Background: Ectodomain shedding by the metalloproteinase family affects axon guidance and neurite outgrowth.
Results: IgLON family members are metalloproteinase substrates that promote neurite outgrowth in a metalloproteinase-dependent manner.
Conclusion: IgON family members are shed from the surface of cortical neurons to promote neurite extension.
Significance: Proteolytic cleavage of IgLON family members could have critical roles in specific targeting and synaptogenesis in cortical neurons.
Keywords: ADAM, Axon, Cell Adhesion, Matrix Metalloproteinase (MMP), Neuron, Shedding, Tissue Inhibitor of Metalloproteinase (TIMP)
Abstract
Matrix metalloproteinases and a disintegrin and metalloproteinases are members of the zinc endopeptidases, which cleave components of the extracellular matrix as well as cell surface proteins resulting in degradation or release of biologically active fragments. Surface ectodomain shedding affects numerous biological processes, including survival, axon outgrowth, axon guidance, and synaptogenesis. In this study, we evaluated the role of metalloproteinases in regulating cortical neurite growth. We found that treatment of mature cortical neurons with pan-metalloproteinase inhibitors or with tissue inhibitors of metalloproteinase-3 reduced neurite outgrowth. Through mass spectrometry, we characterized the metalloproteinase-sensitive cell surface proteome of mature cortical neurons. Members of the IgLON family of glycosylphosphatidylinositol-anchored neural cell adhesion molecules were identified and validated as proteins that were shed from the surface of mature cortical neurons in a metalloproteinase-dependent manner. Introduction of two members of the IgLON family, neurotrimin and NEGR1, in early embryonic neurons was sufficient to confer sensitivity to metalloproteinase inhibitors in neurite outgrowth assays. Outgrowth experiments on immobilized IgLON proteins revealed a role for all IgLON family members in promoting neurite extension from cortical neurons. Together, our findings support a role for metalloproteinase-dependent shedding of IgLON family members in regulating neurite outgrowth from mature cortical neurons.
Introduction
Axon outgrowth is a fundamental process required for proper wiring of the developing brain and for axon regeneration following injury in the adult. During development, gradients of guidance cues bind to cognate receptors on the cell surface to initiate intracellular signals that regulate axon growth (1). Following CNS injury, the environmental cues are mainly repellent, posing an impediment to axon repair, and the intrinsic capacity of the cell to grow is diminished compared with embryonic neurons (2). Therefore, understanding how axonal projections modulate their responses to environmental cues will provide new insights into how the brain achieves functional connectivity and new strategies to promote regeneration following CNS injury. The nervous system has evolved numerous strategies to dynamically regulate how axonal projections respond to the environment. One such mechanism is the regulated proteolytic cleavage of cell surface receptors in a process termed ectodomain shedding (3). Ectodomain shedding can result in insensitivity to ligands in the environment or lead to the formation of biologically active or dominant negative receptor fragments. Shedding has been implicated in a variety of biological processes within the nervous system, including neuronal differentiation, cell migration, neuronal survival, synaptic plasticity, axon guidance, and outgrowth (4–7).
Ectodomain shedding is primarily achieved through the activity of matrix metalloproteinases (MMPs)2 and a disintegrin and metalloproteinase (ADAMs) of the zinc metalloproteinase family (8). These are ubiquitously expressed proteases that include intracellular, cell surface, and secreted family members. Their activity is controlled through removal of a propeptide domain to convert them from inactive zymogens to their mature active form and through the expression of endogenous inhibitors called tissue inhibitors of metalloproteinases (TIMPs) (9). Application of metalloproteinase inhibitors to developing Xenopus laevis results in defects in retinal ganglion cell outgrowth and guidance (10). Similarly, in Drosophila melanogaster, mutations in the ADAM family member Kuzbanian result in severe defects in central nervous system axon guidance (11). Proteolytic cleavage of a number of receptors and cell adhesion molecules affects axon guidance and outgrowth, including L1 cell adhesion molecule and the axon guidance receptors EphA2, Neuropilin-1, Robo, and DCC (5, 12–14). Moreover, Nogo Receptor-1 (NgR1), a receptor for multiple repellent molecules in the adult CNS, is processed by membrane type 3-MMP (15).
In this study, we identify members of the IgLON protein family as metalloproteinase substrates. IgLONs are members of the immunoglobulin (Ig) superfamily of cell adhesion molecules and are the most abundant glycosylphosphatidylinositol-anchored proteins expressed in neurons (16). The IgLON proteins contain three immunoglobulin domains followed by a glycosylphosphatidylinositol anchor protein and possess 6–7 potential glycosylation sites (17). IgLON family members include neurotrimin (NTM), opioid-binding cell adhesion molecule (OBCAM), limbic system-associated membrane protein (LSAMP), and neuronal growth regulator 1 (NEGR1) (16, 18, 19). IgLON proteins form homophilic and heterophilic complexes along the cell surface and with juxtaposed cells to modulate adhesion and neurite outgrowth. Individual IgLON family members can promote or inhibit growth of different types of neurons in part dependent on the complement of IgLON surface expression (20, 21). IgLONs may also play a role in the formation and maintenance of excitatory synapses (22). Our results identify a new family of proteins that are subject to metalloproteinase cleavage and raise the interesting possibility that regulated cleavage of these proteins may play important roles in axon extension and synaptic plasticity.
EXPERIMENTAL PROCEDURES
Animals
Timed pregnant (embryonic day 18–19) female Sprague-Dawley rats were purchased from Charles River Laboratories (Senneville, Quebec, Canada). All animal care and use were in accordance with the McGill University guidelines and approved by the University Animal Care and Use Committee.
Antibodies
For immunofluorescence, the following antibodies were used: mouse and rabbit anti-tubulin βIII from Covance (1:1000, Princeton, NJ) and mouse anti-Myc from Sigma (1:1000). Alexa-fluor secondary antibodies were purchased from Invitrogen (1:1000). For Western blot analysis, the following antibodies were used: anti-NTM (1:100, R&D Systems, Minneapolis, MN); anti-LSAMP (1:100, R&D Systems); anti-OBCAM (1:100, Santa Cruz Biotechnology, Dallas, TX); anti-NEGR1 (1:100, Santa Cruz Biotechnology); mouse and rabbit anti-Myc (1:500, Sigma); anti-GAPDH (1:10000, Abcam, La Jolla, CA); and anti-human IgG (1:1000, Jackson ImmunoResearch, West Grove, PA). HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch.
Plasmids and Cloning
Full-length human cDNA sequences for NTM, OBCAM, LSAMP (OpenBiosystems, Ottawa, Ontario, Canada), and NEGR1 (SinoBiological, Beijing, China) were cloned into the PsecTag-2B vector (Invitrogen) in-frame with the IgK chain leader sequence at their N-terminal ends. The following primers were used to subclone IgLON proteins into the Psectag-2B vector and introduce an Myc (EQKLISEEDL) epitope tag at the N terminus: NTM forward, 5′-GAA AAG CTT GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG AGC GGA GAT GCC ACC TTC-3′, and reverse, 5′-GAA CTC GAG TCA AAA TTT GAG AAG CAG GTG C-3′; OBCAM forward, 5′-GAA GAT ATC GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG AGC GGA GAT GCC ACC TTC-3′, and reverse, 5′-GAA CTC GAG TCA AAA CTT GAT GAA GAA GTG GG-3′; LSAMP forward, 5′-GAA GATATC GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG CGC AGC GTG GAT TTT AAC C-3′, and reverse, 5′-GAA CTC GAG TTA ACA TTT GCT GAG AAG GCA G-3′; and NEGR1 forward, 5′-GAA AAG CTT GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG GTG GAC TTC CCC TGG GCG-3′, and reverse, 5′-GAA GAA TTC TTA TTG TAG AAT GGC ATT CTT CAG GT-3′.
To generate soluble IgLON proteins, the three Ig domains of NTM, OBCAM, LSAMP, and NEGR1 were fused to a human Fc segment encoded by the PFUSE vector (Invitrogen) at their C-terminal ends. The constructs, corresponding to the sequence encoding the ectodomain of NTM, OBCAM, LSAMP, and NEGR1 subcloned into the PFUSE vector, were transiently transfected into HEK293T cells with calcium phosphate (23). Transfected cells were incubated in the serum-free medium, OptiMEM (Invitrogen), and the media were collected 4 days post-transfection. IgLON-Fc proteins were purified by affinity chromatography with protein A-Sepharose beads. After purification, protein concentration was assayed by protein assay and visualized on an 8% SDS-polyacrylamide gel by Coomassie Brilliant Blue Stain.
Cortical Culture
Embryonic day 18–19 (E18–19) rat cortical neurons were dissected in ice-cold Leibovitz (L-15) medium (Invitrogen). Cortical tissue was dissociated in 0.25% trypsin/EDTA (Invitrogen) and gently triturated. Cortical neurons were aged for 8 DIV in culture plates pre-coated with poly-l-lysine (100 μg/ml, Sigma). Culture medium consisted of Neurobasal (Invitrogen), 1% B27 (Invitrogen), 1% N2 (Invitrogen), 50 μg/ml penicillin/streptomycin (Invitrogen) and 2 mm l-glutamine (Invitrogen).
