Background: Null mutants for zebrafish period2 were generated to elucidate its functions.
Results: Both locomotor activity and expression of key circadian clock genes are disrupted in the period2 mutant zebrafish.
Conclusion: Period2 is essential for zebrafish circadian regulation.
Significance: Period2 plays a positive role in the zebrafish circadian clock by enhancing bmal1b expression through binding to nuclear receptor Rorα.
Keywords: Circadian Clock, Gene Regulation, Rev-ErbAα (NR1D1), Transgenic, Zebrafish, Rorα, Period2
Abstract
We report the characterization of a null mutant for zebrafish circadian clock gene period2 (per2) generated by transcription activator-like effector nuclease and a positive role of PER2 in vertebrate circadian regulation. Locomotor experiments showed that per2 mutant zebrafish display reduced activities under light-dark and 2-h phase delay under constant darkness, and quantitative real time PCR analyses showed up-regulation of cry1aa, cry1ba, cry1bb, and aanat2 but down-regulation of per1b, per3, and bmal1b in per2 mutant zebrafish, suggesting that Per2 is essential for the zebrafish circadian clock. Luciferase reporter assays demonstrated that Per2 represses aanat2 expression through E-box and enhances bmal1b expression through the Ror/Rev-erb response element, implicating that Per2 plays dual roles in the zebrafish circadian clock. Cell transfection and co-immunoprecipitation assays revealed that Per2 enhances bmal1b expression through binding to orphan nuclear receptor Rorα. The enhancing effect of mouse PER2 on Bmal1 transcription is also mediated by RORα even though it binds to REV-ERBα. Moreover, zebrafish Per2 also appears to have tissue-specific regulatory roles in numerous peripheral organs. These findings help define the essential functions of Per2 in the zebrafish circadian clock and in particular provide strong evidence for a positive role of PER2 in the vertebrate circadian system.
Introduction
Circadian clocks, which evolved from long and enduring adaptation of organisms to the light/dark cycle on Earth (1–3), can be reset and synchronized by local environmental cues such as light and even food (4, 5) and regulate behavior and physiology with a period of ∼24 h (6–8). The molecular genetic mechanisms underlying circadian regulation have been established by a series of genetic and biochemical studies in several organisms including Cyanobacteria (9), Neurospora crassa (10, 11), Drosophila melanogaster (12, 13), and mice (14–16). In the circadian system, the oscillating pacemaker is regulated by the transcription/translation-based negative feedback loop (17, 18). In flies, CLOCK and CYCLE as positive factors form a heterodimer to activate transcription of period (per)2 and timeless (tim) and PER and TIM as negative factors form a heterodimer to turn off their own transcription (19). Likewise, in mammals, CLOCK and BMAL1 (vertebrate ortholog of fly CYCLE) form a heterodimer to activate transcription of Period and Cryptochrome (Cry) by binding to the E-box elements in the promoter regions of these genes (20–22), and PER and CRY form a heterodimer (PER-CRY) to repress their own transcription (2, 23, 24). Mammals have another loop wherein RORα activates Bmal1 transcription by binding to the ROR/REV-ERB response element (RORE) in the Bmal1 promoter, whereas REV-ERBα competes to bind to RORE to repress Bmal1 transcription (25–27).
The zebrafish (Danio rerio) is an excellent model for circadian studies and is equipped with circadian regulatory components similar to those of flies and mammals (28–31). Light and temperature are crucial circadian entrainment factors in zebrafish (32–34), and in particular, zebrafish peripheral tissues can directly respond to light signals to generate rhythmicity (35, 36). Two light receptors (Tmt opsin and Opsin4.1) were shown to be crucial for light entrainment in zebrafish peripheral circadian clocks (37). In addition, light can entrain the circadian clock to impact the cell cycle and DNA damage repairs in zebrafish (33, 38). Although the transcription/translation-based loops are also thought to operate in zebrafish, there are notable differences in circadian regulation between zebrafish and mammals; for instance, at the transcription level, clock and rorα oscillate in numerous tissues in zebrafish but not in mice (29), and csnk1δ, which encodes for casein kinase δ, also oscillates in zebrafish pineal gland but not in mice (39), emphasizing the necessity and importance to investigate the zebrafish circadian clock to obtain a full understanding of the vertebrate circadian mechanisms.
