Background: Upstream mechanisms of HGF-induced endothelial barrier enhancement are not well understood.
Results: HGF induced IQGAP1 interaction with Rac-specific GEF Asef leading to endothelial barrier enhancement.
Conclusion: IQGAP1-dependent Asef targeting to cortical cytoskeleton represents a novel mechanism of local regulation of Rac and endothelial barrier function.
Significance: Subcellular targeting of small GTPase regulators may represent a novel approach to modulate vascular endothelial permeability.
Keywords: Endothelium, Lung Injury, Permeability, Signal Transduction, Vascular Biology, Rac GTPases
Abstract
Hepatocyte growth factor (HGF) attenuates agonist-induced endothelial cell (EC) permeability and increases pulmonary endothelial barrier function via Rac-dependent enhancement of the peripheral actin cytoskeleton. However, the precise mechanisms of HGF effects on the peripheral cytoskeleton are not well understood. This study evaluated a role for Rac/Cdc42-specific guanine nucleotide exchange factor Asef and the multifunctional Rac effector, IQGAP1, in the mechanism of HGF-induced EC barrier enhancement. HGF induced Asef and IQGAP1 co-localization at the cell cortical area and stimulated formation of an Asef-IQGAP1 functional protein complex. siRNA-induced knockdown of Asef or IQGAP1 attenuated HGF-induced EC barrier enhancement. Asef knockdown attenuated HGF-induced Rac activation and Rac association with IQGAP1, and it abolished both IQGAP1 accumulation at the cell cortical layer and IQGAP1 interaction with actin cytoskeletal regulators cortactin and Arp3. Asef activation state was essential for Asef interaction with IQGAP1 and protein complex accumulation at the cell periphery. In addition to the previously reported role of the IQGAP1 RasGAP-related domain in the Rac-dependent IQGAP1 activation and interaction with its targets, we show that the IQGAP1 C-terminal domain is essential for HGF-induced IQGAP1/Asef interaction and Asef-Rac-dependent activation leading to IQGAP1 interaction with Arp3 and cortactin as a positive feedback mechanism of IQGAP1 activation. These results demonstrate a novel feedback mechanism of HGF-induced endothelial barrier enhancement via Asef/IQGAP1 interactions, which regulate the level of HGF-induced Rac activation and promote cortical cytoskeletal remodeling via IQGAP1-Arp3/cortactin interactions.
Introduction
HGF2 is a multifunctional mesenchyme-derived pleiotropic factor secreted by several cell types, which appears among other bioactive substances in lung circulation under pathological conditions such as acute lung injury, sepsis, lung inflammation, and ventilator-induced lung injury, and it regulates a number of biological events as follows: cell mitogenesis, morphogenesis, organogenesis, and cell survival (1–3). Novel therapeutic strategies using HGF have been suggested for cardiovascular diseases (4, 5). HGF elicits potent angiogenic activities (6) but also exhibits sustained barrier-protective effects on the human pulmonary (7, 8) and cerebral vascular endothelium (9). Binding of HGF to its c-Met receptor stimulates receptor tyrosine kinase activity, followed by the recruitment of multiple Src homology 2 domain-containing signaling molecules, including Grb2-associated binder-1, Grb2, PI 3-kinase, phospholipase Cγ, p60src, Shc, and Shp2 (10, 11). These signaling components are involved in branching morphogenesis (11) and endothelial barrier enhancement (8). HGF barrier-enhancing effects were associated with peripheral actin polymerization mediated by stimulation of PI 3-kinase and Rac GTPase activities leading to accelerated recovery of endothelial integrity altered by barrier disruptive agonists and pathological mechanical forces (8, 12). However, the precise molecular mechanisms of HGF-mediated cytoskeletal remodeling essential for the HGF-induced EC barrier-protective response remain incompletely understood.
Small GTPase Rac activity is associated with lamellipodia and membrane ruffle formation, cell migration, and barrier protection (13, 14). Small GTPases act as molecular switches, cycling between an inactive GDP-bound, and active GTP-bound state. Cycling between the GDP- and GTP-bound states is regulated by guanine nucleotide exchange factors (GEFs), which facilitate exchange of GDP for GTP (15–17). Our previous studies demonstrated the involvement of the Dbl family member Rac-specific GEF Tiam1 in the EC barrier protection induced by several agonists, including HGF (8, 18–20). However, Tiam1 down-regulation did not cause complete suppression of HGF protective effects, suggesting activation of additional mechanisms.
Asef has been recently described as a novel Rac/Cdc42-specific GEF in cancer cells (21). Asef activation by binding to microtubule-associated protein APC promoted intestinal adenoma formation and tumor progression (21). Asef contains a Dbl homology (DH) domain exhibiting GEF activity, a pleckstrin homology domain that determines the subcellular localization and activity by interacting with phosphatidylinositol phosphate, an Src homology 3 autoinhibitory domain, and a region that binds microtubule-associated tumor suppressor protein APC (APC domain) (21). Asef has been proposed to regulate the actin cytoskeleton in epithelial and neuronal cells by activating Rac and Cdc42 GTPases (22). The involvement of Asef in vascular endothelial barrier regulation remains unknown.
IQGAP1 is a multifunctional adaptor protein involved in the coordination of diverse cellular processes, including receptor activation, regulation of MAPK signaling, activity of small GTPases, the regulation of cytoskeletal remodeling, and assembly of cell junctions (23–26). IQGAP1 controls microtubule and actin cytoskeletal dynamics via interactions with small GTPases Rac1 and Cdc42. GTP-bound Rac interacts with IQGAP1 and promotes tethering of actin filaments. IQGAP1 contains a calponin homology domain, four calmodulin-binding IQ domains, a RasGAP-related domain (GRD), and a RasGAP C-terminal domain (27). IQGAP1 has been shown to interact with several target proteins associated with microtubules, adherens junctions, and actin cytoskeleton, including β-catenin, E-cadherin, N-WASP, Arp2/3, cortactin, microtubule-associated plus end tracking proteins (CLIP170, CLASP-2), and others (26).
The reported interaction of IQGAP1 with microtubules via APC (28) raises the possibility of cross0talk between microtubules and actin cytoskeletal regulation. In the cancer Vero cell line, IQGAP1 directly interacts with APC, and an IQGAP1-APC complex co-localizes at the leading edge with Rac and Cdc42 (28) where it may regulate cortical actin dynamics. However, a role of IQGAP1 in HGF-mediated endothelial barrier-protective response has not been yet defined. This study tested the hypothesis that HGF-induced activation of cortical actin dynamics and endothelial barrier enhancement involve Asef activation and functional interactions with IQGAP1, which may be important for local regulation of Rac activity and IQGAP1 interactions with regulators of actin polymerization and cytoskeletal remodeling.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Human HGF was obtained from R&D Systems (Minneapolis, MN). Cell-permeable c-Met kinase inhibitor, N-(3-fluoro-4-(7-methoxy-4-quinolinyl)phenyl)-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-phenyl-2,3-dihydro-1H-pyrazole carboxamide, was from EMD Millipore (Billerica, MA). Texas Red-conjugated phalloidin and Alexa Fluor 488 were purchased form Molecular Probes (Eugene, OR). Arp3 and Rac1 antibodies were purchased from BD Transduction Laboratories (San Diego, CA); Asef, IQGAP1, HA tag, and Myc tag antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); cortactin antibodies were from Millipore (Billerica, MA). Unless otherwise specified, all biochemical reagents, including β-actin and β-tubulin antibodies, were obtained from Sigma. Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (East Rutherford, NJ) and used for experiments at passages 5–7. HA-tagged wild type Asef, dominant negative AsefΔDH, constitutively active AsefΔAPC, activated Asef-D133A/E365A mutant subcloned into pcDNA3.1 vector for mammalian expression, as well as GST-tagged wild type Asef subcloned into pGEX vector for bacterial expression were generated in the Akiyama laboratory and described previously (21, 29). Plasmids encoding Myc-tagged full-length IQGAP1, IQGAP1ΔC mutant encoding an IQGAP1 isoform lacking C-terminal amino acids 1502–1657, and IQGAP1ΔGRD mutant lacking Rac-binding domain (GRD) flanked by amino acids 1025–1238 subcloned into pcDNA3.1 vector (Invitrogen) for mammalian expression were generated in the Sacks laboratory and described elsewhere (30).