Outgrowth Assays
For outgrowth assays with pan-metalloproteinase inhibitors or TIMPs, cortical neurons from E18 to 19 rats and cortical neurons aged for 8 DIV were seeded in a 96-well plate previously coated with poly-l-lysine (PLL) and/or aggrecan (Sigma). To reseed aged cortical neurons, cells were detached from the culture plate with 0.125% trypsin/EDTA. Dissociated aged cortical neurons were washed and seeded in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% FBS (Invitrogen). After 4 h, the medium was replaced to culture medium enriched with pan-metalloproteinase inhibitors, Batimastat (BB-94; 5 μm, Tocris, Ellisville, MO), Ilomastat (GM6001; 20 μm, Calbiochem), Ilomastat-negative control (20 μm, Calbiochem), or endogenous metalloproteinase inhibitors (TIMP1, TIMP2, and TIMP3; 20 μg/ml, R&D Systems). To block neuronal apoptosis, cortical neurons were preincubated with the pan-caspase inhibitor Z-VAD-fmk (50 μm, R&D Systems) for 1 h. The neurons were reseeded in a 96-well plate and exposed to BB-94 in the presence of the pan-caspase inhibitor for 48 h. After 48 h, cortical neurons were fixed in 4% paraformaldehyde, 20% sucrose in PBS for 30 min. Neurons were blocked in 5% BSA and 0.2% Triton X-100 in PBS solution for 1 h and stained for βIII-tubulin antibody and Hoechst 33342 stain (Sigma). For outgrowth assays with immobilized recombinant IgLON proteins, IgLON-Fc proteins in PBS solution were coated in a 96-well plate for 3 h. Aged cortical neurons were seeded in IgLON-Fc-coated wells, fixed, and immunostained after 48 h. Fluorescent images were automatically acquired and analyzed through the MetaXpress software on an ImageXpress system.
Apoptosis Assay
DNA fragmentation was detected by labeling free 3′-OH DNA strands with a TMR red fluorescent marker using a commercially available In Situ Cell Death detection kit (Roche Applied Science). Cortical cultures were counterstained with anti-βIII-tubulin and Hoechst 33342 and imaged using the ImageXpress system microscope. The percentage of TUNEL-positive neurons was determined using the multiwavelength cell scoring module of MetaXpress.
Proteomics
Cortical neurons from E18 to 19 rats were grown for 8 DIV on PLL-coated plates. Cortical neurons were exposed to DMSO vehicle control or BB-94 for 16 h. The following day, cortical neurons were washed with 1 mm MgCl2, 0.1 mm CaCl2 in cold PBS solution and incubated with EZ-link-Sulfo-NHS-LC-biotin (0.5 mg/ml, Thermo Scientific) on ice for 30 mins. Two washes with 10 mm glycine and PBS were performed to remove any unreactive and unbound biotin. Finally, cells were lysed in 1× RIPA buffer, supplemented with 1× Complete EDTA-free proteinase inhibitor mixture (Roche Applied Science). The lysate-containing buffer was transferred into Eppendorf tubes for 30 min, and cell debris was removed by centrifugation. Biotinylated surface proteins were precipitated using streptavidin-agarose beads (Thermo Scientific), resolved on 4–16% gradient SDS-polyacrylamide gels, and stained with Coomassie Brilliant Blue. Up-regulated bands in the BB-94 conditions were processed by trypsin digestion, and the resulting peptide mixtures were analyzed by nanoscale liquid chromatography quadrupole time-of-flight tandem mass spectrometry. Mass spectrometry profiles were analyzed using Scaffold software.
RT-PCR
To assay IgLON expression in cortical neurons, RNA was isolated from 2- and 8-DIV cortical neurons followed by a reverse transcriptase-PCR (RT-PCR). The following primers were used to detect rat IgLON family members: NTM forward, 5′-CAA CCA CCC TAA GAC CTC CA-3′, and reverse, 5′-TGC ACT CAT ACT CGC CTG AC-3′; OBCAM forward, 5′-TCC TCT ACG CTG GGA ATG AC-3′, and reverse, 5′-GTT GGT TCT GGT CTG CCA AT-3′; LSAMP forward, 5′-ATC ACC AGG GAA CAG TCA GG-3′, and reverse, 5′-TCC CGG TAC CAC TCA AAG TC-3′; and NEGR1 forward, 5′-ATG CAG TGC AGA GAA CGA TG-3′, and reverse, 5′-AGT GCT CCT GTG TCA CGT TG-3′.
Immunochemistry
COS-7 cells were seeded on PLL-coated 6- or 12-well plates and cultured in DMEM with 10% FBS. The cells were transiently transfected with Myc-tagged IgLON constructs, using Lipofectamine-2000 (Invitrogen). After 24 h, the medium was refreshed and supplemented with pan-metalloproteinase inhibitors, phorbol 12-myristate 13-acetate (200 nm, Sigma) or PI-PLC (1 unit/ml, Invitrogen). After another 24 h, cell surface biotinylation was performed. For cortical neurons, 3 × 106 neurons were seeded in 60-mm dishes. Biotinylated cell surface proteins and cell lysates were resolved on 8% SDS-polyacrylamide gels and analyzed by Western blot.
For shedding analysis, the culture medium was replaced with serum-free medium (OptiMEM) and supplemented with TPA, pan-metalloproteinase inhibitors, or PI-PLC. After 24 h, supernatants were collected and briefly centrifuged to dispose of residual cell debris. Supernatants were concentrated using column centrifuge filters (10K, Amicon Ultra-15; Millipore, Etobicoke, Ontario, Canada), resolved on 8% SDS-polyacrylamide gels, and analyzed by Western blot. For IgLON shedding analysis in cortical neurons, 5 × 106 neurons were seeded in 10-cm plates. The medium of mature cortical neurons was replaced with Neurobasal medium supplemented with DMSO, BB-94, or PI-PLC. After 6 h, the medium was collected, briefly centrifuged, concentrated, and analyzed by Western blot. Shedding experiments were also conducted on brain extracts, prepared from rat at postnatal day 6. Briefly, cortical tissue was homogenized in ice-cold homogenizing solution (1 mm NaHCO3, 0.2 mm CaCl2, 0.2 mm MgCl2 at pH 7) and centrifuged to dispose of any residual tissue. Supernatants were collected and centrifuged for 45 min at 25,000 × g. The pellet was resuspended in Neurobasal medium supplemented with DMSO, BB-94, or PI-PLC. After 5 h, the medium was centrifuged for 1 h at 100,000 × g. The supernatant and pellet were collected and analyzed by Western blotting.
Electroporation
To express IgLON family members in immature cortical neurons, dissociated cortical neurons were co-electroporated using the Amaxa Nucleofector system (Amaxa kit, VPG-1003; Myrus kit, MIR5011; Program, O-003; Lonza, Allendale, NJ, and Myrus, Madison, WI) according to the manufacturer's protocol. Myc-tagged IgLON family members and a plasmid encoding an enhanced GFP cassette were introduced in a 3:1 ratio, respectively. Surface IgLON expression was assessed through immunostaining of nonpermeabilized cells with mouse or rabbit anti-Myc antibodies (Sigma).
Nucleofected cortical neurons were incubated with an anti-Myc antibody for 1 h at 37 °C and fixed for 15 min with 4% paraformaldehyde, 20% sucrose in PBS solution. Neurons were blocked in 5% BSA in PBS solution for 1 h and incubated with corresponding fluorophore-conjugated secondary antibody and Hoechst 33342 stain. The length of the longest neurite in neurons expressing GFP was measured using the NeuronJ plugin from the ImageJ software.
Statistics
For multiple comparisons, unpaired two-tailed Student t test, one-way ANOVA and two-way ANOVA followed by a Bonferroni post hoc test were performed using GraphPad Prism software.
RESULTS
Inhibitors of Zinc Metalloproteinases Repress Neurite Outgrowth of Cortical Neurons
Ectodomain shedding of a number of receptors and adhesion molecules affects axon growth. To further investigate the role of ectodomain shedding in regulating outgrowth, we measured the length of neurites projecting from rat cortical neurons upon exposure to pan-inhibitors of zinc metalloproteinases. We assessed responses of both embryonic cortical neurons and cortical neurons aged for 8 DIV to sensitize them to repellents in the adult CNS (24). Outgrowth of embryonic cortical neurons was not affected by treatment with BB-94 or GM6001, two broad inhibitors of zinc metalloproteinases (Fig. 1, A and B) (25, 26). In contrast, outgrowth from cortical neurons aged for 8 DIV and re-seeded on a permissive PLL substrate was inhibited by both BB-94 and GM6001 (Fig. 1, A and C). The metalloproteinase inhibitors also mildly attenuated the neurite outgrowth inhibitory effect of the chondroitin sulfate proteoglycan aggrecan (Fig. 1D). Neurons treated with DMSO or the inactive analog of GM6001 (GM-I) were significantly inhibited by 1 or 5 μg/ml aggrecan, respectively, whereas those treated with BB-94 or GM6001 only became responsive to aggrecan at 25 μg/ml, the highest dose tested (Fig. 1, A and D). This suggests that zinc metalloproteinase substrate(s) are important for promoting the intrinsic growth capacity of mature cortical neurons on permissive substrates and also contribute to neurite outgrowth inhibition on chondroitin sulfate proteoglycans. We investigated the molecular mechanism that is responsible for promoting the intrinsic growth capacity of aged cortical neurons.
FIGURE 1.
