed that lacked statistical power to compare the treatments used.18 The largest available registry, the European Acquired Hemophilia Registry (EACH2), was retrospective in part (patients could be enrolled retrospectively for up to 3 years) and did not enroll, in some countries, patients who died.3,9 Patients were treated with IST regimens of variable intensity.9 The data suggested that lower baseline FVIII activity and higher inhibitor concentration were associated with a longer time to inhibitor eradication.9 To our knowledge, this potentially important finding has not changed clinical practice guidelines, possibly because this analysis could not be adjusted for treatment regimen, which may have had an impact on time to remission as well. In fact, EACH2 investigators reported that a combination of steroid and cyclophosphamide resulted in faster remission than steroid alone.9 A meta-analysis of 249 published cases came to the same conclusion,12 whereas the UK surveillance study did not detect a difference between these regimens.8 Of note, the latter study found that combination therapy was used more often in patients with higher inhibitor concentrations, indicating that disease severity may have confounded the effect of treatment. A survey among hemophilia centers in Austria, Germany, and Switzerland showed that many factors, including severity of bleeding, the required duration of expensive hemostatic treatments, and inhibitor concentration influence the physician’s choice of IST.15 If physicians tailor the intensity of treatment to the perceived severity of disease (including suspected prognostic factors), the analysis of prognostic factors can be seriously confounded, unless it is stratified for treatment. We therefore aimed to study prognostic factors in a group of patients treated according to a uniform IST protocol.
The GTH-AH 01/2010 treatment protocol was designed in 2008. It was influenced by consensus recommendations and data from the UK surveillance study indicating that steroid alone vs steroid plus cyclophosphamide were treatments of similar efficacy and safety. We followed a simple escalation strategy, starting with steroid alone (weeks 1-3), adding cyclophosphamide (weeks 4-6) and rituximab (weeks 7-10), as long as PR was not achieved. Escalation had to be withheld per protocol if patients showed a continuous increase of FVIII, which turned out to be a feasible strategy. Further exceptions were allowed per protocol in the presence of contraindications. Importantly, however, no choice of treatment was allowed because of perceived severity of disease, bleeding tendency, laboratory parameters, or other baseline characteristics. Enrolment was strictly prospective, and all cases from study sites were excluded if the site did not follow the protocol. Outcomes achieved with the GTH protocol were largely similar to other recent studies: CR was achieved by 61% of patients (UK surveillance study 71%,8 French Surveillance des Auto antiCorps au cours de l’Hémophilie Acquise [SACHA] study 61% [reported after 1 year],10 EACH2 study 72%)3; mortality was 33% (United Kingdom 43%, SACHA 33%, EACH2 26%); death due to infection occurred in 16% of the whole cohort (United Kingdom 11%, SACHA 12%, EACH2 4%); and death due to bleeding occurred in 2.9% of the whole cohort (United Kingdom 9.1%, SACHA 3.5%, EACH2 4.5%). Compared with the historic collection by Green and Lechner,6 who found in 22% their patients death directly or indirectly attributable to the presence the inhibitor, we recognize infection as the major cause of death in AHA today.
Our prognostic factor analysis demonstrates a significant effect of baseline FVIII activity on the time to achieve PR or CR. Even more relevant for clinical practice, baseline FVIII activity and inhibitor concentration could be used to define a subgroup of patients with better prognosis. This group, characterized by a lower inhibitor titer (<20 BU/mL) and a higher baseline FVIII activity (≥1 IU/dL), comprised about one-third of patients and was found to have >50% chance of achieving PR with steroids alone by day 21. Patients not belonging to this group had a <10% chance of achieving PR early as further supported by ROC analysis. Given the risk of prolonged IST exposure, it appears justified to consider more aggressive regimens in the latter patients, eg, by starting a combination of steroids and other immunosuppressants from the beginning. This is supported by observations in other disease areas, eg, rheumatoid arthritis, demonstrating that not only intensity but also duration of IST determined the risk of serious infection.19
GTH 01/2010 is the first study to prospectively assess predictors of survival in AHA. It was not unexpected that malignancy and poor WHO-PS at baseline were associated with reduced OS. More surprisingly, baseline FVIII activity was an independent and strong predictor of OS. This was not due to bleeding, as bleeding was not a frequent cause of death and the number or severity of bleeds did not correlate with risk of death (data not shown). More likely, a lower FVIII activity may be a surrogate marker for a more robust autoimmune reaction that could be more difficult to treat and require longer IST, thus implying a greater risk of infection. In fact, infections were significantly associated with mortality in our study.
It should be noted that the prognostic baseline characteristics assessed here would not necessarily have the same impact on patients treated with different regimens. Using more intense treatment at baseline for patients with a low FVIII activity could increase the risk of mortality from other causes, eg, in people of advanced age or with comorbidities. Therefore, the analysis of prognostic risk factors should be continued in future studies.
In conclusion, our study established clinically useful prognostic factors for remission and survival of AHA. However, it also confirmed that current IST regimens often require a very long time to remission and that side effects still cause considerable morbidity and mortality. The challenge for future studies will be to develop IST regimens that reduce the burden of side effects, potentially by tailoring their intensity to prognostic baseline characteristics established in the current study.
Acknowledgments
This study was conducted by the Thrombosis and Haemostasis Society of the German-speaking countries (GTH e.V.). Contributing investigators are listed in supplemental “Appendix.” The authors thank Ingrid Pabinger, GTH president 2007-2011, for support and seminal contributions during study design and initiation. The authors acknowledge the contribution of all study sites, local study coordinators, and Susanne Bartels, central study coordinator at Hannover Medical School.