Analysis of EC Permeability
Endothelial permeability to macromolecules was monitored by express permeability testing assay (XPerT) (31, 32) available from Millipore (Vascular Permeability Imaging Assay, catalog no. 17-10398). This assay is based on high affinity binding of cell-impermeable avidin-conjugated FITC-labeled tracer to the biotinylated extracellular matrix proteins immobilized on the surface covered with EC monolayers. In permeability visualization experiments, 15 min after EC stimulation with HGF, FITC-avidin solution was added directly to the culture medium for 3 min before termination of the experiment. Unbound FITC-avidin was washed out with PBS (pH 7.4, 37 °C); cells were fixed with 3.7% formaldehyde in PBS (10 min at room temperature), and visualization of FITC-avidin on the bottoms of coverslips was performed using Nikon imaging system Eclipse TE 300 (Nikon, Tokyo, Japan) equipped with a digital camera (DKC 5000, Sony, Tokyo, Japan); ×10 objective lenses were used. Images were processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). For the permeability assay in 96-well plates, cells were seeded on biotinylated gelatin-coated plates (3 × 104 cells/well) and grown for 48–72 h prior to testing. FITC-avidin solution was added directly to the culture medium at the final concentration 25 μg/ml for 3 min before termination of the experiment unless otherwise specified. Unbound FITC-avidin was washed out with 200 μl of PBS, pH 7.4, 37 °C (two cycles, 10 s each). Finally, 100 μl of PBS was added to each well, and the fluorescence of matrix-bound FITC-avidin was measured on Victor X5 Multilabel Plate Reader (PerkinElmer Life Sciences) using an excitation wavelength of 485 nm and emission wavelength of 535 nm.
Immunofluorescence Staining
Endothelial cells plated on glass coverslips were treated with the agonist of interest, fixed in 3.7% formaldehyde solution in PBS for 10 min at 4 °C, washed three times with PBS, permeabilized with 0.1% Triton X-100 in PBS/Tween (PBST) for 30 min at room temperature, and blocked with 2% BSA in PBST for 30 min. Incubations with primary antibody of interest were performed in blocking solution (2% BSA in PBST) for 1 h at room temperature, followed by staining with Alexa 488-conjugated secondary antibodies. Actin filaments were stained with Texas Red-conjugated phalloidin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Japan) as described elsewhere (33, 34).
Co-immunoprecipitation, Differential Protein Fractionation, and Immunoblotting
After agonist stimulation, cells were washed in cold PBS and lysed on ice with cold TBS/Nonidet P-40 lysis buffer (20 mm Tris, pH 7.4, 150 mm NaCl, 1% Nonidet P-40) supplemented with protease and phosphatase inhibitor mixtures (Roche Applied Science). Clarified lysates were then incubated with antibodies to Myc tag, IQGAP1, or Asef overnight at 4 °C and washed 3–4 times with TBS/Nonidet P-40 lysis buffer, and the complexes were analyzed by Western blotting using appropriate antibodies. In fractionation studies, cytosolic (soluble) and membrane/cytoskeletal (particulate) fractions were isolated as described previously (19, 35). Protein extracts were separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and probed with specific antibodies. Equal protein loading was verified by reprobing membranes with antibody to β-actin or specific protein of interest.
Rac and Asef Activation Assays
Rac activation was evaluated in pulldown assays using agarose beads with immobilized PAK1-PBD (36). In brief, after stimulation, cell lysates were collected, and GTP-bound Rac was captured using pulldown assays with immobilized PAK1-PBD agarose. Levels of activated Rac were evaluated by Western blot analysis and normalized to the total protein content. Active Asef was affinity-precipitated from cell lysates according to a previously described protocol (37) using the Rac (G15A) mutant kindly provided by K. Szaszi (St. Michael's Hospital, Toronto, Canada). This mutant cannot bind nucleotide and therefore has high affinity for activated GEFs (38). Activated Asef in Rac (G15A) pulldowns was detected by Western blotting and normalized to total Asef in cell lysates for each sample. Precipitation with glutathione-Sepharose beads containing no fusion proteins resulted in no Asef precipitation.
siRNA and DNA Transfections
Asef-specific and c-Met-specific sets of three pre-designed StealthTM select siRNA duplexes were purchased from Invitrogen in ready to use, desalted, deprotected, and annealed double strand form. Nonspecific RNA (Dharmacon) was used as a control treatment. Transfection of EC with siRNA was performed as described previously (39). After 72 h of transfection, cells were used for experiments or harvested for Western blot verification of specific protein depletion. Transient transfections of plasmid DNA were performed using PolyJet In Vitro Transfection Reagent (Signagen, Rockville, MD) according to manufacturer's protocol. After 24 h of transfection, cells were treated with the agonist of interest and used for experiments.
Bacterial Pulldown and Protein Overlay Assay
GST-tagged IQGAP1 in pGEX vector was used for bacterial expression in BL21-AI Escherichia coli strain. GST fusion protein was isolated (40) using glutathione resin (Clontech) and stored as 50% glycerol slurry. After stimulation with agonist, endothelial monolayers were washed with PBS and incubated on ice for 15 min with lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1.5 mm MgCl2, 1 mm EDTA, 1% Triton X-100, and 10% glycerol). Lysate was clarified by centrifugation and incubated with glutathione resin loaded with GST-IQGAP1 (2 h, 4 °C). The resin was then collected by centrifugation and washed three times with lysis buffer, and the amount of Rac bound to IQGAP1 beads was evaluated by Western blot analysis. Agarose beads without IQGAP1 were used as control for nonspecific binding. For protein overlay assay, recombinant proteins expressed in E. coli system were eluted from the beads using elution buffer containing 20 mm HEPES, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol, and 30 mm reduced glutathione. The assay was performed using Bio-Dot Microfiltration apparatus (Bio-Rad) according to the manufacturer's protocol with minor modifications. Briefly, bait proteins were immobilized onto nitrocellulose membrane. After a 30-min incubation with blocking buffer containing 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2 mm dithiothreitol, and 2% nonfat dry milk, the membranes were incubated with the protein of interest in the blocking buffer for 4 h at 4 °C. After three rounds of washing with washing buffer containing 20 mm HEPES, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol, and 0.1% Tween 20, the membranes were incubated appropriate primary antibody, and standard Western blotting procedure was performed to detect interacting proteins.
Statistical Analysis
Results are expressed as means ± S.D. of three to six independent experiments. Stimulated samples were compared with controls by unpaired Student's t tests. For multiple-group comparisons, a one-way variance analysis (ANOVA), followed by the post hoc Fisher's test were used. p < 0.05 was considered statistically significant.
RESULTS
HGF Induces Accumulation and Co-localization of Asef and IQGAP1 at the Cell Periphery
HGF stimulation activates peripheral cytoskeletal dynamics in vascular EC in a Rac-dependent fashion (8). This study used human pulmonary artery endothelial cells to characterize functional interactions between the Rac-specific guanine nucleotide exchange factor Asef and Rac/Cdc42 effector IQGAP1.
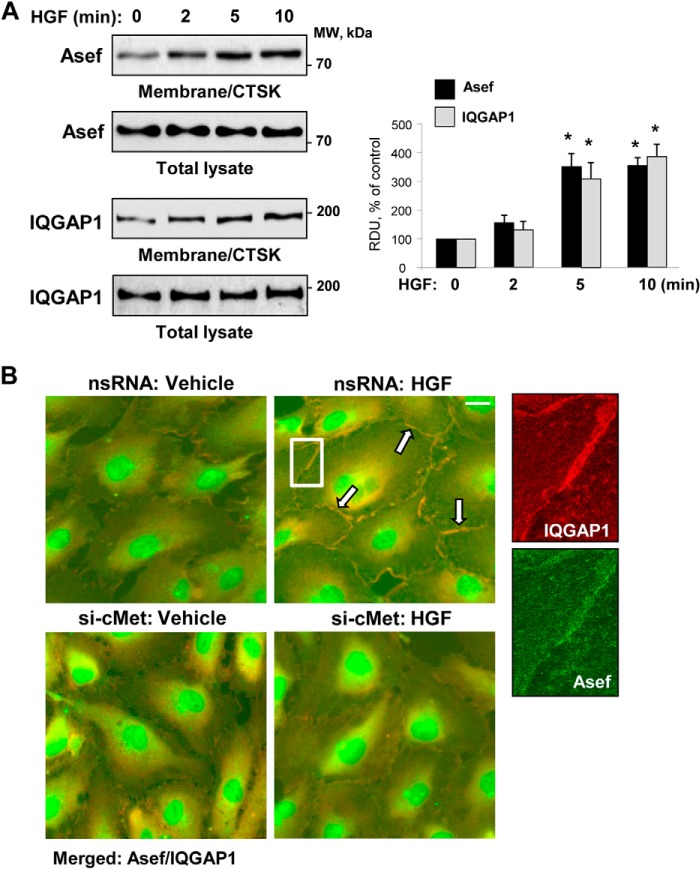
Subcellular fractionation assays showed increased levels of Asef and IQGAP1 in membrane/cytoskeletal fractions collected from HGF-stimulated endothelial cells (Fig. 1A). Immunofluorescence staining of Asef and IQGAP1 showed their accumulation at the cell periphery upon HGF stimulation (higher magnification insets in Fig. 1B). Merged images show Asef and IQGAP1 co-localization at peripheral areas of adjacent cells (Fig. 1B, upper panels). Knockdown of HGF receptor c-Met using siRNA approach inhibited HGF-induced co-localization of IQGAP1 and Asef at the cell junction area (Fig. 1B, lower panels). Asef antibody also decorated nuclear and perinuclear regions in control and HGF-stimulated cells, which likely represents nonspecific staining.