Mammalian Period2 as a canonical component of the circadian clock plays important roles in the circadian clock (21, 40), sleeping (41), metabolism (42), and carcinogenesis (43). In humans, a PER2 missense mutation abolishes phosphorylation by CK1δ (44) and results in familial advanced sleep phase syndrome (45). Mouse Per2 is a light-responded gene, and its circadian phase and amplitude of expression in the suprachiasmatic nuclei can be altered by different light/dark cycles (46). Zebrafish per2 is also a light-regulated gene, and its expression is significantly damped under constant darkness (32). Using transgenic fish and stably transfected cell line-based assays, a light-responsive module composed of D-box and E-box motifs within the per2 promoter was identified (32). In addition, zebrafish per2 is required for expression of the clock-controlled arylalkylamine N-acetyltransferase 2 (aanat2) (47, 48) encoding the rate-limiting enzyme for melatonin synthesis (48).
Despite all this important progress concerning zebrafish per2, there have been no stable genetic mutants for zebrafish per2, which are critical for determining its roles in zebrafish circadian regulation as well as other life processes. Recently, genome-editing tools, zinc finger nuclease, transcription activator-like effector nuclease (TALEN), and CRISPR/Cas9 have been developed to generate site-specific DNA double strand breaks that trigger the endogenous nonhomologous end joining DNA repair pathway to induce indel mutations in targeted genes in numerous species including zebrafish (49–51). Here we have successfully generated two lines of per2-null mutants with TALEN. Characterization of per2 mutant zebrafish showed that Per2 is essential for maintaining rhythmicity of zebrafish locomotor activities and expression of circadian clock genes as well as a circadian clock-controlled gene. We also determined that Per2 plays dual roles in the zebrafish circadian clock, not only repressing expression of E-box-containing genes but also promoting expression of RORE-containing genes. The positive role of PER2 in Bmal1 expression is conserved from zebrafish to mice with the difference that zebrafish Per2 enhances bmal1b expression through its binding of Rorα rather than Rev-erbα, whereas the enhancement of Bmal1 expression by mouse PER2 still requires RORα mediation even though it binds to REV-ERBα rather than RORα. Zebrafish Per2 seems to have distinct regulatory functions in the different peripheral organs. These results should help elucidate the essential functions of Per2 in the zebrafish circadian clock and provide critical evidence for a positive role of PER2 in the vertebrate circadian system.
EXPERIMENTAL PROCEDURES
Zebrafish Maintenance and Embryo Production
Zebrafish wild-type AB strain and per2 mutant lines were raised on a 14-h/10-h light/dark (LD) cycle at 28 °C in the Soochow University Zebrafish Facility according to standard protocols (52). Embryos were produced by pair mating, maintained in culture dishes, and used for experiments at specified stages.
TALEN Construction and Microinjection
TALEN target sites were designed using the web tool TALE-NT (53). TALEN expression vectors were constructed using the “unit assembly” method with Sharkey-AS and Sharkey-R forms of FokI cleavage domains as described previously (54). Briefly, TALEN expression vectors were linearized by NotI and used as templates for capped TALEN mRNA synthesis with the SP6 mMESSAGE mMACHINE kit (Ambion). Capped TALEN mRNAs encoding each monomer were simultaneously microinjected into one-cell zebrafish embryos.
Analysis of Mutagenesis Frequencies and Identification of per2 Mutants
The injected embryos were maintained in E3 medium (5 mm NaCl, 0.17 mm KCl, 0.33 mm CaCl2, 0.33 mm MgSO4) at 28.5 °C. The genomic DNAs of five groups (five embryos each) were extracted at 2 days postfertilization. A 259-base pair (bp) DNA fragment containing the per2 target site was amplified by PCR (primers used are listed in Table 1). 7 μl of the PCR product was digested using BslI (New England Biolabs) at 55 °C for 3 h. The intensities of cleaved and uncleaved bands were quantified with NIH ImageJ and used for estimating mutagenesis frequencies. The uncleaved bands were recovered after gel electrophoresis and cloned into pMD-19T (Takara) for sequencing analysis. To identify germ line-transmitted mutations, the microinjected founder (F0) embryos were raised to adulthood. The F0 fish were then outcrossed with wild-type zebrafish to produce F1. From each cross, 10 F1 embryos were collected for genomic DNA extraction and enzymatic digestion (data not shown). Siblings of the F1 embryos that carry heritable mutations were raised to adulthood, and an individual F1 fish was reidentified via PCR amplification and sequencing with fin clipped DNAs. Homozygous per2 mutant fish were generated by crossing of the male and female fish carrying the same mutation. Two per2-null mutant lines were established (see Fig. 1D).
TABLE 1.