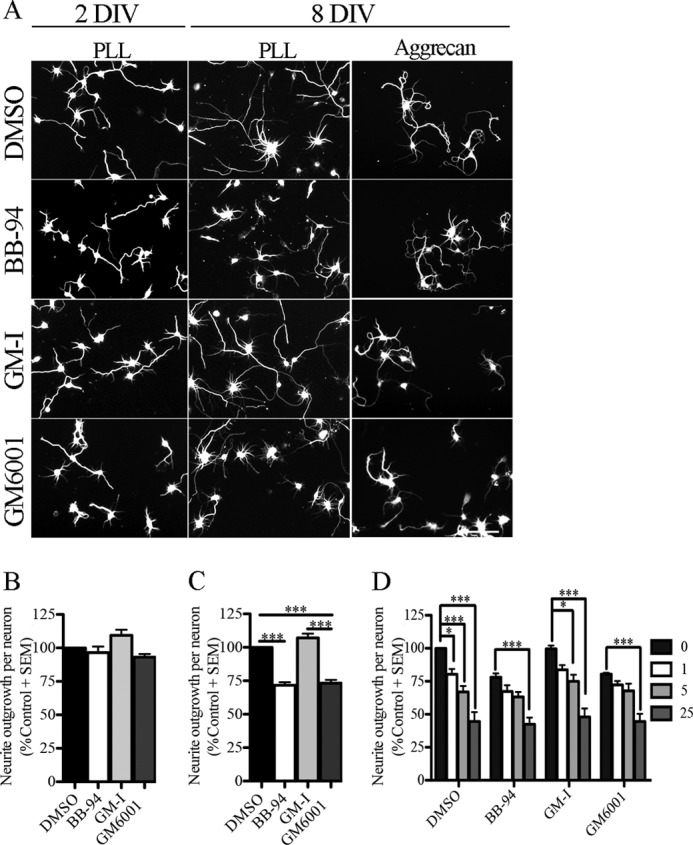
Pan-metalloproteinase inhibitors repress neurite outgrowth in mature cortical neurons. A, rat cortical neurons from two developmental stages (2 DIV and 8 DIV) seeded on a PLL and an aggrecan (5 μg/ml) substrate and treated with a vehicle control (DMSO), metalloproteinase inhibitors (BB-94 and GM601), and a GM6001-Inactive control (GM-I). Neuronal projections were visualized with βIII-tubulin staining. B and C, outgrowth quantification from 2-DIV (B) and 8-DIV (C) cortical neurons. Outgrowth was normalized to the DMSO control for each experiment. D, outgrowth quantification from 8-DIV cortical neurons seeded on PLL and aggrecan substrates (1, 5, and 25 μg/ml) and treated with DMSO, BB-94, GM-I, and GM6001. Outgrowth data were normalized to DMSO control on PLL substrate. n = 4–8 from independent cultures. Data shown as mean ± S.E. *, p < 0.05; ***, p < 0.001 by one- and two-way ANOVA, followed by Bonferroni post hoc test. Scale bar, 100 μm.
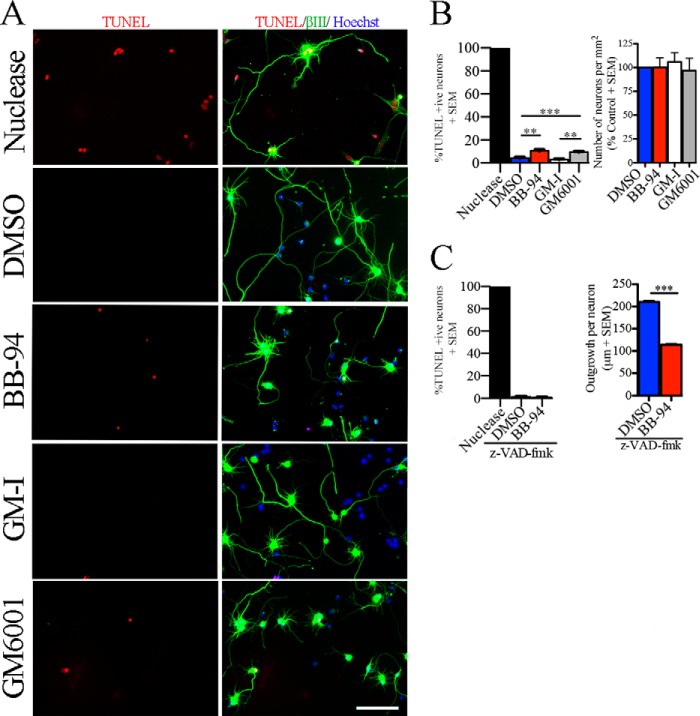
To determine whether metalloproteinase inhibitors suppress neurite extension in mature cortical neurons through indirect effects on cellular adhesion or viability, we quantified the total number of neurons and the number of apoptotic neurons following treatment with BB-94 or GM6001. The metalloproteinase inhibitors had no effect on the number of neurons that adhered to the substrate (Fig. 2, A and B). To assay for cell viability, TUNEL TMR-red staining was performed, and the cultures were co-stained with a nuclear and a neuronal marker (Fig. 2A). Both BB-94 and GM6001 had a small but significant effect on the number of TUNEL-positive neurons (Fig. 2B). In control conditions, we found 3–4% of apoptotic neurons, whereas BB-94 increased the number to 10%, and GM6001 increased the number to 9%. We therefore tested the effects of BB-94 in the presence of the pan-caspase inhibitor Z-VAD-fmk to block apoptosis (27). Z-VAD-fmk blocked the BB-94-dependent increase in cell death but did not attenuate the neurite outgrowth inhibitory effect of BB-94 leading us to conclude that the neurite outgrowth inhibitory effect of the metalloproteinase inhibitors is not an indirect effect of reduced cell viability (Fig. 2C).
FIGURE 2.
Metalloproteinase inhibitors inhibit growth independently of adhesion or cell death. A, TUNEL assay was performed in aged rat cortical neurons (8 DIV) seeded on a PLL substrate and treated with a vehicle control (DMSO), metalloproteinase inhibitors (BB-94 and GM6001), and GM-I. Neurons were stained with anti-βIII-tubulin, Hoechst and TUNEL. As a positive control for the TUNEL assay, neurons were pretreated with a DNA nuclease. B, total number of neurons and apoptotic neurons present after indicated treatments. For TUNEL assays, the data are shown as a percentage of TUNEL-positive neurons. For total number of neurons, data have been normalized to control DMSO. C, TUNEL assay and neurite outgrowth quantification of mature cortical neurons treated with DMSO and BB-94 in the presence of the pan-caspase inhibitor (Z-VAD-fmk). n = 3–4 from independent cultures. Data are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001 by one-way ANOVA followed by Bonferroni post hoc test (B) and Student's t test (C). Scale bar, 100 μm.
ADAM Family Member Promotes Neurite Outgrowth of Mature Cortical Neurons
We then determined the type of metalloproteinase responsible for promoting cortical neurite outgrowth by treating cells with TIMPs, endogenous inhibitors of zinc metalloproteinases. Individual TIMPs vary in their selectivity (28). TIMP1 inhibits ADAM10 and MMPs except for most membrane-type MMPs. TIMP2 inhibits all metalloproteinases, and TIMP3 inhibits the metalloproteinases and some ADAM family members. When mature cortical neurons were exposed to recombinant TIMPs, TIMP3 selectively and significantly inhibited neurite outgrowth (Fig. 3, A and B). Similar to the pan-metalloproteinase inhibitors, TIMP3 had no effect on cell adhesion and a modest effect on cell survival (Fig. 3, C, and D). The selective effect of TIMP3 implicates an ADAM family member in enhancing outgrowth in mature cortical neurons.
FIGURE 3.
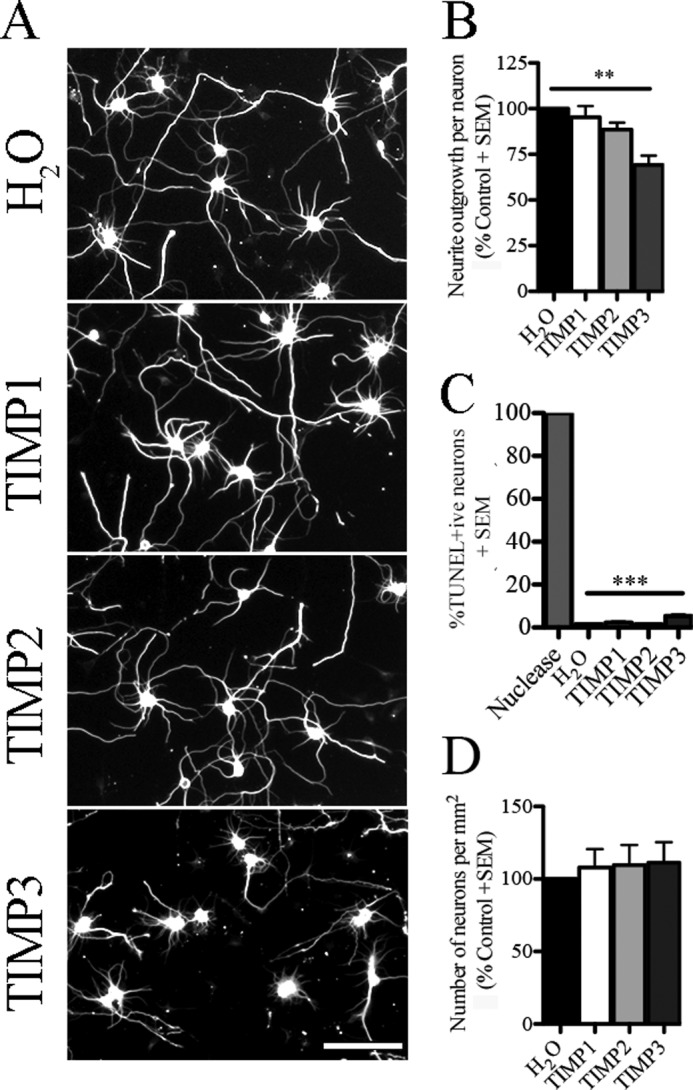
ADAM family member promotes neurite outgrowth in aged cortical neurons. A, aged cortical neurons (8 DIV) seeded on a PLL substrate and treated with control (H2O) or endogenous metalloproteinase inhibitors (TIMP1–3). Neuronal projections were visualized with βIII-tubulin staining. B, neurite outgrowth quantification of aged cortical neurons exposed to soluble recombinant TIMPs and control conditions. Data have been normalized to the control condition. C, TUNEL assay was performed in aged cortical neurons treated with TIMPs or H2O control. DNA nuclease was used as a positive control. Data are shown as a percentage of TUNEL-positive neurons. D, number of neurons present on the PLL substrate after treatment with soluble TIMPs and control condition. n = 3–4 from independent cultures. Data are mean ± S.E. **, p < 0.01; ***, p < 0.001 by one-way ANOVA followed by Bonferroni post hoc test. Scale bar, 100 μm.