FIGURE 1.
HGF induces peripheral accumulation and co-localization of Asef and IQGAP1. A, EC were stimulated with HGF (50 ng/ml) for the time periods indicated. The content of Asef and IQGAP1 was determined by Western blot analysis of membrane/cytoskeletal fractions (CTSK) with specific antibodies and normalized to the total protein. Bar graphs depict quantitative analysis of Western blot data at membrane/cytoskeletal fractions; n = 4; *, p < 0.05 versus nonstimulated conditions. RDU, relative density units. B, HPAEC were transfected with c-Met-specific siRNA or nonspecific RNA and stimulated with HGF (50 ng/ml, 10 min). The cells were fixed and subjected to double immunofluorescence staining for Asef (green) and IQGAP1 (red). Merged images depict areas of HGF-induced protein co-localization that appear in yellow and are marked by arrows. Shown are representative results of three independent experiments; bar, 5 μm. Higher magnification insets show details of localization of Asef and IQGAP1 at the cell periphery of HGF-stimulated EC transfected with nonspecific RNA.
HGF-induced Enhancement of Endothelial Barrier Is Mediated by Asef and IQGAP1
The role of Asef and IQGAP1 in HGF-induced EC barrier enhancement was examined using siRNA knockdown approach. Visualization of local areas with increased or decreased EC permeability for macromolecules was performed in control EC monolayers and in EC with siRNA-based Asef or IQGAP1 knockdown (Fig. 2A). In control conditions, basal accumulation of FITC-labeled tracer was observed at sites underlying the cell-cell junction area and reflecting basal level of mass transport across the cell-cell junction area. HGF significantly decreased the basal EC monolayer permeability for FITC-labeled avidin. The barrier enhancing effect of HGF was abrogated in EC monolayers with Asef or IQGAP1 knockdown (Fig. 2A). The bar graph in Fig. 2B represents a quantitative analysis of EC permeability changes by measurements of fluorescence of accumulated FITC-avidin in 96-well plates with EC monolayers using a microplate reader, as described under “Experimental Procedures.”
FIGURE 2.
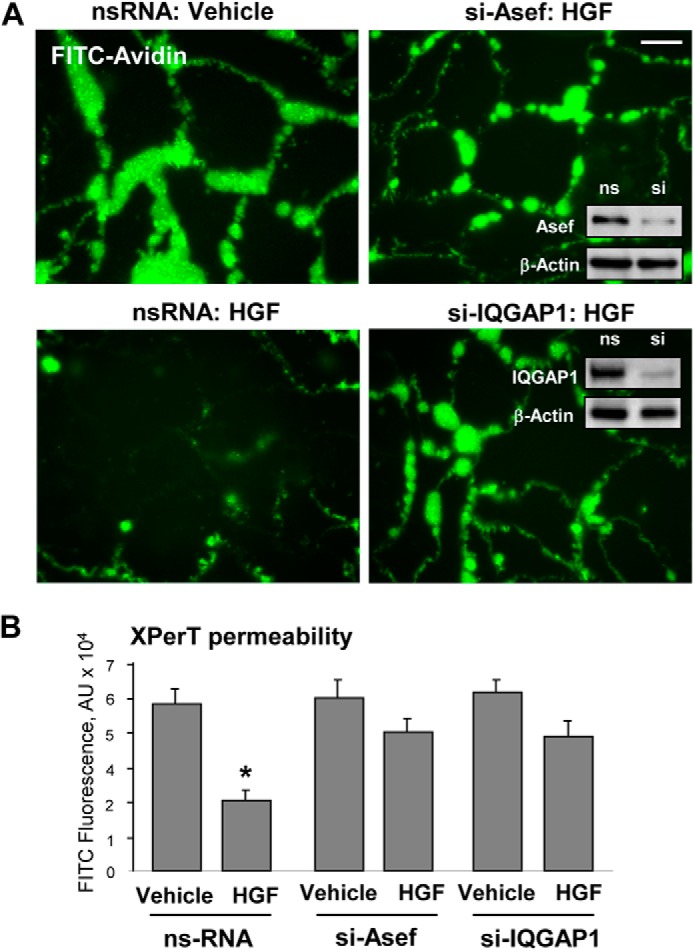
Asef and IQGAP1 knockdown attenuate HGF-induced EC barrier enhancement. A, HPAEC grown on glass coverslips with immobilized biotinylated gelatin (0.25 mg/ml) and transfected with Asef-specific and IQGAP1-specific siRNA or nonspecific (ns) RNA were stimulated with vehicle or HGF (50 ng/ml) followed by addition of FITC-avidin (25 μg/ml, 3 min). Unbound FITC-avidin was removed, and FITC fluorescence signal was visualized by fluorescence microscopy; bar, 10 μm. Insets, siRNA-mediated protein depletion was confirmed by Western blotting. B, permeability measurements. Bar graph depicts quantitative analysis of FITC fluorescence in control and stimulated EC monolayers in 96-well format measured in microplate reader. Results are represented as mean ± S.D.; *, p < 0.05, n = 6. RDU, relative density units.
HGF Stimulates Asef, Rac, and Asef-dependent Rac Association with IQGAP1
We next tested whether HGF induces interactions between Asef and IQGAP1. Co-immunoprecipitation assays with IQGAP1 antibody showed that endogenous Asef and IQGAP1 associate in cells (Fig. 3A). Incubation with HGF revealed a time-dependent increase in Asef content in immunocomplexes from HGF-stimulated cells. Similar results were obtained in reverse co-immunoprecipitation assays with Asef antibody and detection of co-precipitated IQGAP1 (Fig. 3B). HGF-induced Asef-IQGAP1 association was dependent on activation of HGF receptor c-Met and was abolished by preincubation with the cell-permeable c-Met kinase inhibitor carboxamide (Fig. 3).
FIGURE 3.
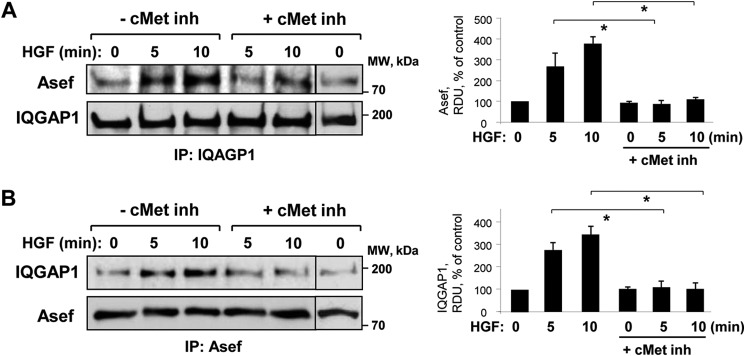
HGF induces interactions between Asef and IQGAP1. Cells were stimulated with vehicle or HGF (50 ng/ml) with or without pretreatment with cell-permeable c-Met kinase inhibitor (inh) (carboxamide 50 nm, 30 min). Co-immunoprecipitation assays using antibodies to IQGAP1 (A) or Asef (B) were performed, and Asef and IQGAP1 content in the immunoprecipitates (IP) was detected using appropriate antibody. Bar graphs depict quantitative analysis of Western blot data; n = 3; *, p < 0.05 versus HGF alone.