All primers used in the study
| Gene | Primer sequence | Accession no. | Note |
|---|---|---|---|
| per2 | AATCCCACGGAAATGAATCTC | ENSDART00000148788 | TALEN target fragment |
| ATCAGGCGACACAAGCTGAC | |||
| per2 | CGAATTCGCATGTCTAAAGACCTGGATTC | ENSDART00000148788 | cDNA cloning |
| TGGTACCTCAGGTGTCTGGACCGGGACAGC | |||
| rev-erbα | TGGATCCGCCACCATGACTTTACTGGGGCTCAAC | ENSDART00000126282 | cDNA cloning |
| GAAGCTTGGCATCAATGCGGAAAGACAG | |||
| rorαa | TGAATTCGCCACCATGTCAGAGTCACATGGATTC | ENSDART00000148537 | cDNA cloning |
| AGGATCCCCCGTCAACGGGCATCGACTG | |||
| rorαb | ACTCGAGGCCACCATGTACTTAATGATCACAGC | ENSDART00000019140 | cDNA cloning |
| AGGATCCGGCCTCCTGAGGCAGAATCTG | |||
| aanat2 promoter | AGGTACCAGTAGCCTAGAGCGCCATCTG | ENSDART00000018205 | Promoter cloning |
| CCTCGAGCTCCTCGCCTTTAACCCTCC | |||
| bmal1b promoter | AGGTACCACATGTTGGGGAAACTGGAG | ENSDART00000098259 | Promoter cloning |
| TCTCGAGATGAAGAAGGCAGGGAAGCAC | |||
| per1b | AGGAAGGCTGACAGATGATGAATG | ENSDART00000011082 | qRT-PCR |
| CCAGAGTGGGCTAAAGCGAAGTA | |||
| per2 | ACGAGGACAAGCCAGAGGAACG | ENSDART00000148788 | qRT-PCR |
| GCACTGGCTGGTGATGGAGA | |||
| per3 | GTTCTGGCGGAGTAATGGAG | ENSDART00000024304 | qRT-PCR |
| TGACGACGTTTTACTGGTGC | |||
| cry1aa | GACGCACAGCAGATAACAGGACG | ENSDART00000034401 | qRT-PCR |
| CAAAATGTAACAACTCGGGAAAAGG | |||
| cry1ba | TCGCCAAGTGCATAATTGGA | ENSDART00000100222 | qRT-PCR |
| GTGTGTCTCCCGAGGAAGGA | |||
| cry1bb | TCTACCAACAACTGTCCCGCTAC | ENSDART00000125347 | qRT-PCR |
| GCCATCCCATTTCCATTCCC | |||
| bmal1b | CCCTCTAGCTGTGGCTCAAG | ENSDART00000098259 | qRT-PCR |
| TCCCGCCATTGGACATCTTT | |||
| aanat2 | AGGACGCCATCAGTGTGTTT | ENSDART00000018205 | qRT-PCR |
| CTGGCCCAGGAAAACAAGTA | |||
| β-Actin | ACGAACGACCAACCTAAACTCT | ENSDART00000054987 | qRT-PCR |
| TTAGACAACTACCTCCCTTTGC | |||
| Luciferase | GTGTCCGATTCAGTCATGCC | Transgenic fish identification | |
| CTGGTTGTTTCTGTCAGGCC |
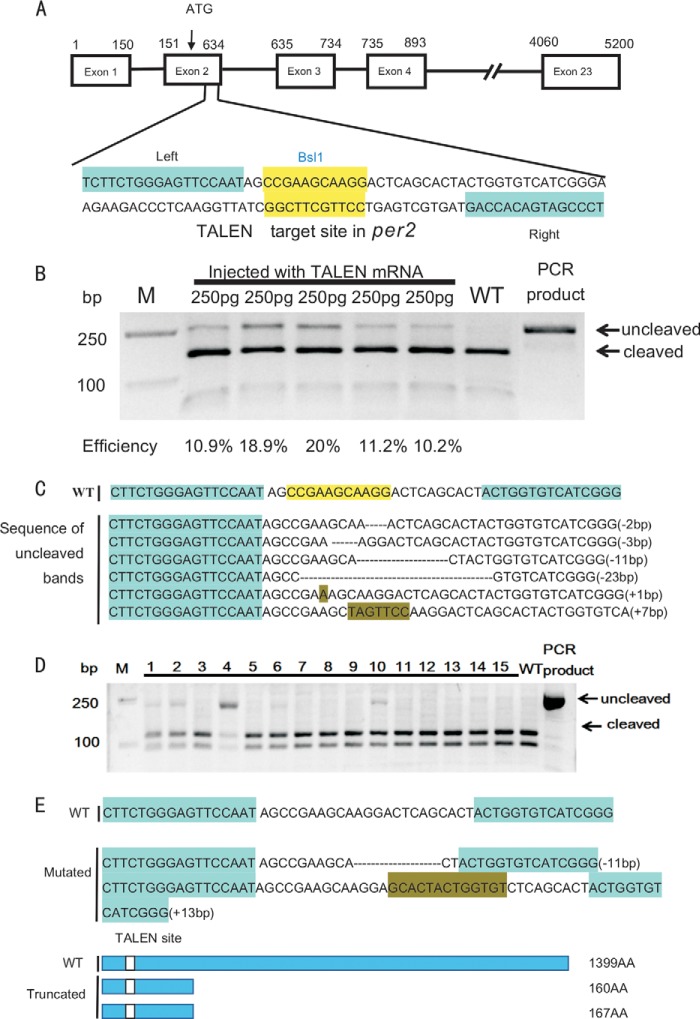
FIGURE 1.