Funding for this study was obtained from GTH e.V. and by unrestricted educational grants from Novo Nordisk Pharma GmbH (Mainz, Germany) and Baxter Deutschland GmbH (Unterschleißheim, Germany).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.T., P.K., A.H.-K., and R.K. designed the protocol; A.T., P.K., R.K., S.G., K.H., U.G., R.E.S., J.S., A.H.-K., R.G., P.N., C.v.A., W.M., K.S., U.S., D.M., R.U.T., U.K., M.K., and K.L. enrolled patients, collected data, and critically reviewed the data; A.T., J.-M.B., and S.W. managed data; A.T., A.K., E.B., and J.-M.B. performed statistical analysis; A.T., R.U.T., P.K., R.E.S., and R.K. wrote the initial draft of the manuscript; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Tiede, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl Neuberg Str 1, 30625 Hannover, Germany; e-mail: tiede.andreas@mh-hannover.de.
References
- 1.Collins PW. Therapeutic challenges in acquired factor VIII deficiency. Hematology Am Soc Hematol Educ Program. 2012;2012:369–374. doi: 10.1182/asheducation-2012.1.369. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Mannucci PM. Acquired haemophilia A: a 2013 update. Thromb Haemost. 2013;110(6):1114–1120. doi: 10.1160/TH13-05-0363. [DOI] [PubMed] [Google Scholar]
- 3.Knoebl P, Marco P, Baudo F, et al. EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost. 2012;10(4):622–631. doi: 10.1111/j.1538-7836.2012.04654.x. [DOI] [PubMed] [Google Scholar]
- 4.Baudo F, Collins P, Huth-Kühne A, et al. EACH2 registry contributors. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120(1):39–46. doi: 10.1182/blood-2012-02-408930. [DOI] [PubMed] [Google Scholar]
- 5.Tengborn L, Baudo F, Huth-Kühne A, et al. EACH2 registry contributors. Pregnancy-associated acquired haemophilia A: results from the European Acquired Haemophilia (EACH2) registry. BJOG. 2012;119(12):1529–1537. doi: 10.1111/j.1471-0528.2012.03469.x. [DOI] [PubMed] [Google Scholar]
- 6.Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb Haemost. 1981;45(3):200–203. [PubMed] [Google Scholar]
- 7.Gheisari R, Bomke B, Hoffmann T, Scharf RE. Clinical features and outcome of acquired haemophilia A. Interim analysis of the Düsseldorf study. Hamostaseologie. 2010;30(3):156–161. [PubMed] [Google Scholar]
- 8.Collins PW, Hirsch S, Baglin TP, et al. UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109(5):1870–1877. doi: 10.1182/blood-2006-06-029850. [DOI] [PubMed] [Google Scholar]
- 9.Collins P, Baudo F, Knoebl P, et al. EACH2 registry collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood. 2012;120(1):47–55. doi: 10.1182/blood-2012-02-409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borg JY, Guillet B, Le Cam-Duchez V, Goudemand J, Lévesque H SACHA Study Group. Outcome of acquired haemophilia in France: the prospective SACHA (Surveillance des Auto antiCorps au cours de l’Hémophilie Acquise) registry. Haemophilia. 2013;19(4):564–570. doi: 10.1111/hae.12138. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Xue F, Shi H, et al. Acquired hemophilia a: retrospective analysis of 49 cases from a single Chinese hemophilia center. Clin Appl Thromb Hemost. 2015;21(1):35–40. doi: 10.1177/1076029613488937. [DOI] [PubMed] [Google Scholar]
- 12.Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol. 2003;121(1):21–35. doi: 10.1046/j.1365-2141.2003.04162.x. [DOI] [PubMed] [Google Scholar]
- 13.Huth-Kühne A, Baudo F, Collins P, et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica. 2009;94(4):566–575. doi: 10.3324/haematol.2008.001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins P, Baudo F, Huth-Kühne A, et al. Consensus recommendations for the diagnosis and treatment of acquired hemophilia A. BMC Res Notes. 2010;3:161. doi: 10.1186/1756-0500-3-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiede A, Huth-Kühne A, Oldenburg J, et al. Immunosuppressive treatment for acquired haemophilia: current practice and future directions in Germany, Austria and Switzerland. Ann Hematol. 2009;88(4):365–370. doi: 10.1007/s00277-008-0665-7. [DOI] [PubMed] [Google Scholar]
- 16.Tiede A, Werwitzke S, Scharf RE. Laboratory diagnosis of acquired hemophilia A: limitations, consequences, and challenges. Semin Thromb Hemost. 2014;40(7):803–811. doi: 10.1055/s-0034-1390004. [DOI] [PubMed] [Google Scholar]
- 17.W Collins P, Chalmers E, Hart D, et al. United Kingdom Haemophilia Centre Doctors’ Organization. Diagnosis and management of acquired coagulation inhibitors: a guideline from UKHCDO. Br J Haematol. 2013;162(6):758–773. doi: 10.1111/bjh.12463. [DOI] [PubMed] [Google Scholar]
- 18.Green D, Rademaker AW, Briët E. A prospective, randomized trial of prednisone and cyclophosphamide in the treatment of patients with factor VIII autoantibodies. Thromb Haemost. 1993;70(5):753–757. [PubMed] [Google Scholar]
- 19.Dixon WG, Abrahamowicz M, Beauchamp ME, et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis. 2012;71(7):1128–1133. doi: 10.1136/annrheumdis-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]