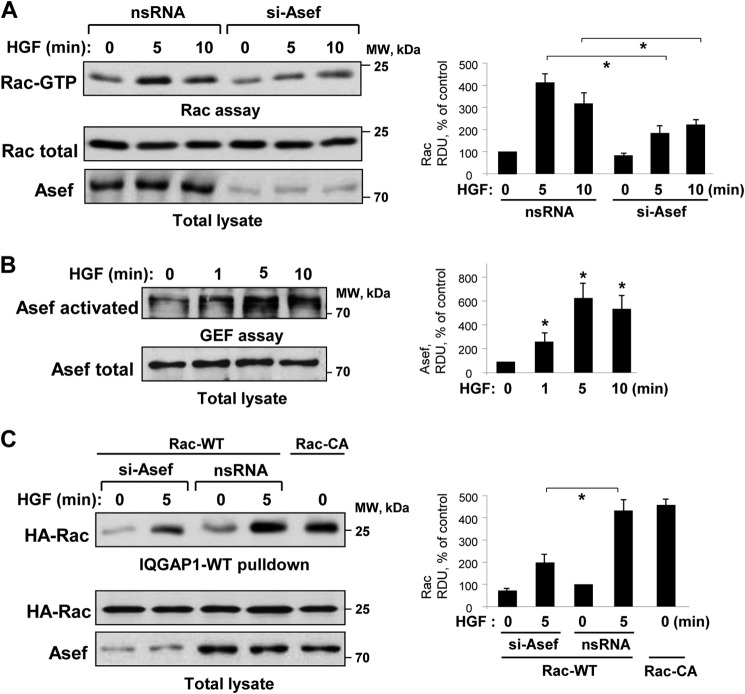
The HGF-induced endothelial barrier protective response is associated with activation of Rac1 GTPase signaling, but the precise mechanisms of Rac1 activation remain elusive. Asef knockdown by Asef-specific siRNA significantly attenuated the HGF-induced activation of Rac monitored by a Rac-GTP pulldown assay (Fig. 4A). HGF rapidly stimulated Asef nucleotide exchange activity that was evaluated by a pulldown assay with the immobilized RacG15A mutant described under “Experimental Procedures” (Fig. 4B). Pulldown assays with cell lysates from control and HGF-stimulated EC monolayers using beads with immobilized IQGAP1 showed increased Rac binding to immobilized IQGAP1 incubated with HGF-stimulated pulmonary EC lysates. This effect is presumably due to increased levels of activated GTP-loaded Rac in HGF-stimulated cells leading to increased interaction of Rac-GTP with IQGAP1. HGF-induced Rac-IQGAP1 binding was attenuated in HPAEC with Asef knockdown (Fig. 4C). Cells expressing constitutively activated Rac (Rac-CA) were used as a positive control.
FIGURE 4.
HGF activates Asef and Rac1 and induces Asef-dependent Rac1/IQGAP1 interaction. A, HPAEC were transfected with 100 nm Asef-specific siRNA or nonspecific (ns) RNA and stimulated with HGF (50 ng/ml) for 0, 5, or 10 min. Rac activation was determined by Rac-GTP pulldown assay. The content of activated Rac was normalized to the total Rac content in EC lysates. Bar graphs depict quantitative analysis of Western blot data; n = 4; *, p < 0.05 versus nonspecific RNA. B, HPAEC were stimulated with HGF (50 ng/ml) for the indicated periods of time. Asef activation was determined in pulldown assay with immobilized RacG15A and evaluated by increased Asef association with RacG15A. Content of activated Asef was normalized to the total Asef content in EC lysates. Bar graphs depict quantitative analysis of Western blot data; n = 3; *, p < 0.05 versus vehicle. C, HPAEC transfected with plasmid encoding wild type or constitutively active (CA) HA-tagged Rac1 were treated with Asef-specific siRNA or nonspecific RNA and stimulated with HGF (50 ng/ml, 5 min). Cell lysates were added to agarose beads with immobilized recombinant IQGAP1. After washing, bound Rac was detected by immunoblotting with Rac antibody. The content of IQGAP1-bound Rac was normalized to the total recombinant Rac content in the EC lysates. Bar graphs depict quantitative analysis of Western blot data; n = 3; *, p < 0.05 versus nonspecific RNA. RDU, relative density units.
Asef Mediates IQGAP1 Peripheral Accumulation and Interaction with Cytoskeletal Effectors
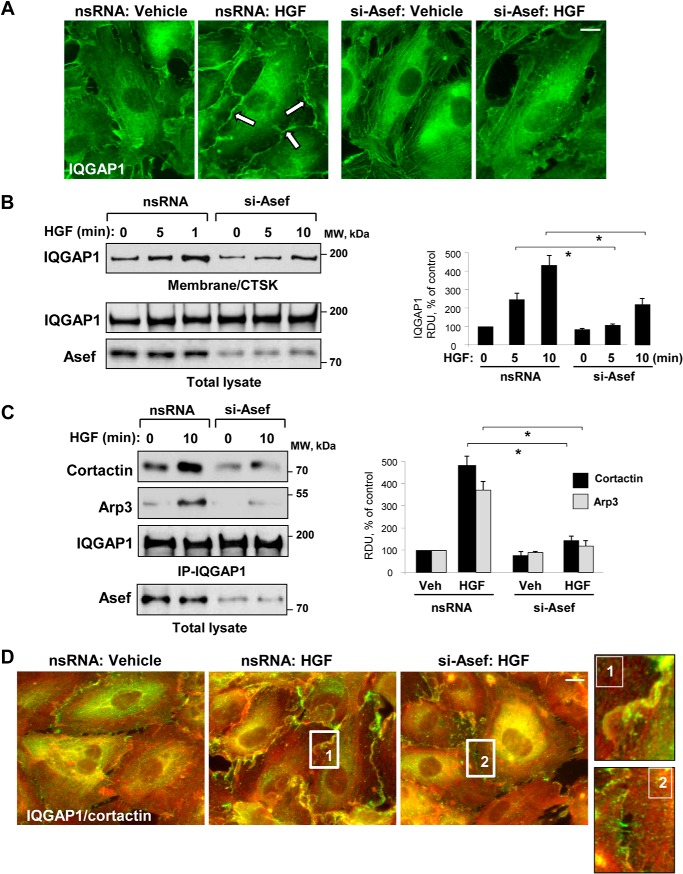
The potential role of Asef in the mechanism by which HGF induced IQGAP1 peripheral translocation was tested by immunofluorescence staining of IQGAP1 in control cells and EC monolayers with Asef knockdown (Fig. 5A). Asef knockdown attenuated peripheral accumulation of IQGAP1 in response to HGF. The Asef-dependent mechanism of HGF-induced IQGAP1 translocation to subcortical compartment was verified in a biochemical assay. Asef knockdown abolished HGF-induced accumulation of IQGAP1 in the membrane/cytoskeletal fraction, as detected by immunoblotting (Fig. 5B).
FIGURE 5.
Asef mediates HGF-induced peripheral localization of IQGAP1. HPAEC were transfected with Asef-specific siRNA or nonspecific (ns) RNA and stimulated with HGF (50 ng/ml). A, IQGAP1 localization was analyzed by immunofluorescence staining with IQGAP1 antibody; bar, 5 μm. B, IQGAP1 accumulation in membrane/cytoskeletal fraction (CTSK) was monitored by Western blot and normalized to the total protein. Bar graphs depict quantitative analysis of Western blot data; n = 4; p < 0.05 versus nonspecific RNA. C, after stimulation of control and Asef-depleted EC monolayers with HGF (50 ng/ml), co-immunoprecipitation (IP) assays were performed using IQGAP1 antibody. The presence of Arp3 and cortactin in immune complexes was determined by immunoblotting with corresponding antibody. Reprobing with IQGAP1 antibody was used as a normalization control. Shown are representative results of three independent experiments. D, HGF-induced redistribution of IQGAP1 and cortactin to lamellipodia-like structures was examined by immunofluorescence staining with IQGAP1 (red) and cortactin (green) antibody. Shown are merged images. Higher magnification insets show details of IQGAP1 and cortactin localization at the cell cortical areas of control and Asef-depleted EC upon stimulation with HGF. Shown are representative results of three independent experiments; Veh, vehicle; bar, 5 μm. RDU, relative density units.
Several cytoskeletal Rac effectors, such as the Arp2/3 complex, p21Arc, and cortactin, are intimately involved in cortical actin rearrangement and regulation of actin polymerization (41–43). Asef-dependent IQGAP1 interaction with cytoskeletal effectors in HGF-stimulated EC was further tested in co-immunoprecipitation assays with an IQGAP1 antibody. HGF increased co-immunoprecipitation of cortactin and Arp3 with IQGAP1, which was abolished by Asef knockdown (Fig. 5C). Immunofluorescence co-staining of IQGAP1 and cortical actin-binding protein cortactin in control and HGF-stimulated EC showed HGF-induced accumulation and co-localization of IQGAP1 and cortactin, which was again inhibited by Asef knockdown (Fig. 5D). There was no significant difference between IQGAP1 and cortactin localization in nonstimulated EC transfected with nonspecific or Asef-specific siRNA (data not shown).