Generation of zebrafish per2 mutant. A, the TALEN sites targeting the second exon of per2 gene. The left and right TALEN sites are highlighted in cyan, and the BslI site in the spacer in yellow. B, TALEN efficiencies shown by gel analysis. The targeted fragment was PCR-amplified from pooled genomic DNAs of five embryos microinjected with capped TALEN mRNAs at a concentration of 250 ng and then digested with BslI. The uncleaved and cleaved PCR products are indicated. Mutagenesis efficiencies were estimated by the ratios of intensities of uncleaved bands and the sum of cleaved bands and uncleaved bands quantified with NIH ImageJ software. WT, wild type; M, marker. C, types of indel mutations in the per2 TALEN target site shown by representative sequencing results of the uncleaved PCR fragments. D, screening of heritable mutants. F0 founder fish were out-crossed with wild-type fish to produce F1, and the DNAs extracted from F1 embryos were used for identifying mutant fish. Two of 15 fish were found to carry heritable mutations (lanes 4 and 10). E, two mutated fish lines. One has an 11-bp deletion, the other has a 13-bp insertion (upper), and both are frameshift mutations that result in truncated proteins (lower). AA, amino acids.
Behavioral Analysis for Zebrafish
Locomotor activity analysis was performed as described previously with some modifications (39). On the 4th day postfertilization, larvae were singly placed into each well of the 48-well plate. Locomotor activities of larvae were monitored and recorded for 4 consecutive days using an automated video tracking system (Videotrack, ViewPoint Life Sciences, Montreal, Canada) and analyzed with Zebralab3.10 software (ViewPoint Life Sciences). Locomotor activities were measured from day 5 to day 8 postfertilization as the total distance moved by one larva during 10-min time windows. The data are presented as a moving distance average for each group (n = 24). The period length of each larval locomotor trace was retrieved by a fit to a damped cosine curve using non-linear least square fitting with the CellulaRhythm R script (55). Statistical analysis of period length differences between the treatment groups was performed with one-way analysis of variance followed by Dunnett's posttest comparing each sample group with the control group (55).
RNA Extraction and Quantitative Real Time PCR (qRT-PCR)
Total RNAs were extracted from ∼30 larvae of homozygous per2 or wild type at 4-h intervals from 120 to 148 h postfertilization under LD or DD conditions and from adult organs including the brain, muscle, heart, and liver under LD using TRIzol (Invitrogen) reagent, respectively. qRT-PCR was performed in an ABI StepOnePlus instrument with the SYBR Green detection system (Invitrogen). PCR thermal profiles were 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Experiments were performed in triplicates, each with at least two different biological samples for corresponding genotypes and developmental stages. All results were normalized to the expression level of the housekeeping gene β-actin. qRT-PCR results are shown as a relative expression level calculated using the 2−ΔΔCT method. p values were analyzed with one-way analysis of variance test or Student's t test. All primers used are listed in Table 1.
DNA Constructs
A 405-bp fragment of the zebrafish aanat2 promoter region containing one E′-box (−76 bp) and a 1,700-bp fragment of zebrafish bmal1b promoter region containing two RORE boxes (−22 and +18 bp) were isolated and cloned into the luciferase reporter pGL4.17 vector (Promega). The resulting DNA constructs were named as aanat2-luc and bmal1b-luc, respectively. Full-length cDNAs of zebrafish bmal1b and clock1a have been cloned into pcDNA3.1 previously (56). Full-length cDNAs of zebrafish per2, rev-erbα, rorαa, and rorαb genes were PCR-amplified from zebrafish embryonic/larval cDNAs and cloned into the pCMV-HA vector or the pcDNA3.1-Myc/His expression vector, respectively. The stop codon TAA sequence at the 3′-ends of each cDNA was removed to fuse with the Myc/His tag in the pcDNA3.1-Myc/His vector. All DNA constructs were confirmed by DNA sequencing. The primers used are listed in Table 1.