Members of the IgLON Family of Cell Adhesion Molecules Are Shed from the Surface of Cortical Neurons by Metalloproteinases
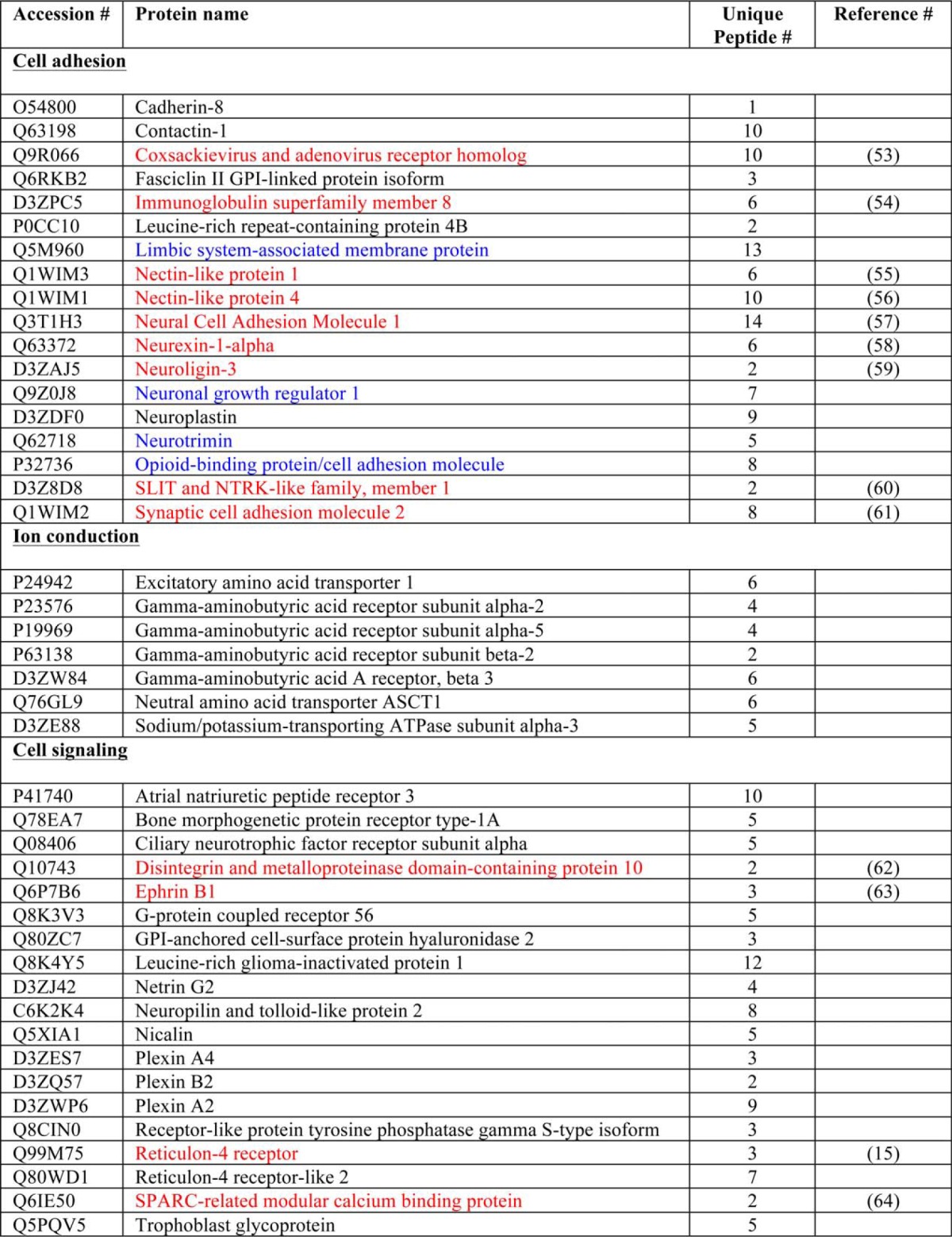
In the interest of identifying metalloproteinase substrates that may be responsible for the intrinsic ability of cortical neurons to extend neurites, we identified proteins that are shed from the surface of cortical neurons in a metalloproteinase-dependent manner. We biotinylated cell surface proteins in mature cortical neurons cultured in the presence or absence of the BB-94. We then washed the cells and precipitated cell surface-biotinylated proteins with streptavidin beads, separated them by SDS-PAGE, and stained them with Coomassie Brilliant Blue stain. Seven bands were markedly more intense in samples treated with BB-94 suggesting that they contained metalloproteinase substrates that were enriched on the cell surface upon metalloproteinase inhibition (Fig. 4). These bands were excised from the gel and analyzed by tandem mass spectrometry. Peptides from 44 proteins were identified, 13 of which have been previously described to undergo ectodomain shedding (Table 1). A survey of the proteins revealed functions in cell adhesion, ion conduction, and cell signaling. Numerous proteins have roles in regulating synaptogenesis. Notably, four of the proteins identified belong to the IgLON subgroup of the immunoglobulin superfamily cell adhesion molecules.
FIGURE 4.
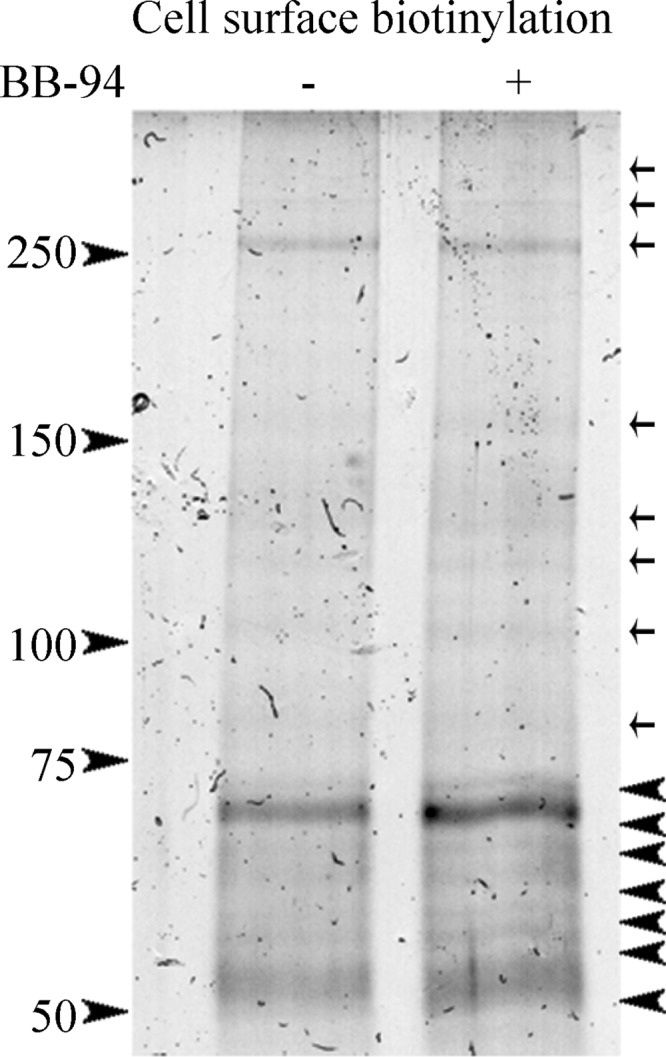
Proteomic analysis in cortical neurons identifies IgLON family members as novel metalloproteinase substrates. A cell surface biotinylation was performed on mature cortical neurons treated with a control condition (DMSO) and metalloproteinase inhibitor (BB-94). Cells were washed, and cell surface biotinylated proteins were precipitated with streptavidin beads, separated by SDS-PAGE, and stained with Coomassie Brilliant Blue stain. Seven up-regulated bands (arrowheads) in the BB-94 treatment compared with control conditions were excised and sent for a proteomic analysis. Arrows define protein bands that are insensitive to metalloproteinase activity.
TABLE 1.
Mass spectrometry results
A list of potential metalloproteinase substrates from a proteomic analysis performed on seven sample bands up-regulated in a cell surface biotinylation (Fig. 4). A list of 44 potential metalloproteinase substrates were obtained from a proteomic analysis. From this list, 13 peptides were previously identified as metalloproteinases substrates (red) and four members of a family of adhesion proteins (IgLONs), as potential metalloproteinase substrate candidates (blue).
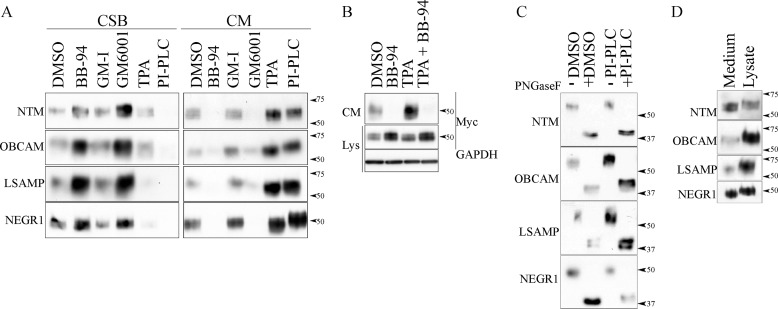
To validate that IgLON family members are indeed processed by metalloproteinase activity, we transfected COS-7 cells with Myc-tagged IgLON constructs and analyzed both the conditioned media and biotinylated cell surface proteins. All IgLON family members could be detected on the cell surface and in the conditioned media from the transfected COS-7 cells (Fig. 5A). The bands that we detect correspond to the glycosylated forms of the IgLONs, which migrate with molecular masses between 50 and 68 kDa, demonstrating that transfected IgLONs are properly glycosylated and traffic to the cell surface. When the cells were exposed to BB-94 or GM6001, IgLON family members accumulated on the cell surface and were weak or undetectable in the conditioned media. Furthermore, treatment of cells with the phorbol ester TPA, which increases levels of metalloproteinase activity, resulted in a loss of the IgLONs from the cell surface and accumulation in the conditioned media (29). The effect of TPA was blocked with BB-94 confirming that the enhanced shedding was metalloproteinase-dependent (Fig. 5B). We also treated neurons with PI-PLC, an enzyme that cleaves glycosylphosphatidylinositol-anchored proteins. IgLONs in the conditioned media following PI-PLC treatment migrate slightly higher than the metalloproteinase-dependent shed product (Fig. 5A). The distinction between the product generated by metalloproteinases and PI-PLC was even more apparent when the deglycosylating agent peptide:N-glycosidase F was added to the conditioned media (Fig. 5C) (30). Following treatment with peptide:N-glycosidase, the IgLONs migrate near their predicted molecular masses of 37 kDa, and the endogenously cleaved products migrate more rapidly than full-length IgLONs released by PI-PLC. It was also apparent that the product in the conditioned media migrated more rapidly than the product from cell lysates (Fig. 5D). We conclude that IgLON proteins are cleaved close to the membrane to shed an ectodomain fragment that is near full length.