Asef- IQGAP1 Functional Interaction Promotes HGF-induced Rac Activity and IQGAP1 Stimulation of Cytoskeletal Effectors
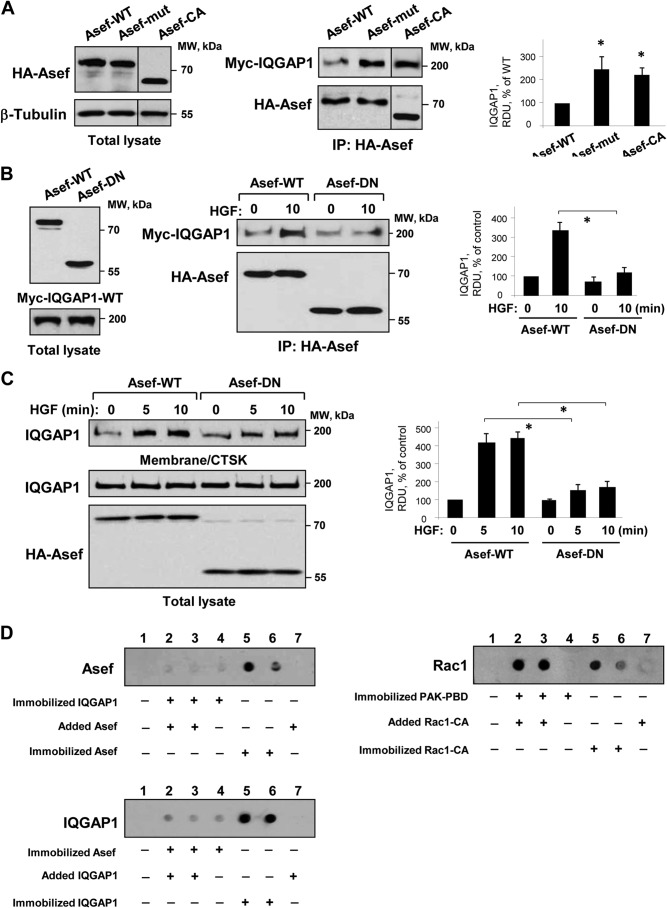
The results described above demonstrate the HGF-induced Asef-IQGAP1 association. However, whether this functional interaction depends on Asef activation is unclear. To address this question, we expressed in pulmonary EC the wild type Asef, constitutively active Asef mutant Asef-D133A/E365A with point mutations at Asp-133 and Glu-365 E365A (29), or dominant negative AsefΔDH mutant with deleted DH domain (Fig. 6, A and B), and we examined their association with ectopically expressed Myc-tagged IQGAP1 under control and HGF-stimulated conditions. Co-immunoprecipitation assays showed increased association of IQGAP1 with activated Asef-D133A/E365A or constitutively active AsefΔAPC mutants in nonstimulated cells (Fig. 6A) or wild type Asef in HGF-stimulated cells (Fig. 6B), whereas interaction of the dominant negative Asef mutant with IQGAP1 in HGF-stimulated cells was significantly attenuated (Fig. 6B) and also suppressed HGF-induced translocation of IQGAP1 to the membrane/cytoskeletal fraction (Fig. 6C). These results indicate that Asef nucleotide exchange activity is essential for HGF-induced IQGAP1 activation and intracellular redistribution.
FIGURE 6.
Role of Asef activation in the HGF-induced interaction with IQGAP1 and IQGAP1 membrane translocation. A, left panel, ectopic expression of HA-tagged wild type (WT) Asef, activated point mutated Asef-D133A/E365A mutant (Asef-mut), or activated truncated AsefΔAPC (Asef-CA) mutant in pulmonary EC was verified by immunoblotting using anti-HA antibodies. Right panel, EC were co-transfected with HA-Asef-WT, HA-Asef-D133A/E365A, or AsefΔAPC mutant and Myc-IQGAP1, followed by immunoprecipitation (IP) with HA tag antibody. Myc-IQGAP1 content in immunoprecipitates was monitored by immunoblotting with the Myc tag antibody. Probing with HA tag antibody was used as a normalization control. Bar graphs depict quantitative analysis of Western blot data; n = 3; *, p < 0.05 versus Asef WT. B, left panel, ectopic expression of HA-tagged wild type (WT) or dominant negative (DN) Asef mutants, and Myc-tagged wild type IQGAP1 in pulmonary EC was verified by immunoblotting using anti-HA or anti-Myc antibodies. Right panel, EC were co-transfected with Asef-WT or Asef-DN mutant and IQGAP1, and HGF-induced protein association was examined by immunoprecipitation assays with HA tag antibody. IQGAP1 content in immunoprecipitates was monitored by immunoblotting with Myc tag antibody. Probing with HA tag antibody was used as a normalization control. Bar graphs depict quantitative analysis of Western blot data; n = 4; *, p < 0.05 versus Asef WT. C, EC were transfected with wild type or DN-Asef, and HGF-induced translocation of IQGAP1 to membrane/cytoskeletal (CTSK) fraction was examined by Western blot analysis. Probing of total lysates with IQGAP1 antibody and HA tag to detect recombinant Asef was used as a normalization control. Bar graphs depict quantitative analysis of Western blot data; n = 3; *, p < 0.05 versus Asef WT. D, Asef-IQGAP1 binding assay. IQGAP1, Asef, and PAK-PBD expressed in E. coli were immobilized on nitrocellulose membrane followed by incubation with Asef, IQGAP1, or Rac1-CA, respectively. After washing steps, interaction of Asef, IQGAP1, or Rac1-CA with the corresponding nitrocellulose-immobilized binding partners was detected by immunoblotting with the corresponding antibody. Shown are representative results of three independent experiments. RDU, relative density units.
The next experiments investigated whether IQGAP1/Asef interactions are direct. Wild type IQGAP1 and Asef were expressed in the bacterial system, and interactions between IQGAP1 and Asef were examined using protein overlay assay. IQGAP1 (Fig. 6D, upper panel) or Asef (Fig. 6D, middle panel) were immobilized on nitrocellulose membranes followed by incubation with Asef or IQGAP1, respectively. Both assays showed no interaction between IQGAP1 and Asef. In contrast, strong protein/protein interactions were detected between constitutively active Rac1 and its binding target, PAK-PBD, which were used as a positive control (Fig. 6D, lower panel).
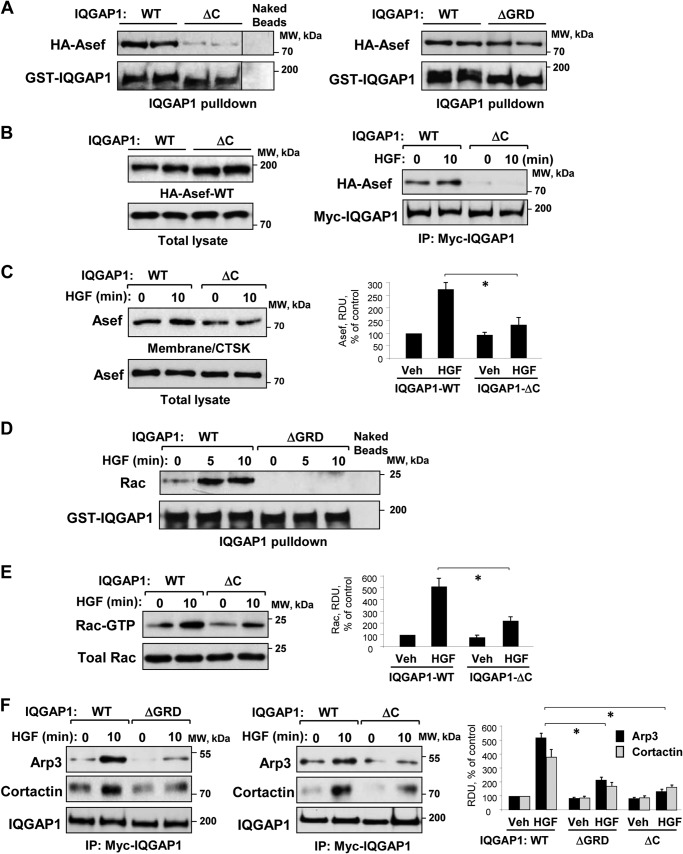
Among several IQGAP1 functional domains, the C-terminal domain is implicated in the IQGAP1 interactions with actin-binding and microtubule-associated proteins, including APC (26, 44, 45). To evaluate a role for the C terminus of IQGAP1 in its association with Asef, we used agarose beads conjugated with bacterially expressed recombinant wild type IQGAP1 or IQGAP1-ΔC mutant. This truncated mutant lacks amino acids 1502–1657 at the C terminus. Analysis was performed by pulldown of overexpressed wild type Asef from EC lysates. Asef was found to associate with wild type IQGAP1 but not with IQGAP1-ΔC beads or naked beads without conjugated IQGAP1 (Fig. 7A, left panels). Experiments with the IQGAP1-ΔGRD mutant lacking the Ras GTPase-binding site did not reveal significant differences in Asef binding, as compared with wild type controls (Fig. 7A, right panels). These data suggest that the GRD domain is not essential for Asef-IQGAP1complex formation.