DNA Site-directed Mutagenesis
The mutagenized DNA vectors were constructed by PCR-based site-directed mutagenesis. PCR was performed with KOD Plus DNA polymerase (Toyobo). DpnI (New England Biolabs) restriction enzyme-treated PCR products were transformed into Escherichia coli. Positive clones were selected and verified by sequencing.
Cell Transfection and Luciferase Reporter Assays
Human embryonic kidney (HEK) 293T cells were used for cell transfection assays. HEK 293T cells were cultured in DMEM containing 10% serum and penicillin-streptomycin in 24-well plates. Transfection was done with Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. Reporter gene assays were performed with the Dual-Luciferase reporter assay system (Promega) using 100 ng each for bmal1b-luc, rorαa, rorαb, and rev-erbα and 200 ng for per2. 2 ng of pRL-TK vector was added for control, and pCDNA3.1-Myc/His was added to bring to the same amount. Each experiment was conducted in triplicate.
Co-immunoprecipitation Assay and Western Blotting
One day before transfection, HEK 293T cells were seeded in 10-cm Petri dishes. Thirty-four hours following transfection, cells were lysed in radioimmune precipitation assay buffer with protease inhibitor (Sigma). Lysates were released with protein G-Sepharose beads (GE Healthcare) and then incubated with rabbit polyclonal anti-HA antibodies (Protech) or anti-His antibodies (Protech). After washing five times, the precipitates were resuspended in SDS-PAGE sample buffer, boiled for 3 min, and resolved by 8% SDS-PAGE followed by Western blot analysis using mouse monoclonal anti-His antibody or anti-HA antibody (Protech). Immunoreactive bands were detected by ECL reagents (Biological Industries).
Generation of bmal1b-luc Transgenic Fish and in Vivo Measurement of Bioluminescence Rhythms
The vector bmal1b-luc was linearized by KpnI digestion and microinjected into one- to two-cell zebrafish embryos. Injected embryos were raised to adulthood and individually bred to wild-type fish or pairwise bred to each other. Transgenic progeny were identified by PCR using a pair of primers (Table 1). Transgenic embryos or larval fish were placed individually in a well of a 96-well with 200 μl of Holtfreter solution (7.0 g of NaCl, 0.4 g of sodium bicarbonate, 0.2 g of CaCl2, and 0.1 g of KCl (pH 7.0) in 2 liters of double distilled H2O) aerated overnight and containing 0.5 mm d-luciferin potassium salt (BBI). The monitoring of bioluminescence was performed with a Luminoskan Ascent microplate luminometer (Thermo), and data analysis was performed according to the protocol described previously (55).
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed according to the manufacturer's protocol (Millipore's ChIP assay kit). Briefly, a group of 200 capped per2 mRNA-injected larvae and control injected larvae at 5 days postfertilization was collected and cross-linked in 2% formaldehyde at room temperature for 30 min, and then a volume of 1.25 m glycine was added to stop cross-linking followed by PBS washes (three times, each for 10 min). We used purified rabbit or mouse IgG (Invitrogen) as a negative control. ChIP PCRs were performed using primers flanking the E-boxes or RORE sites as well as primers not flanking the E-boxes or the RORE sites in the promoter regions of annat2 or bmal1b as controls. Primers used for the ChIP PCR are listed in Table 1.
Statistical Analysis
Groups of data are presented as mean ± S.E. We performed statistical analyses with analysis of variance or the unpaired two-tailed Student's t test. All statistical analyses were performed using SPSS 16.0 software, and p < 0.05 was regarded as a statistically significant difference.
RESULTS
Generation of Zebrafish per2 Mutants
Using the software TALE-NT (53), we selected a TALEN pair targeting the second exon (containing the start codon ATG) of zebrafish per2, and within the targeted 57-bp fragment, there is a BslI restriction site for evaluating mutagenesis efficiency and subsequent mutant identification (Fig. 1A). We then used the “unit assembly” method (54) to construct the two arms of the per2 TALEN. The capped mRNAs of the two TALEN arms were microinjected into one-cell embryos at a concentration of 250 pg. To evaluate the mutagenesis efficiency, a 259-bp genomic DNA fragment containing the target site was PCR-amplified from five groups of injected embryos and a control group of embryos (five embryos each). Enzymatic digestion of the PCR-amplified fragments with BslI showed that the efficiencies of the five groups are 10.5, 18.9, 20, 11.2, and 10.2%, respectively, with an average of 14.2% (Fig. 1B). We cloned the uncleaved PCR fragments into the sequencing vector pMD-19T. Single clone sequencing revealed six different types of indel mutations in the per2 TALEN target site in F0 (Fig. 1C). The siblings of these microinjected F0 embryos were raised to adulthood. Out-crossing F0 fish with wild-type fish produced F1 embryos. Two of 15 fish examined with PCR amplification and BslI digestion of DNAs extracted from their F1 embryos were found to carry heritable mutations (Fig. 1D). DNA sequencing showed that one fish carried a 11-bp deletion and the other carried a 13-bp insertion, which both result in frameshift mutations: the 11-bp deletion mutation might encode a truncated protein with only 160 amino acids, and the 13-bp insertion mutation might encode a truncated protein with only 167 amino acids (Fig. 1E). The homozygous mutant fish with the 11-bp deletion were used primarily for all subsequent experiments.