FIGURE 5.
IgLON family members are shed by metalloproteinases. A, to validate the processing of IgLON family members, myc-IgLON constructs were expressed in COS-7 cells. Biotinylated cell surface proteins (CSB) and conditioned medium (CM) of transduced COS-7 cells were analyzed by Western blotting after treatment with DMSO, GM-I, BB-94, GM6001, TPA, or PI-PLC. B, to validate the processing of IgLON family member by metalloproteinase activity, the medium of COS-7 cells expressing the Myc-tagged IgLON family member NEGR1 was analyzed after treatment with TPA in the presence or absence of BB-94. C, to identify the size of the cleaved IgLON fragment, the medium of COS-7 cells, transduced with myc-IgLON constructs and treated with DMSO and PI-PLC, was concentrated and deglycosylated with peptide:N-glycosidase F (PNGaseF). D, conditioned media and lysates of COS-7 cells transduced with myc-IgLON constructs were analyzed by SDS-PAGE and Western blotting with IgLON-specific antibodies. Western blots are representative of 3–6 independent experiments.
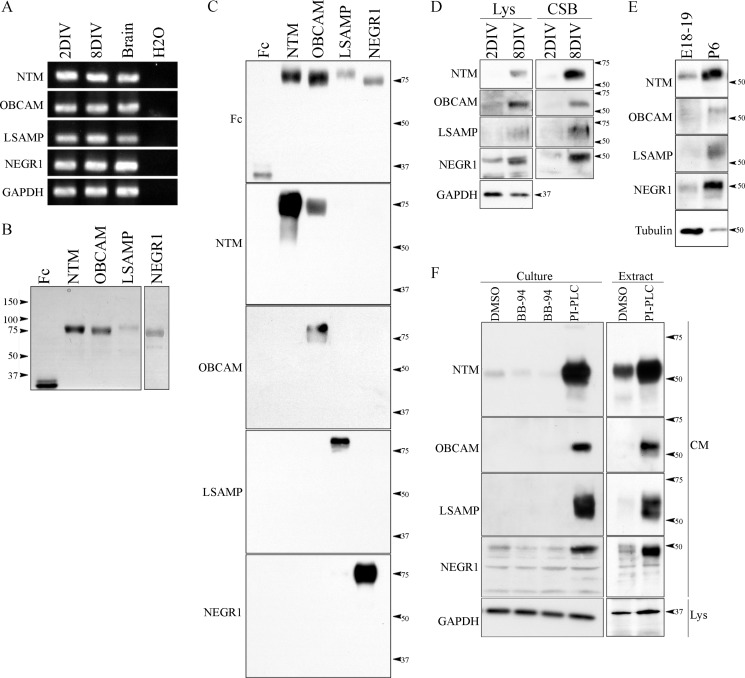
Metalloproteinase activity in any given cell type is a function of the expression of mature metalloproteinases as well as the expression of TIMPs. We next characterized the shedding profile of IgLONs from cortical neurons to determine whether they were good candidate substrates to promote the intrinsic growth capacity of the aged cortical neurons. We analyzed the expression pattern of IgLON family members in cortical neurons cultured for 2 and 8 DIV. mRNA for all four IgLON family members was detectable in both stages of cortical neurons by RT-PCR (Fig. 6A). We then acquired commercial antibodies to individual IgLON family members and confirmed that they detected recombinant protein (Fig. 6, B and C). The anti-NTM antibody showed some cross-reactivity to OBCAM, whereas the other anti-IgLON antibodies were specific. Using these antibodies to probe cortical lysates, we found that IgLON proteins were weakly expressed or absent in 2 DIV cortical neurons both in the cell lysate and at the cell surface and were up-regulated at 8 DIV (Fig. 6D). The up-regulation in IgLON protein expression was also apparent in cortex isolated from rat brain ruling out the possibility that this is an effect of the cell culture (Fig. 6E). The expression profile of the IgLONs correlated with the metalloproteinase sensitivity of the cortical neurons in neurite outgrowth assays (Fig. 1).
FIGURE 6.
IgLON expression and processing is developmentally regulated in cortical neurons. A, reverse transcription PCR (RT-PCR) was performed in cortical neurons from two developmental stages (2 and 8 DIV). B, recombinant IgLON-Fc proteins were purified from the conditioned media of transfected HEK293T cells and analyzed by SDS-PAGE and Coomassie Brilliant Blue stain. C, specificity of commercially available IgLON antibodies was validated by Western blot against soluble recombinant IgLON-Fc proteins. D and E, developmental expression of IgLON family members was assayed in cortical neurons. Lysates (Lys) and biotinylated cell surface proteins (CSB) were collected from 2- and 8-DIV cortical neurons and lysates from the cortex of embryonic (E18–19) and postnatal (P6) stage rats. The expression of IgLON family members was determined by Western blot using commercially available IgLON antibodies. F, to validate processing of IgLON family members by metalloproteinase activity in cortical neurons, the medium of aged cortical neurons and the medium of membrane extracts from postnatal stages of brain cortex were collected. Commercially available IgLON antibodies were used to detect cleaved IgLON fragments in the medium. Anti-GAPDH and tubulin antibodies were used as loading controls. Western blots are representative of 3–4 independent experiments.
We then used these antibodies to probe conditioned media from cortical neurons cultured for 8 DIV. When cortical neurons were treated with PI-PLC, all IgLON family members were enriched in the conditioned media indicating that they are all expressed on the cell surface of these neurons (Fig. 6F). In the conditioned media from untreated cortical neurons, NTM and NEGR1 were present, and levels were reduced by treatment with BB-94 demonstrating that these two family members undergo constitutive shedding in mature cortical neurons (Fig. 6F). LSAMP and OBCAM were not detectable in the conditioned media from resting cortical neurons leading us to conclude that these IgLON family members undergo little shedding in these conditions or that they are unstable upon their release. To validate the processing of NTM and NEGR1 by metalloproteinases in vivo, we collected the conditioned media from brain extracts. All IgLON family members were detected in the conditioned media, and similar to the culture experiment, NTM and NEGR1 were most robustly shed (Fig. 6F).
NTM and NEGR1 Sensitize Immature Cortical Neurons to BB-94
We next asked whether introducing IgLONs into 2 DIV cortical neurons would be sufficient to sensitize the neurons to a metalloproteinase inhibitor in the neurite outgrowth assay. 2-DIV cortical neurons were nucleofected with individual IgLON constructs. We confirmed that Myc-tagged IgLON constructs were expressed at the cell surface of the neurons by performing immunofluorescence staining on cortical neurons prior to permeabilization (Fig. 7A). Introduction of NTM or NEGR1, but not OBCAM or LSAMP, was sufficient to sensitize these neurons to the outgrowth inhibitory activity of the metalloproteinase inhibitor BB-94 implicating shedding of these IgLON family members in regulating the intrinsic growth capacity of neurons (Fig. 7B). Introduction of the IgLONs in the absence of BB-94 did not impact outgrowth suggesting that BB-94 leads to an accumulation of NTM and NEGR1 on the cell surface that impedes neurite extension.
FIGURE 7.
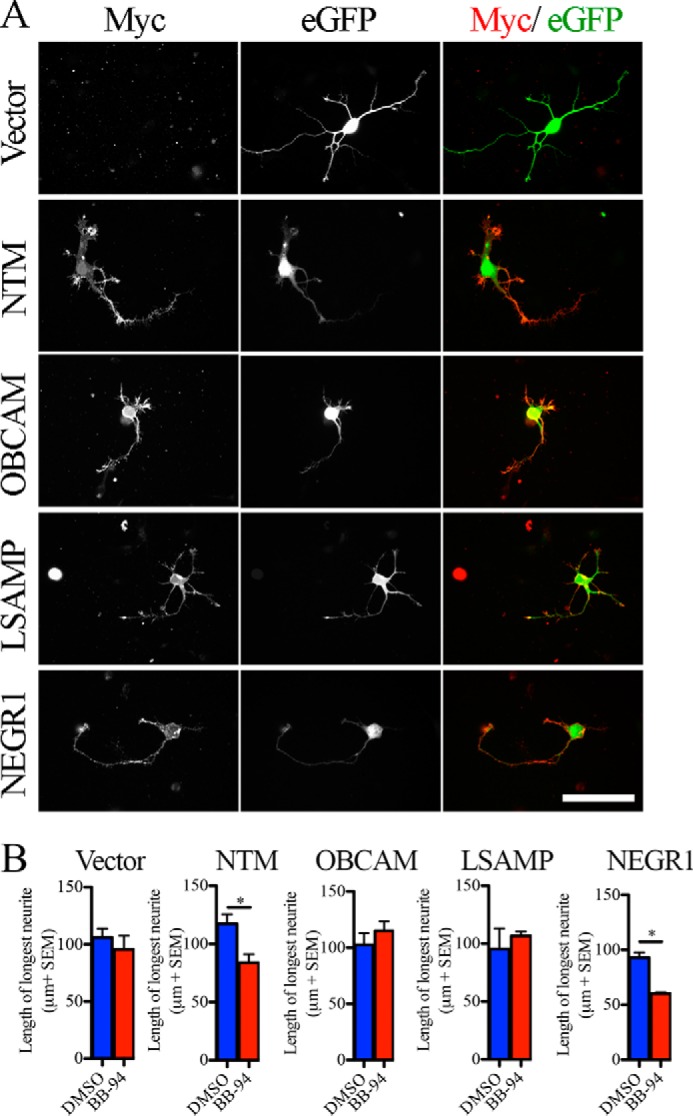
Surface IgLON expression sensitizes 2-DIV cortical neurons to metalloproteinase inhibitors. A, Myc-IgLON family members were co-electroporated with enhanced GFP (eGFP) in dissociated E18–19 rat cortical neurons. Surface myc-IgLON expression was detected with anti-Myc antibody in the absence of permeabilization. B, outgrowth assay was performed in enhanced GFP-positive cortical neurons transduced with myc-IgLON constructs treated with DMSO or BB-94. n = 3–4 from independent cultures. Data are mean ± S.E., *, p < 0.05, by Student's t test. Scale bar, 50 μm.