FIGURE 7.
Role of IQGAP1 domains in HGF-induced interaction with Asef, Rac activation, and interaction with Arp3 and cortactin. A, EC lysates with expressed HA-Asef-WT were incubated with agarose beads conjugated with wild type (WT) IQGAP1, IQGAP1ΔC, or IQGAP1ΔGRD. After washing, bound Asef was detected by immunoblotting. B, left panel, ectopic expression of Myc-tagged wild type IQGAP1 and IQGAP1ΔC mutant and wild type (HA-Asef-WT) recombinant Asef in pulmonary EC. Right panel, EC were co-transfected with Myc-IQGAP1 wild type (IQGAP1-WT) or Myc-IQGAP1ΔC mutant and HA-Asef-WT followed by stimulation with HGF (50 ng/ml, 10 min) and immunoprecipitation (IP) with Myc antibody. Asef content in immunocomplexes was detected by immunoblotting with HA tag antibody. C, EC with ectopic expression of with wild type (WT) IQGAP1 and the IQGAP1ΔC-truncated mutant were stimulated with HGF (50 ng/ml, 10 min). The content of Asef in membrane/cytoskeletal (CTSK) fractions was determined by Western blot analysis and normalized to the total Asef in cell lysates. Bar graphs depict quantitative analysis of Western blot data; n = 3; *, p < 0.05 versus IQGAP1 WT. Veh, vehicle. D, EC were stimulated with HGF (50 ng/ml) for 5 or 10 min. EC lysates were incubated with agarose beads conjugated with IQGAP1-WT or IQGAP1ΔGRD. After washing steps, immobilized Rac was detected by immunoblotting. Nonconjugated beads were used as nonspecific binding control. Probing of membranes with GST antibody was used as a normalization control. E, Rac activation assays in whole cell lysates were performed as described under “Experimental Procedures.” Bar graphs depict quantitative analysis of Western blot data; n = 3; p < 0.05 versus IQGAP1 WT. F, EC were transfected with myc-IQGAP1-WT, myc-IQGAP1ΔGRD mutant lacking Rac interaction domain, or Myc-IQGAP1ΔC and stimulated with HGF (50 ng/ml, 10 min). The levels of Arp3 and cortactin co-precipitated with recombinant IQGAP1 were detected by immunoblotting with the appropriate antibody. Bar graphs depict quantitative analysis of Western blot data; n = 4; *, p < 0.05 versus IQGAP1 WT. RDU, relative density units.
To further test the role of the IQGAP1 C-terminal domain in HGF-induced IQGAP1/Asef interactions, we expressed wild type IQGAP1 or IQGAP1-ΔC in pulmonary EC. In contrast to cells expressing wild type IQGAP1, Asef was not found in immunoprecipitates from EC-expressing IQGAP1-ΔC (Fig. 7B). Because immunoprecipitation of recombinant IQGAP1 was performed using Myc tag antibody, interference of endogenous IQGAP1 in this assay was excluded. Complementary experiments demonstrated reduced peripheral translocation of Asef in response to HGF in the cells transfected with IQGAP1-ΔC (Fig. 7C). These results further support the key role of the IQGAP1 C-terminal domain in the HGF-induced enhancement of IQGAP1-Asef protein association.
IQGAP1 binds preferentially to activated Rac1 and Cdc42 via Ras GTPase-activating protein-related domain (GRD) flanked by amino acids 1025–1238 (23). In this study, HGF induced interaction of Rac with wild type IQGAP1 but not with the IQGAP1-ΔGRD deletion mutant (Fig. 7D). Interestingly, analysis of HGF-stimulated EC-expressing wild type IQGAP1 or IQGAP1-ΔC mutants showed attenuated Rac activation in cells expressing IQGAP1-ΔC (Fig. 7E). Taken together with the results in Fig. 7B, these data suggest a role for Asef/IQGAP1 interaction in Rac activation by HGF.
IQGAP1 interaction with activated GTPase results in IQGAP1-mediated activation of downstream effectors. Expression of wild type IQGAP1 or IQGAP1-truncated mutant lacking the GRD domain (IQGAP1-ΔGRD) in pulmonary EC showed that HGF-induced association of Arp3 and cortactin with IQGAP1-ΔGRD was decreased (Fig. 7F). Importantly, co-precipitation of Arp3 and cortactin with IQGAP1-ΔC was also decreased. These data indicate allosteric regulation of IQGAP1 interactions with actin cytoskeletal proteins.
DISCUSSION
The main finding of this study is the HGF-induced formation of the Asef-IQGAP1 signaling complex regulating remodeling of peripheral actin in the endothelial monolayer and enhancement of the endothelial barrier. Molecular inhibition of Asef and IQGAP1 attenuated HGF-induced EC barrier enhancement. Conversely, HGF-induced formation of the Asef-IQGAP1 complex further stimulated HGF-induced Rac signaling and increased IQGAP1 interaction with the actin-binding proteins cortactin and Arp3, which are involved in cortical actin polymerization and peripheral cytoskeletal dynamics. As Asef is capable of activating both, Rac and Cdc42, which may interact with IQGAP1 and trigger cell junction and cytoskeletal remodeling and regulate EC permeability (46, 47), we do not exclude additional involvement of Asef-activated Cdc42 in the cytoskeletal remodeling and barrier regulation. However, this study focused on Rac as the best described regulator of EC monolayer integrity.
HGF-induced Asef-IQGAP1 association required active Asef and was significantly but not completely suppressed by expression of a dominant negative Asef mutant or Asef siRNA. Previous studies reporting an additional role of another Rac GEF, Tiam1, in the mediation of HGF-protective responses in vascular endothelium (8, 48) may explain the incomplete inhibition of HGF responses by Asef knockdown shown in this study.
Asef activity was also essential for HGF-induced accumulation of IQGAP1 at the cell periphery. Although HGF also activates Tiam1 (8, 48), our results suggest that IQGAP1 redistribution and interaction with the actin cytoskeleton effectors are at least in part regulated by Asef. These data suggest that Asef and Tiam1 may be involved in different aspects of the HGF-induced effects, and further studies are warranted to investigate the interplay between Tiam1 and Asef in the HGF-induced regulation of peripheral cytoskeletal remodeling and the EC permeability response.
Asef forms a complex with a microtubule-binding protein APC (22). In turn, APC interacts the with IQGAP1 C terminus (25, 26). Our experiments with the expression of the IQGAP1ΔC mutant show an abrogated IQGAP1/Asef interaction and suggest the critical importance of the IQGAP1 C-terminal domain in the complex formation with Asef indirectly, via linker protein(s), i.e. APC. How may HGF increase Asef-IQGAP1 association? We speculate that HGF may promote peripheral microtubule growth leading to increased availability of the APC/Asef pool to the IQGAP1 localized at the cortical region. This possibility is supported by our most recent study showing Rac-dependent formation of an IQGAP1 functional complex containing microtubule plus-end binding protein EB1 and cortactin (49). The precise mechanisms of IQGAP1-Asef capturing at the cell periphery require further elucidation.
Another interesting question is the mechanism of suppression of HGF-induced Rac activation by an IQGAP1ΔC mutant. Previous studies showed that the deletion of any of three IQGAP1 functional domains, the C terminus, GRD, or IQ domains, abolished Cdc42 binding to IQGAP1 (50). Overexpression of the IQGAP1, but not IQGAP1-ΔGRD, mutant increased basal Cdc42 activation. These findings suggest an allosteric mechanism of IQGAP1 interaction with Cdc42, which may also apply to the IQGAP1 interaction with Rac1. Although these findings demonstrate why Rac may not bind to IQGAP1-ΔC, they do not explain why cells expressing IQGAP1-ΔC did not develop full Rac activation in response to HGF. The impaired Rac activation in this condition may be explained by Asef mis-targeting caused by forced expression of IQGAP1ΔC mutant, which does not capture Asef at the cell cortical location. As a result, Asef in HGF-stimulated cells expressing IQGAP1ΔC becomes unloaded prematurely due to peripheral microtubule depolymerization reflecting a basic feature of microtubule dynamic instability, and therefore, it does not reach its destination at the cell cortex. Asef also does not become properly activated, which impairs its regulation of local Rac signaling and peripheral actin remodeling. Asef activity in the IQGAP1 complex may be also stimulated by IQGAP1-assisted translocation to the cell membrane, where Asef may interact via its pleckstrin homology domain with PIP3, the phosphoinositide product of PI 3-kinase (51). Interaction with PIP3 is necessary to activate the catalytic activity of the DH domains of some GEFs (16).