Disrupted Rhythmicity of Locomotor Activities and Altered Expression of Key Circadian Clock Genes and a Circadian Clock-controlled Gene in per2 Mutant Zebrafish
Locomotor activities of zebrafish larvae exhibit robust circadian rhythmicity and peak during the subjective day (57). To determine whether Per2 affects locomotor activity rhythms, behavior analyses were performed for per2 mutant and wild-type larvae starting at 96 h postfertilization under LD and DD conditions. Under the LD condition, the locomotor activities of per2 mutant larvae were significantly reduced compared with wild types (Fig. 2A); specifically there was an ∼30% reduction in the total moving distance during the 3 days examined (Fig. 2B). Under the DD condition, per2 mutant larvae displayed an approximately 2-h phase delay (Fig. 2C) and an ∼1.1-h lengthened periodicity (Fig. 2D). These results indicate that the locomotor activity rhythms are disrupted in per2 knock-out fish.
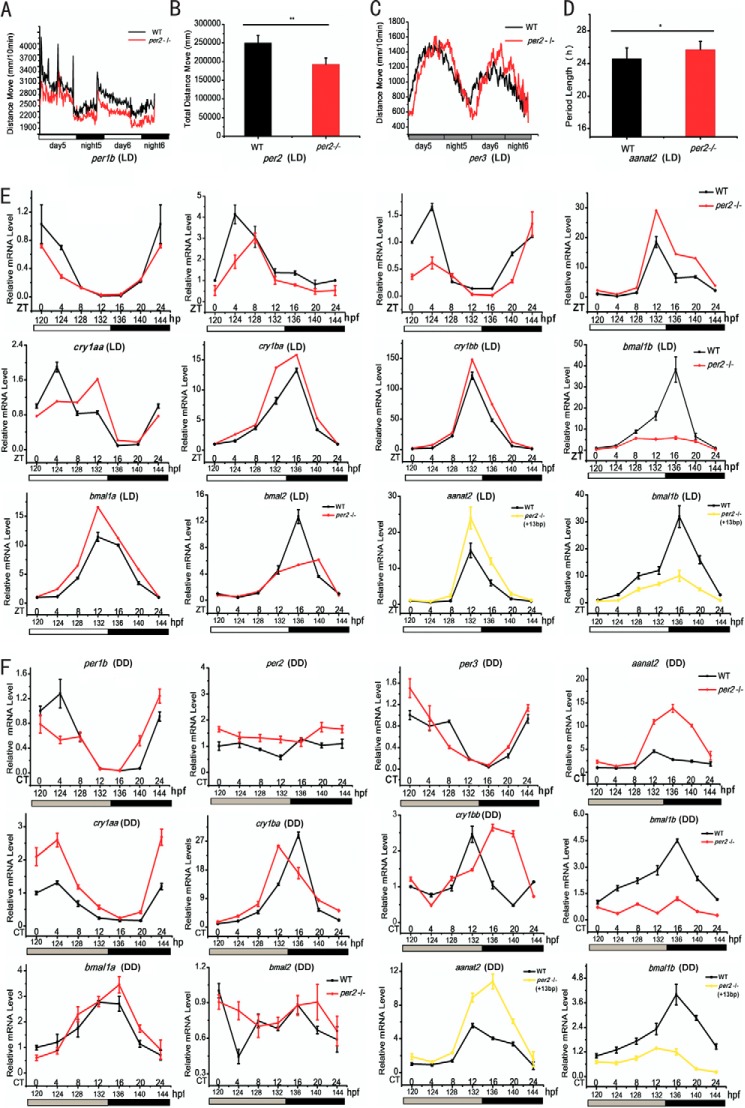
FIGURE 2.