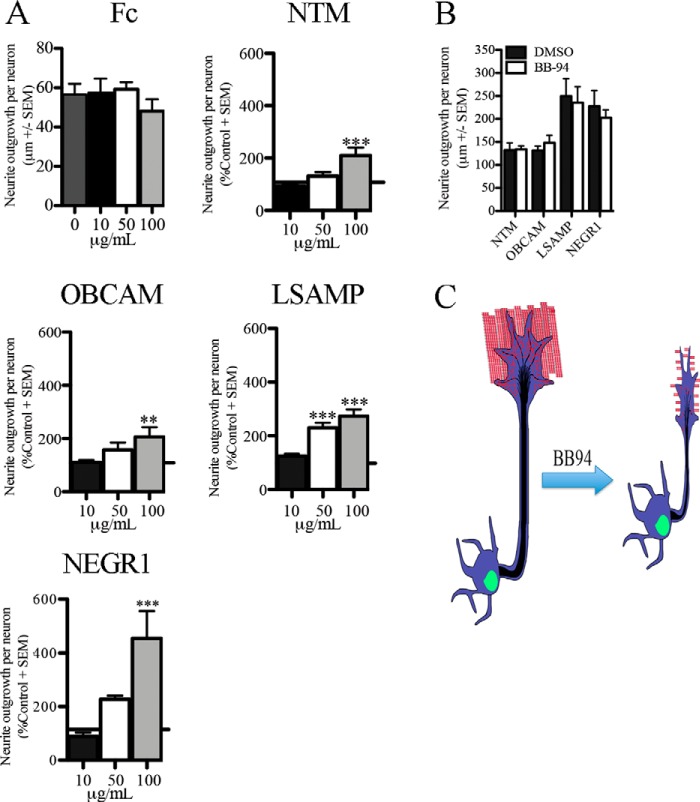
Immobilized Full-length IgLON Family Members Promote Neurite Outgrowth from 8-DIV Cortical Neurons
IgLON family members act through homophilic and heterophilic interactions and through interactions with other receptors to promote adhesion and to promote or repress growth (31, 32). The effects of IgLON family members vary depending on the cell type and stage (20, 33). We coated IgLONs as substrates and tested their effects on neurite outgrowth from 8-DIV cortical neurons (20). We observed a dose-dependent increase in neurite outgrowth with recombinant NTM, OBCAM, LSAMP, and NEGR1 compared with control substrate (Fig. 8A). This finding supports a model whereby IgLONs shed from the surface of mature cortical neurons could generate a growth-permissive substrate for outgrowth. We then treated mature cortical neurons grown on IgLON substrates with BB-94. We found that outgrowth of cortical neurons grown on IgLON substrates was insensitive to BB-94 suggesting that the substrate can overcome inhibitory effects of cell surface IgLONs (Fig. 8B). Together, our data demonstrate that IgLON shedding promotes the growth of cortical neurons by forming a growth-permissive substrate for cortical neurons and by relieving a neurite outgrowth inhibitory signal transduced by a NTM- or NEGR1-containing cell surface complex (Fig. 8C).
FIGURE 8.
Immobilized IgLON family members promote neurite outgrowth in cortical neurons. A, outgrowth quantification of mature cortical neurons seeded on Fc-IgLON substrates (10, 50, and 100 μg/ml). Data were normalized to control Fc substrate. B, outgrowth quantification of mature cortical neurons seeded on IgLON substrates (100 μg/ml) and exposed to DMSO or BB-94. Data are shown as mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001 by one-way (B) and two-way (C) ANOVA, followed by Bonferroni post hoc test. C, schematic representation of the role of IgLON processing in neurite outgrowth.
DISCUSSION
Ectodomain shedding is an important regulatory mechanism that can desensitize a cell to a ligand and generate biologically active fragments. Here, we report that members of the IgLON family of cell adhesion molecules are shed from the surface of mature cortical neurons through ADAM-dependent proteolysis. Release of IgLON proteins from the surface of mature cortical neurons promotes their outgrowth likely by providing a permissive substrate at the leading edge of the neuronal growth cone and through the relief of a neurite outgrowth inhibitory signal from cell surface IgLONs.
Ectodomain Shedding of IgLON Family Members in Neurite Outgrowth
Previous studies have uncovered important aspects of metalloproteinase activity in neurite outgrowth. One major aspect is the enrichment of the extracellular matrix with functionally active fragments to enhance neurite outgrowth. For example, cleavage of L1-CAM proteins promotes neurite outgrowth in cerebellar granule cells (4), whereas cleavage of neuronal cell adhesion molecule (NCAM) promotes neurite outgrowth in hippocampal neurons (34). The growth-promoting effect of the L1-CAM and NCAM shed products is mediated through homophilic interactions and activation of the integrin receptor (12, 35, 36). Although we have not characterized the site of metalloproteinase cleavage, our experiments reveal that near full-length IgLON family members are processed in a metalloproteinase-dependent manner, similar to L1-CAM and NCAM, to facilitate neuronal extension in cortical neurons. The ability of substrate-bound IgLONs to promote outgrowth of mature cortical neurons supports the idea that shed IgLONs signal through homophilic-heterophilic-IgLON interactions or alternative receptor molecules to promote growth (Fig. 8). IgLON overexpression did not promote neurite growth on its own suggesting that insufficient concentrations of IgLONs were generated in the vicinity of the growth cone to generate a growth-permissive substrate. By mass spectrometry, we found that all IgLON family members were enriched on the cell surface following treatment with metalloproteinase inhibitors. In contrast, only NTM and NEGR1 could be detected in conditioned media from cortical neurons by Western blotting. This may reflect limited stability of OBCAM and LSAMP upon their release from the cell surface.
Our data also support the idea that shedding of NTM and NEGR1 acts to relieve an outgrowth inhibitory signal transduced from the cell surface. Introduction of NTM and NEGR1 into 2-DIV cortical neurons, which do not express endogenous IgLON at the cell surface, sensitized neurons to the outgrowth inhibitory effect of BB-94 but did not affect outgrowth in the absence of BB-94 (Fig. 7). If the sensitivity to BB-94 resulted from a failure to present growth-promoting fragments in the vicinity of the growth cone, then it follows that transfection of IgLONs themselves should have had a growth-promoting effect. Because they did not, we reason that BB-94 leads to the enrichment of a cell surface complex that limits the extension of cortical neurons. This complex could be formed by IgLON homodimers or heterodimers or by IgLON proteins in complex with a co-receptor (31, 32). IgLON overexpression likely fails to inhibit outgrowth in the absence of BB-94 because it is constitutively shed. Mature cortical neurons grown on IgLON substrates were insensitive to BB-94 suggesting that the growth promoting activity of shed IgLONs overcomes the influence of any cell surface inhibitory signals. A tempting speculation is that substrate-bound IgLONs may complex with cell surface IgLONs to displace a co-receptor that is required for transducing neurite outgrowth inhibitory signals intracellularly.
IgLON Shedding by the ADAM Family of Metalloproteinases
The selective effect of TIMP3 on neurite extension implicates a member of the ADAM family of metalloproteinases as likely mediators of NTM and NEGR1 processing. NTM has also been identified as a potential BACE-1 substrate raising the possibility that it is a substrate of multiple proteases (37). Thirty seven ADAM family members are expressed in mice, 12 of which contain proteolytic activity (38). Among the best characterized ADAM proteases, ADAM10, ADAM17, and ADAM8 are observed to regulate axon extension. Proteolytic cleavage of NCAM and L1-CAM by ADAM10 and ADAM17 is involved in axon growth, and ectodomain shedding of CHL1 by ADAM8 promotes neurite outgrowth in cerebellar granule cells (4, 34, 39). Although we have not conducted a comprehensive analysis of all ADAM family members, we did find that knockdown of ADAM10 had no impact on IgLON shedding (data not shown).
Physiological Role of IgLON Shedding
It is noteworthy that metalloproteinase inhibitors selectively affected the outgrowth of aged cortical neurons, although the immature neurons were unaffected (Fig. 1). The developmentally regulated response of the cortical neurons correlates with an increase in expression of IgLON proteins at the cell surface. The developmental increase in IgLON expression is consistent with previous reports analyzing the expression of several IgLON family members in tissue from several regions of the rodent brain (16, 40). In the course of postnatal development, axons continue to grow in complexity and length to establish connections and form the laminated cortical structure. The delayed expression of IgLON family members raises the possibility that IgLON shedding may play roles in late stages of cortical neuron outgrowth or synaptogenesis rather than the initial outgrowth of cortical neurons. Axonal LSAMP expression plays an important role in the specific targeting of dopaminergic axons to the lateral subdomain of the habenula (41). Furthermore, down-regulation of NEGR1 expression both in vitro and in vivo was observed to decrease the overall complexity of neurite arborization suggesting a role in axon targeting and stability rather than overall outgrowth (42). IgLON family members are also expressed at synaptic sites (22, 40, 43). Overexpression of individual IgLON family members differentially affects the number of dendritic synapses that form in cultured hippocampal neurons, whereas NEGR1 and OBCAM loss of function results in a reduction in synapse formation on dendrites (42, 44, 45). Proteolytic cleavage of IgLON family members could thus have critical roles in specific targeting and synaptogenesis of cortical neurons similar to roles of other synaptic cell adhesion molecules, including NCAM, L1-CAM, and N-cadherin (46–48). The physiological role of IgLON shedding is a particularly interesting question in the context of the recent classification of IgLONs as potential candidate genes underlying aspects of autism spectrum disorder and cancer susceptibility (49–52).