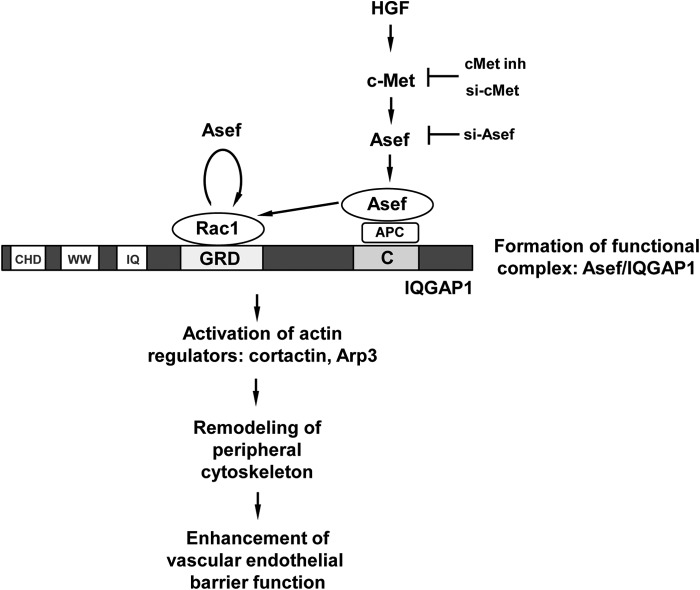
Our data show that HGF stimulation increases Rac binding to IQGAP1, which is controlled by HGF-activated Asef. This interaction promotes IQGAP1 association with cortactin and Arp3, proteins known to activate peripheral cytoskeletal remodeling and EC barrier enhancement (8, 42, 52). IQGAP1 interacts with Rac and Cdc42 GTPases via GRD, and this interaction leads to IQGAP1 activation and interaction with effector proteins (23). Interestingly, deletion of the GRD or C-terminal domain attenuated IQGAP1 interactions with cortactin and Arp3, and deletion of the C-terminal domain also decreased Rac activation caused by HGF. These effects suggest that IQGAP1 stimulates local Rac activity by association with Asef via the C-terminal domain. In addition, binding of activated Rac to the IQGAP1 GRD stimulates peripheral cytoskeletal remodeling. These results suggest a dual role for IQGAP1 as both mediator and effector of Rac signaling, which requires functional interactions with Asef. Based on our findings, we propose a model of Asef/IQGAP1-dependent control of vascular endothelial integrity by HGF (Fig. 8). HGF-induced activation of the c-Met receptor stimulates activation of Asef leading to formation of a functional Asef-IQGAP1 complex via APC at the C-terminal domain of IQGAP1. As a result, stimulation of Rac activity by Asef stimulates IQGAP1 interactions with the actin-binding proteins cortactin and Arp3 leading to remodeling of the peripheral cytoskeleton and enhancement of the vascular endothelial barrier. In addition to the C terminus of IQGAP1, activated Asef may also interact with Rac, which is in turn associated with the GRD domain of IQGAP1. Association of activated GEFs with their small GTPase targets has been detected in pulldowns and published in the literature (19, 53). This may explain the increased association of IQGAP1 with constitutively active Asef mutants lacking the APC-binding domain (Fig. 6A). In contrast, under basal conditions the Asef/IQGAP1 interactions are not significantly affected in the cells overexpressing IQGAP1, which lacks the GRD domain (Fig. 7A).
FIGURE 8.
HGF mediates vascular endothelial barrier enhancement effects via stimulation of Asef/IQGAP1 interactions. In vascular endothelium, HGF induces c-Met-dependent activation and peripheral translocation of Rac-specific Asef. At the cell periphery, Asef forms a functional complex with IQGAP1 via C-terminal (RasGAP C terminus) domain. These interactions are indirect and may be mediated by Asef-IQGAP1 linker APC. Activated Asef induces local Rac activation and its association with the GRD (Ras GTPase-activating protein-related) domain of IQGAP1, which leads to enhanced cortical actin polymerization and endothelial barrier enhancement. In addition, Asef may also interact with GRD-associated Rac and promote additional Rac activation. CHP, calponin homology domain; WW, polyproline binding region; IQ, IQ domain containing four IQ motifs.
A recent study demonstrated a functional role of IQGAP1 in the Rac- and phospholipase D-dependent peripheral targeting of the NADPH oxidase subunit p47PHOX in pulmonary EC exposed to hyperoxia (54). Hyperoxia-mediated IQGAP1 activation through Rac1 was critical for p47PHOX translocation accompanied by peripheral accumulation of tyrosine-phosphorylated cortactin and p47PHOX-dependent ROS formation in human lung endothelial cells. Taken together with the suggested role of IQGAP1 in control of adherens junctions, microtubule capturing, and the results of this study, these findings illustrate a wide variety of IQGAP1 cellular functions.
In conclusion, these results demonstrate a novel role for the Asef-IQGAP1 functional complex mediating the HGF-induced EC barrier enhancement. Activation of Asef-IQGAP1 signaling may represent a general mechanism of local Rac regulation in agonist-stimulated cells leading to barrier enhancement or other physiological responses.
This work was supported, in whole or in part, by National Institutes of Health Grants HL89257 and HL107920 from NHLBI and by the Intramural Research Program of the National Institutes of Health.
- HGF
- hepatocyte growth factor
- EC
- endothelial cell
- HPAEC
- human pulmonary artery endothelial cell
- GEF
- guanine nucleotide exchange factor
- DH
- Dbl homology
- GRD
- RasGAP-related domain
- APC
- adenomatous polyposis coli
- PI 3-kinase
- phosphatidylinositol 3-kinase.
REFERENCES
- 1. Matsumoto K., Nakamura T. (1996) Emerging multipotent aspects of hepatocyte growth factor. J. Biochem. 119, 591–600 [DOI] [PubMed] [Google Scholar]
- 2. Rosen E. M., Goldberg I. D. (1995) Scatter factor and angiogenesis. Adv. Cancer Res. 67, 257–279 [DOI] [PubMed] [Google Scholar]
- 3. Zhang L., Himi T., Morita I., Murota S. (2000) Hepatocyte growth factor protects cultured rat cerebellar granule neurons from apoptosis via the phosphatidylinositol-3 kinase/Akt pathway. J. Neurosci. Res. 59, 489–496 [DOI] [PubMed] [Google Scholar]
- 4. Aoki M., Morishita R., Taniyama Y., Kaneda Y., Ogihara T. (2000) Therapeutic angiogenesis induced by hepatocyte growth factor: potential gene therapy for ischemic diseases. J. Atheroscler. Thromb. 7, 71–76 [DOI] [PubMed] [Google Scholar]
- 5. Tomita N., Morishita R., Higaki J., Ogihara T. (2000) Novel molecular therapeutic approach to cardiovascular disease based on hepatocyte growth factor. J. Atheroscler. Thromb. 7, 1–7 [DOI] [PubMed] [Google Scholar]
- 6. Bussolino F., Di Renzo M. F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P. M. (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 119, 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu F., Schaphorst K. L., Verin A. D., Jacobs K., Birukova A., Day R. M., Bogatcheva N., Bottaro D. P., Garcia J. G. (2002) Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3β. FASEB J. 16, 950–962 [DOI] [PubMed] [Google Scholar]
- 8. Birukova A. A., Alekseeva E., Mikaelyan A., Birukov K. G. (2007) HGF attenuates thrombin-induced permeability in the human pulmonary endothelial cells by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 21, 2776–2786 [DOI] [PubMed] [Google Scholar]
- 9. Date I., Takagi N., Takagi K., Kago T., Matsumoto K., Nakamura T., Takeo S. (2004) Hepatocyte growth factor attenuates cerebral ischemia-induced learning dysfunction. Biochem. Biophys. Res. Commun. 319, 1152–1158 [DOI] [PubMed] [Google Scholar]
- 10. Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graziani A., Panayotou G., Comoglio P. M. (1994) A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261–271 [DOI] [PubMed] [Google Scholar]
- 11. Schaeper U., Gehring N. H., Fuchs K. P., Sachs M., Kempkes B., Birchmeier W. (2000) Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149, 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birukova A. A., Moldobaeva N., Xing J., Birukov K. G. (2008) Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am. J. Physiol. Lung. Cell Mol. Physiol. 295, L612–L623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burridge K., Wennerberg K. (2004) Rho and Rac take center stage. Cell 116, 167–179 [DOI] [PubMed] [Google Scholar]
- 14. Birukov K. G., Zebda N., Birukova A. A. (2013) Barrier enhancing signals in pulmonary edema. Compr. Physiol. 3, 429–484 [DOI] [PubMed] [Google Scholar]
- 15. Boguski M. S., McCormick F. (1993) Proteins regulating Ras and its relatives. Nature 366, 643–654 [DOI] [PubMed] [Google Scholar]
- 16. Zheng Y. (2001) Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 26, 724–732 [DOI] [PubMed] [Google Scholar]
- 17. Bishop A. L., Hall A. (2000) Rho GTPases and their effector proteins. Biochem. J. 348, 241–255 [PMC free article] [PubMed] [Google Scholar]