Disrupted rhythms of locomotor activities and expression of key circadian clock genes as well as a circadian clock-controlled gene in homozygous per2 mutant zebrafish. Under the LD condition, per2 mutant larvae display lower activity amplitude than wild types as shown by the line chart (A) and histogram of the total moving distances during the 3 days (B). Under the DD condition, per2 mutant fish show a 2-h phase delay in comparison with wild types as shown by the line chart (C) and histogram of the average period during the 3 days (D). Behavioral assays were done with an automated video tracking system (Videotrack) and analyzed with Zebralab3.10 software (see “Experimental Procedures” for details). E and F, disrupted expression of key circadian clock genes and a circadian clock-controlled gene in per2 mutant fish. Shown are qRT-PCR analyses of key circadian clock genes (per1b, per2, per3, cry1aa, cry1ba, cry1bb, bmal1a, bmal1b, and bmal2) and a circadian clock-controlled gene (aanat2) in wild-type and per2 mutant fish under LD (E) and DD conditions (F). WT is shown in black, 11-bp deletion mutant is shown in red, and 13-bp insertion mutant is shown in yellow. RNA extraction was done with TRIzol, and qRT-PCR experiments were performed with an ABI StepOnePlus instrument and the SYBR Green detection system (see “Experimental Procedures” for details). *, p < 0.05; **, p < 0.01. Error bars represent S.E.
We also examined expression of key circadian clock genes and a circadian clock-controlled gene in per2 mutant fish. Zebrafish possess four per genes, per1a and per1b (co-orthologs of mammalian Per1) and per2 and per3 (single orthologs of mammalian Per2 and Per3) (58). Under the LD condition, per2 exhibited robust oscillation in wild types but still oscillates with much damped amplitude in per2 mutant zebrafish, whereas under the DD condition, per2 became arrhythmic in both wild-type and per2 mutant zebrafish (Fig. 2, E and F). Compared with wild types, per1b and per3 were significantly down-regulated in per2 mutant fish under both LD and DD conditions (Fig. 2, E and F), suggesting that Per2 plays a positive role in regulation of these two zebrafish per genes. Similar to per genes, cry genes are negative regulators in the transcription/translational feedback loop (59). Although mice have two Cry genes (59), zebrafish have six cry genes (31, 56, 60, 61). Under both LD and DD, cry1aa, cry1ba, and cry1bb were primarily up-regulated in per2 mutant fish (Fig. 2, E and F), suggesting that Per2 plays a repressive role in regulation of these cry genes. Intriguingly, bmalb gene, one of the two co-orthologs of mouse Bmal1 (62), was down-regulated in per2 mutant under both LD and DD conditions (Fig. 2, E and F), suggesting that Per2 plays a positive role in bmalb expression. The other two bmal genes (bmal1a and bmal2) also had disrupted expression patterns in per2 mutant under both LD and DD conditions (Fig. 2, E and F).
Melatonin plays an important role in the endogenous circadian clock system in vertebrates (63). Melatonin rhythms are generated by the oscillating rate-limiting enzyme aralkylamine N-acetyltransferase (AANAT) in the pineal gland (47, 64). Mammals have only a single Aanat gene that is expressed in both the pineal gland and the retina (65). In zebrafish, there are two copies of aanat genes, aanat1 and aanat2. Although aanat1 is expressed only in the retina, aanat2 is expressed in the pineal gland and in the retina at relatively lower levels (66). aanat2 was significantly up-regulated under both LD and DD conditions in per2 mutant fish (Fig. 2, E and F), suggesting that Per2 plays a repressive role in regulation of aanat2.
We also characterized another null per2 mutant line carrying the 13-bp insertion and found that it displays a similar behavioral phenotype (data not shown) and altered gene expression (Fig. 2, E and F) like the 11-bp deletion mutant line. These results demonstrate that like mammalian Per2 zebrafish per2 is also essential for the zebrafish circadian clock.
Dual Roles of Per2 Are Mediated by E-boxes and RORE Boxes in Zebrafish
Our qRT-PCR analysis showed that aanat2 is up-regulated but bmal1b is down-regulated in per2 mutant fish (Fig. 2, E and F), indicating that Per2 represses aanat2 expression but enhances bmal1b expression in zebrafish. To investigate the role of Per2 in zebrafish aanat2 expression, a 405-bp aanat2 promoter containing one E-box was isolated and cloned into the pGL4.17 vector (Fig. 3A). The full-length cDNAs of per2, bmal1b, and clock1a were cloned into the pcDNA3.1-Myc/His vector, respectively. In vitro cell transfection showed that aanat2 is significantly up-regulated by a combination of Clock1a and Bmal1b but repressed by Per2, and Per2 could no longer inhibit aanat2 when the E-box in the aanat2 promoter was mutated (Fig. 3C). In addition, ChIP assays showed that Per2 can bind to the E-box in the aanat2 promoter (Fig. 4E). These results demonstrate that zebrafish Per2 can repress the gene expression through the E-box elements, which is consistent with mouse PER2 function (67).