Acknowledgments
We thank Wayne Sossin and Phil Barker for comments on the manuscript.
This work was supported by grants from the Canadian Institutes of Health Research and the McGill Program in Neuroengineering and by a Canada Research Chair (to A. E. F.).
- MMP
- matrix metalloproteinase
- TIMP
- tissue inhibitor of metalloproteinase
- ADAM
- a disintegrin and metalloproteinase
- ANOVA
- analysis of variance
- PLL
- poly-l-lysine
- LSAMP
- limbic system-associated membrane protein
- NTM
- neurotrimin
- OBCAM
- opioid-binding cell adhesion molecule
- PI-PLC
- phosphatidylinositol phospholipase C
- DIV
- days in vitro
- TPA
- 12-O-tetradecanoylphorbol-13-acetate
- GM-I
- N-t-butoxycarbonyl-l-leucyl-l-tryptophan methylamide
- NCAM
- neuronal cell adhesion molecule
- Z-VAD-fmk
- benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone.
REFERENCES
- 1. Kolodkin A. L., Tessier-Lavigne M. (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb. Perspect. Biol. 3, a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nash M., Pribiag H., Fournier A. E., Jacobson C. (2009) Central nervous system regeneration inhibitors and their intracellular substrates. Mol. Neurobiol. 40, 224–235 [DOI] [PubMed] [Google Scholar]
- 3. Hooper N. M., Karran E. H., Turner A. J. (1997) Membrane protein secretases. Biochem. J. 321, 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maretzky T., Schulte M., Ludwig A., Rose-John S., Blobel C., Hartmann D., Altevogt P., Saftig P., Reiss K. (2005) L1 is sequentially processed by two differently activated metalloproteases and presenilin/γ-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol. Cell. Biol. 25, 9040–9053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romi E., Gokhman I., Wong E., Antonovsky N., Ludwig A., Sagi I., Saftig P., Tessier-Lavigne M., Yaron A. (2014) ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A. Nat. Commun. 5, 4058. [DOI] [PubMed] [Google Scholar]
- 6. Vaillant C., Meissirel C., Mutin M., Belin M. F., Lund L. R., Thomasset N. (2003) MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Mol. Cell. Neurosci. 24, 395–408 [DOI] [PubMed] [Google Scholar]
- 7. Zolkiewska A. (2008) ADAM proteases: ligand processing and modulation of the Notch pathway. Cell. Mol. Life Sci. 65, 2056–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yong V. W. (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 6, 931–944 [DOI] [PubMed] [Google Scholar]
- 9. Brew K., Nagase H. (2010) The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta 1803, 55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webber C. A., Hocking J. C., Yong V. W., Stange C. L., McFarlane S. (2002) Metalloproteases and guidance of retinal axons in the developing visual system. J. Neurosci. 22, 8091–8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fambrough D., Pan D., Rubin G. M., Goodman C. S. (1996) The cell surface metalloprotease/disintegrin Kuzbanian is required for axonal extension in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 93, 13233–13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mechtersheimer S., Gutwein P., Agmon-Levin N., Stoeck A., Oleszewski M., Riedle S., Postina R., Fahrenholz F., Fogel M., Lemmon V., Altevogt P. (2001) Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J. Cell Biol. 155, 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janes P. W., Saha N., Barton W. A., Kolev M. V., Wimmer-Kleikamp S. H., Nievergall E., Blobel C. P., Himanen J. P., Lackmann M., Nikolov D. B. (2005) Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123, 291–304 [DOI] [PubMed] [Google Scholar]
- 14. Galko M. J., Tessier-Lavigne M. (2000) Function of an axonal chemoattractant modulated by metalloprotease activity. Science 289, 1365–1367 [DOI] [PubMed] [Google Scholar]
- 15. Ferraro G. B., Morrison C. J., Overall C. M., Strittmatter S. M., Fournier A. E. (2011) Membrane-type matrix metalloproteinase-3 regulates neuronal responsiveness to myelin through Nogo-66 receptor 1 cleavage. J. Biol. Chem. 286, 31418–31424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Struyk A. F., Canoll P. D., Wolfgang M. J., Rosen C. L., D'Eustachio P., Salzer J. L. (1995) Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J. Neurosci. 15, 2141–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh S., Hachisuka A., Kawasaki N., Hashii N., Teshima R., Hayakawa T., Kawanishi T., Yamaguchi T. (2008) Glycosylation analysis of IgLON family proteins in rat brain by liquid chromatography and multiple-stage mass spectrometry. Biochemistry 47, 10132–10154 [DOI] [PubMed] [Google Scholar]
- 18. Horton H. L., Levitt P. (1988) A unique membrane protein is expressed on early developing limbic system axons and cortical targets. J. Neurosci. 8, 4653–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kimura Y., Katoh A., Kaneko T., Takahama K., Tanaka H. (2001) Two members of the IgLON family are expressed in a restricted region of the developing chick brain and neural crest. Dev. Growth Differ. 43, 257–263 [DOI] [PubMed] [Google Scholar]
- 20. Gil O. D., Zanazzi G., Struyk A. F., Salzer J. L. (1998) Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J. Neurosci. 18, 9312–9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lodge A. P., Howard M. R., McNamee C. J., Moss D. J. (2000) Co-localisation, heterophilic interactions and regulated expression of IgLON family proteins in the chick nervous system. Brain Res. Mol. Brain Res. 82, 84–94 [DOI] [PubMed] [Google Scholar]
- 22. Chen S., Gil O., Ren Y. Q., Zanazzi G., Salzer J. L., Hillman D. E. (2001) Neurotrimin expression during cerebellar development suggests roles in axon fasciculation and synaptogenesis. J. Neurocytol. 30, 927–937 [DOI] [PubMed] [Google Scholar]
- 23. Jordan M., Schallhorn A., Wurm F. M. (1996) Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24, 596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huebner E. A., Kim B. G., Duffy P. J., Brown R. H., Strittmatter S. M. (2011) A multi-domain fragment of Nogo-A protein is a potent inhibitor of cortical axon regeneration via Nogo receptor 1. J. Biol. Chem. 286, 18026–18036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies B., Brown P. D., East N., Crimmin M. J., Balkwill F. R. (1993) A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 53, 2087–2091 [PubMed] [Google Scholar]
- 26. Grobelny D., Poncz L., Galardy R. E. (1992) Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 31, 7152–7154 [DOI] [PubMed] [Google Scholar]
- 27. Huang X., Lu Z., Lv Z., Yu T., Yang P., Shen Y., Ding Y., Fu D., Zhang X., Fu Q., Yu Y. (2013) The Fas/Fas ligand death receptor pathway contributes to phenylalanine-induced apoptosis in cortical neurons. PLoS One 8, e71553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker A. H., Edwards D. R., Murphy G. (2002) Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 115, 3719–3727 [DOI] [PubMed] [Google Scholar]
- 29. Park M. J., Park I. C., Hur J. H., Rhee C. H., Choe T. B., Yi D. H., Hong S. I., Lee S. H. (2000) Protein kinase C activation by phorbol ester increases in vitro invasion through regulation of matrix metalloproteinases/tissue inhibitors of metalloproteinases system in D54 human glioblastoma cells. Neurosci. Lett. 290, 201–204 [DOI] [PubMed] [Google Scholar]
- 30. Tarentino A. L., Gómez C. M., Plummer T. H., Jr. (1985) Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry 24, 4665–4671 [DOI] [PubMed] [Google Scholar]
- 31. Akeel M., McNamee C. J., Youssef S., Moss D. (2011) DIgLONs inhibit initiation of neurite outgrowth from forebrain neurons via an IgLON-containing receptor complex. Brain Res. 1374, 27–35 [DOI] [PubMed] [Google Scholar]
- 32. McNamee C. J., Youssef S., Moss D. (2011) IgLONs form heterodimeric complexes on forebrain neurons. Cell Biochem. Funct. 29, 114–119 [DOI] [PubMed] [Google Scholar]
- 33. Reed J., McNamee C., Rackstraw S., Jenkins J., Moss D. (2004) Diglons are heterodimeric proteins composed of IgLON subunits, and Diglon-CO inhibits neurite outgrowth from cerebellar granule cells. J. Cell Sci. 117, 3961–3973 [DOI] [PubMed] [Google Scholar]
- 34. Kalus I., Bormann U., Mzoughi M., Schachner M., Kleene R. (2006) Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J. Neurochem. 98, 78–88 [DOI] [PubMed] [Google Scholar]
- 35. Diestel S., Hinkle C. L., Schmitz B., Maness P. F. (2005) NCAM140 stimulates integrin-dependent cell migration by ectodomain shedding. J. Neurochem. 95, 1777–1784 [DOI] [PubMed] [Google Scholar]
- 36. Hinkle C. L., Diestel S., Lieberman J., Maness P. F. (2006) Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM). J. Neurobiol. 66, 1378–1395 [DOI] [PubMed] [Google Scholar]
- 37. Kuhn P. H., Koroniak K., Hogl S., Colombo A., Zeitschel U., Willem M., Volbracht C., Schepers U., Imhof A., Hoffmeister A., Haass C., Rossner S., Bräse S., Lichtenthaler S. F. (2012) Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 31, 3157–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber S., Saftig P. (2012) Ectodomain shedding and ADAMs in development. Development 139, 3693–3709 [DOI] [PubMed] [Google Scholar]