- 18. Birukova A. A., Zagranichnaya T., Alekseeva E., Bokoch G. M., Birukov K. G. (2008) Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J. Cell Physiol. 215, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birukova A. A., Zagranichnaya T., Fu P., Alekseeva E., Chen W., Jacobson J. R., Birukov K. G. (2007) Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 313, 2504–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singleton P. A., Chatchavalvanich S., Fu P., Xing J., Birukova A. A., Fortune J. A., Klibanov A. M., Garcia J. G., Birukov K. G. (2009) Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ. Res. 104, 978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawasaki Y., Sato R., Akiyama T. (2003) Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat. Cell Biol. 5, 211–215 [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki Y., Senda T., Ishidate T., Koyama R., Morishita T., Iwayama Y., Higuchi O., Akiyama T. (2000) Asef, a link between the tumor suppressor APC and G-protein signaling. Science 289, 1194–1197 [DOI] [PubMed] [Google Scholar]
- 23. Swart-Mataraza J. M., Li Z., Sacks D. B. (2002) IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 277, 24753–24763 [DOI] [PubMed] [Google Scholar]
- 24. Kaibuchi K., Kuroda S., Fukata M., Nakagawa M. (1999) Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr. Opin. Cell Biol. 11, 591–596 [DOI] [PubMed] [Google Scholar]
- 25. Noritake J., Watanabe T., Sato K., Wang S., Kaibuchi K. (2005) IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 118, 2085–2092 [DOI] [PubMed] [Google Scholar]
- 26. White C. D., Erdemir H. H., Sacks D. B. (2012) IQGAP1 and its binding proteins control diverse biological functions. Cell. Signal. 24, 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown M. D., Sacks D. B. (2006) IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 16, 242–249 [DOI] [PubMed] [Google Scholar]
- 28. Watanabe T., Wang S., Noritake J., Sato K., Fukata M., Takefuji M., Nakagawa M., Izumi N., Akiyama T., Kaibuchi K. (2004) Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell 7, 871–883 [DOI] [PubMed] [Google Scholar]
- 29. Murayama K., Shirouzu M., Kawasaki Y., Kato-Murayama M., Hanawa-Suetsugu K., Sakamoto A., Katsura Y., Suenaga A., Toyama M., Terada T., Taiji M., Akiyama T., Yokoyama S. (2007) Crystal structure of the rac activator, Asef, reveals its autoinhibitory mechanism. J. Biol. Chem. 282, 4238–4242 [DOI] [PubMed] [Google Scholar]
- 30. Briggs M. W., Li Z., Sacks D. B. (2002) IQGAP1-mediated stimulation of transcriptional co-activation by β-catenin is modulated by calmodulin. J. Biol. Chem. 277, 7453–7465 [DOI] [PubMed] [Google Scholar]
- 31. Dubrovskyi O., Birukova A. A., Birukov K. G. (2013) Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab. Invest. 93, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian X., Tian Y., Gawlak G., Sarich N., Wu T., Birukova A. A. (2014) Control of vascular permeability by atrial natriuretic peptide via GEF-H1-dependent mechanism. J. Biol. Chem. 289, 5168–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Birukova A. A., Birukov K. G., Smurova K., Adyshev D., Kaibuchi K., Alieva I., Garcia J. G., Verin A. D. (2004) Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 18, 1879–1890 [DOI] [PubMed] [Google Scholar]
- 34. Birukova A. A., Smurova K., Birukov K. G., Kaibuchi K., Garcia J. G., Verin A. D. (2004) Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc. Res. 67, 64–77 [DOI] [PubMed] [Google Scholar]
- 35. Birukova A. A., Malyukova I., Poroyko V., Birukov K. G. (2007) Paxillin-β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L199–L211 [DOI] [PubMed] [Google Scholar]
- 36. Birukov K. G., Bochkov V. N., Birukova A. A., Kawkitinarong K., Rios A., Leitner A., Verin A. D., Bokoch G. M., Leitinger N., Garcia J. G. (2004) Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 95, 892–901 [DOI] [PubMed] [Google Scholar]
- 37. Waheed F., Dan Q., Amoozadeh Y., Zhang Y., Tanimura S., Speight P., Kapus A., Szászi K. (2013) Central role of the exchange factor GEF-H1 in TNF-α-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells. Mol. Biol. Cell 24, 1068–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. García-Mata R., Wennerberg K., Arthur W. T., Noren N. K., Ellerbroek S. M., Burridge K. (2006) Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406, 425–437 [DOI] [PubMed] [Google Scholar]
- 39. Birukova A. A., Fu P., Xing J., Yakubov B., Cokic I., Birukov K. G. (2010) Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 298, L837–L848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vikis H. G., Guan K. L. (2004) Glutathione-S-transferase-fusion based assays for studying protein-protein interactions. Methods Mol. Biol. 261, 175–186 [DOI] [PubMed] [Google Scholar]
- 41. Borisy G. G., Svitkina T. M. (2000) Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104–112 [DOI] [PubMed] [Google Scholar]
- 42. Pollard T. D., Borisy G. G. (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 43. Uruno T., Liu J., Zhang P., Fan Yx, Egile C., Li R., Mueller S. C., Zhan X. (2001) Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3, 259–266 [DOI] [PubMed] [Google Scholar]
- 44. Watanabe T., Noritake J., Kakeno M., Matsui T., Harada T., Wang S., Itoh N., Sato K., Matsuzawa K., Iwamatsu A., Galjart N., Kaibuchi K. (2009) Phosphorylation of CLASP2 by GSK-3β regulates its interaction with IQGAP1, EB1 and microtubules. J. Cell Sci. 122, 2969–2979 [DOI] [PubMed] [Google Scholar]
- 45. Le Clainche C., Schlaepfer D., Ferrari A., Klingauf M., Grohmanova K., Veligodskiy A., Didry D., Le D., Egile C., Carlier M. F., Kroschewski R. (2007) IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J. Biol. Chem. 282, 426–435 [DOI] [PubMed] [Google Scholar]
- 46. Kouklis P., Konstantoulaki M., Vogel S., Broman M., Malik A. B. (2004) Cdc42 regulates the restoration of endothelial barrier function. Circ. Res. 94, 159–166 [DOI] [PubMed] [Google Scholar]
- 47. Broman M. T., Kouklis P., Gao X., Ramchandran R., Neamu R. F., Minshall R. D., Malik A. B. (2006) Cdc42 regulates adherens junction stability and endothelial permeability by inducing α-catenin interaction with the vascular endothelial cadherin complex. Circ. Res. 98, 73–80 [DOI] [PubMed] [Google Scholar]
- 48. Singleton P. A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J. G. (2007) CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J. Biol. Chem. 282, 30643–30657 [DOI] [PubMed] [Google Scholar]
- 49. Tian Y., Tian X., Gawlak G., O'Donnell J. J., 3rd, Sacks D. B., Birukova A. A. (2014) IQGAP1 regulates endothelial barrier function via EB1-cortactin crosstalk. Mol. Cell. Biol. 34, 3546–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sokol S. Y., Li Z., Sacks D. B. (2001) The effect of IQGAP1 on Xenopus embryonic ectoderm requires Cdc42. J. Biol. Chem. 276, 48425–48430 [DOI] [PubMed] [Google Scholar]
- 51. Kawasaki Y., Tsuji S., Sagara M., Echizen K., Shibata Y., Akiyama T. (2009) Adenomatous polyposis coli and Asef function downstream of hepatocyte growth factor and phosphatidylinositol 3-kinase. J. Biol. Chem. 284, 22436–22443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dudek S. M., Birukov K. G., Zhan X., Garcia J. G. (2002) Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 298, 511–519 [DOI] [PubMed] [Google Scholar]
- 53. Kakiashvili E., Speight P., Waheed F., Seth R., Lodyga M., Tanimura S., Kohno M., Rotstein O. D., Kapus A., Szászi K. (2009) GEF-H1 mediates tumor necrosis factor-α-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J. Biol. Chem. 284, 11454–11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Usatyuk P. V., Gorshkova I. A., He D., Zhao Y., Kalari S. K., Garcia J. G., Natarajan V. (2009) Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J. Biol. Chem. 284, 15339–15352 [DOI] [PMC free article] [PubMed] [Google Scholar]