- 39. Naus S., Richter M., Wildeboer D., Moss M., Schachner M., Bartsch J. W. (2004) Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J. Biol. Chem. 279, 16083–16090 [DOI] [PubMed] [Google Scholar]
- 40. Miyata S., Matsumoto N., Taguchi K., Akagi A., Iino T., Funatsu N., Maekawa S. (2003) Biochemical and ultrastructural analyses of IgLON cell adhesion molecules, Kilon and OBCAM in the rat brain. Neuroscience 117, 645–658 [DOI] [PubMed] [Google Scholar]
- 41. Schmidt E. R., Brignani S., Adolfs Y., Lemstra S., Demmers J., Vidaki M., Donahoo A. L., Lilleväli K., Vasar E., Richards L. J., Karagogeos D., Kolk S. M., Pasterkamp R. J. (2014) Subdomain-mediated axon-axon signaling and chemoattraction cooperate to regulate afferent innervation of the lateral habenula. Neuron 83, 372–387 [DOI] [PubMed] [Google Scholar]
- 42. Pischedda F., Szczurkowska J., Cirnaru M. D., Giesert F., Vezzoli E., Ueffing M., Sala C., Francolini M., Hauck S. M., Cancedda L., Piccoli G. (2014) A cell surface biotinylation assay to reveal membrane-associated neuronal cues: Negr1 regulates dendritic arborization. Mol. Cell. Proteomics 13, 733–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zacco A., Cooper V., Chantler P. D., Fisher-Hyland S., Horton H. L., Levitt P. (1990) Isolation, biochemical characterization and ultrastructural analysis of the limbic system-associated membrane protein (LAMP), a protein expressed by neurons comprising functional neural circuits. J. Neurosci. 10, 73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hashimoto T., Maekawa S., Miyata S. (2009) IgLON cell adhesion molecules regulate synaptogenesis in hippocampal neurons. Cell Biochem. Funct. 27, 496–498 [DOI] [PubMed] [Google Scholar]
- 45. Yamada M., Hashimoto T., Hayashi N., Higuchi M., Murakami A., Nakashima T., Maekawa S., Miyata S. (2007) Synaptic adhesion molecule OBCAM; synaptogenesis and dynamic internalization. Brain Res. 1165, 5–14 [DOI] [PubMed] [Google Scholar]
- 46. Brennaman L. H., Maness P. F. (2008) Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule. Mol. Cell. Neurosci. 37, 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malinverno M., Carta M., Epis R., Marcello E., Verpelli C., Cattabeni F., Sala C., Mulle C., Di Luca M., Gardoni F. (2010) Synaptic localization and activity of ADAM10 regulate excitatory synapses through N-cadherin cleavage. J. Neurosci. 30, 16343–16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsumoto-Miyai K., Ninomiya A., Yamasaki H., Tamura H., Nakamura Y., Shiosaka S. (2003) NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J. Neurosci. 23, 7727–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Rubeis S., He X., Goldberg A. P., Poultney C. S., Samocha K., Cicek A. E., Kou Y., Liu L., Fromer M., Walker S., Singh T., Klei L., Kosmicki J., Shih-Chen F., Aleksic B., Biscaldi M., Bolton P. F., Brownfeld J. M., Cai J., Campbell N. G., Carracedo A., Chahrour M. H., Chiocchetti A. G., Coon H., Crawford E. L., Crooks L., Curran S. R., Dawson G., Duketis E., Fernandez B. A., Gallagher L., Geller E., Guter S. J., Hill R. S., Ionita-Laza J., Jimenz Gonzalez P., Kilpinen H., Klauck S. M., Kolevzon A., Lee I., Lei I., Lei J., Lehtimäki T., Lin C. F., Ma'ayan A., Marshall C. R., McInnes A. L., Neale B., Owen M. J., Ozaki N., Parellada M., Parr J. R., Purcell S., Puura K., Rajagopalan D., Rehnström K., Reichenberg A., Sabo A., Sachse M., Sanders S. J., Schafer C., Schulte-Rüther M., Skuse D., Stevens C., Szatmari P., Tammimies K., Valladares O., Voran A., Wang L. S., Weiss L. A., Jeremy Willsey A., Yu T. W., Yuen R. K., DDD Study; Homozygosity Mapping Collaborative for Autism; UK10K Consortium, Cook E. H., Freitag C. M., Gill M., Hultman C. M., Lehner T., Palotie A., Schellenberg G. D., Sklar P., State M. W., Sutcliffe J. S., Walsh C. A., Scherer S. W., Zwick M. E., Barrett J. C., Cutler D. J., Roeder K., Devlin B., Daly M. J., Buxbaum J. D. (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pinto D., Delaby E., Merico D., Barbosa M., Merikangas A., Klei L., Thiruvahindrapuram B., Xu X., Ziman R., Wang Z., Vorstman J. A., Thompson A., Regan R., Pilorge M., Pellecchia G., Pagnamenta A. T., Oliveira B., Marshall C. R., Magalhaes T. R., Lowe J. K., Howe J. L., Griswold A. J., Gilbert J., Duketis E., Dombroski B. A., De Jonge M. V., Cuccaro M., Crawford E. L., Correia C. T., Conroy J., Conceição I. C., Chiocchetti A. G., Casey J. P., Cai G., Cabrol C., Bolshakova N., Bacchelli E., Anney R., Gallinger S., Cotterchio M., Casey G., Zwaigenbaum L., Wittemeyer K., Wing K., Wallace S., van Engeland H., Tryfon A., Thomson S., Soorya L., Rogé B., Roberts W., Poustka F., Mouga S., Minshew N., McInnes L. A., McGrew S. G., Lord C., Leboyer M., Le Couteur A. S., Kolevzon A., Jiménez González P., Jacob S., Holt R., Guter S., Green J., Green A., Gillberg C., Fernandez B. A., Duque F., Delorme R., Dawson G., Chaste P., Café C., Brennan S., Bourgeron T., Bolton P. F., Bolte S., Bernier R., Baird G., Bailey A. J., Anagnostou E., Almeida J., Wijsman E. M., Vieland V. J., Vicente A. M., Schellenberg G. D., Pericak-Vance M., Paterson A. D., Parr J. R., Oliveira G., Nurnberger J. I., Monaco A. P., Maestrini E., Klauck S. M., Hakonarson H., Haines J. L., Geschwind D. H., Freitag C. M., Folstein S. E., Ennis S., Coon H., Battaglia A., Szatmari P., Sutcliffe J. S., Hallmayer J., Gill M., Cook E. H., Buxbaum J. D., Devlin B., Gallagher L., Betancur C., Scherer S. W. (2014) Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 94, 677–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prasad A., Merico D., Thiruvahindrapuram B., Wei J., Lionel A. C., Sato D., Rickaby J., Lu C., Szatmari P., Roberts W., Fernandez B. A., Marshall C. R., Hatchwell E., Eis P. S., Scherer S. W. (2012) A discovery resource of rare copy number variations in individuals with autism spectrum disorder. G3 2, 1665–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Minhas H. M., Pescosolido M. F., Schwede M., Piasecka J., Gaitanis J., Tantravahi U., Morrow E. M. (2013) An unbalanced translocation involving loss of 10q26.2 and gain of 11q25 in a pedigree with autism spectrum disorder and cerebellar juvenile pilocytic astrocytoma. Am. J. Med. Genet. Part A 161A, 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Houri N., Huang K. C., Nalbantoglu J. (2013) The Coxsackievirus and Adenovirus Receptor (CAR) undergoes ectodomain shedding and regulated intramembrane proteolysis (RIP). PLoS One 8, e73296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stipp C. S., Kolesnikova T. V., Hemler M. E. (2001) EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J. Biol. Chem. 276, 40545–40554 [DOI] [PubMed] [Google Scholar]
- 55. Tanaka Y., Irie K., Hirota T., Sakisaka T., Nakanishi H., Takai Y. (2002) Ectodomain shedding of nectin-1α by SF/HGF and TPA in MDCK cells. Biochem. Biophys. Res. Commun. 299, 472–478 [DOI] [PubMed] [Google Scholar]
- 56. Fabre-Lafay S., Garrido-Urbani S., Reymond N., Gonçalves A., Dubreuil P., Lopez M. (2005) Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-α-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 280, 19543–19550 [DOI] [PubMed] [Google Scholar]
- 57. Shichi K., Fujita-Hamabe W., Harada S., Mizoguchi H., Yamada K., Nabeshima T., Tokuyama S. (2011) Involvement of matrix metalloproteinase-mediated proteolysis of neural cell adhesion molecule in the development of cerebral ischemic neuronal damage. J. Pharmacol. Exp. Ther. 338, 701–710 [DOI] [PubMed] [Google Scholar]
- 58. Saura C. A., Servián-Morilla E., Scholl F. G. (2011) Presenilin/γ-secretase regulates neurexin processing at synapses. PLoS One 6, e19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee H., Lee E. J., Song Y. S., Kim E. (2014) Long-term depression-inducing stimuli promote cleavage of the synaptic adhesion molecule NGL-3 through NMDA receptors, matrix metalloproteinases and presenilin/γ-secretase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kajiwara Y., Buxbaum J. D., Grice D. E. (2009) SLITRK1 binds 14-3-3 and regulates neurite outgrowth in a phosphorylation-dependent manner. Biol. Psychiatry 66, 918–925 [DOI] [PubMed] [Google Scholar]
- 61. Bajor M., Kaczmarek L. (2013) Proteolytic remodeling of the synaptic cell adhesion molecules (CAMs) by metzincins in synaptic plasticity. Neurochem. Res. 38, 1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tousseyn T., Thathiah A., Jorissen E., Raemaekers T., Konietzko U., Reiss K., Maes E., Snellinx A., Serneels L., Nyabi O., Annaert W., Saftig P., Hartmann D., De Strooper B. (2009) ADAM10, the rate-limiting protease of regulated intramembrane proteolysis of Notch and other proteins, is processed by ADAMS-9, ADAMS-15, and the γ-secretase. J. Biol. Chem. 284, 11738–11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tanaka M., Sasaki K., Kamata R., Sakai R. (2007) The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J. Cell Sci. 120, 2179–2189 [DOI] [PubMed] [Google Scholar]
- 64. Sage E. H., Reed M., Funk S. E., Truong T., Steadele M., Puolakkainen P., Maurice D. H., Bassuk J. A. (2003) Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J. Biol. Chem. 278, 37849–37857 [DOI] [PubMed] [Google Scholar]