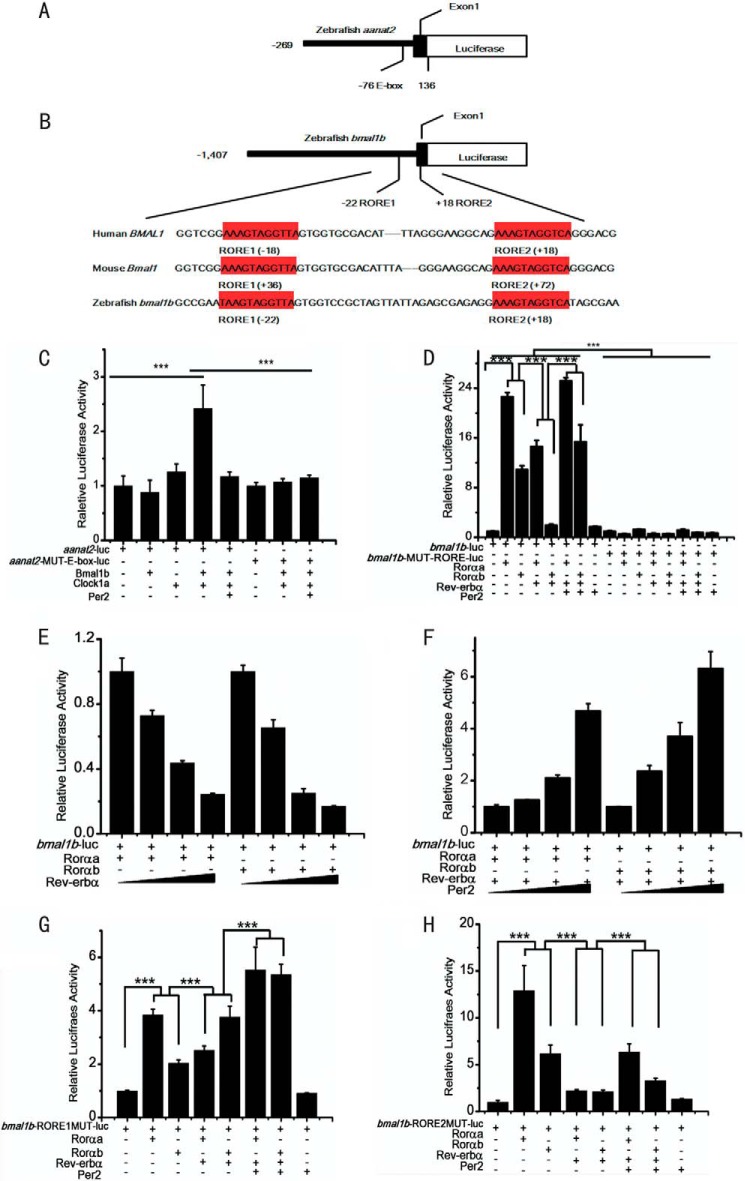
FIGURE 3.
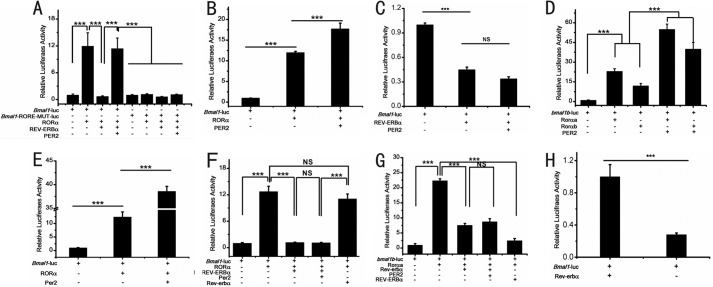
Dual roles of zebrafish Per2 are mediated by E-boxes and RORE boxes. A, schematic of the DNA construct aanat2-luc wherein the aanat2 promoter contains one E-box. B, schematic of the DNA construct bmal1b-luc wherein the bmal1b promoter harbors the two RORE motifs (in red), which are identical in human, mouse, and zebrafish. C, Per2 inhibits the Clock1a-Bmal1b heterodimer-mediated aanat2 transcription through E-box. The activities of the E-box containing aanat2 promoter are activated by co-transfection of Clock1a and Bmal1b but repressed by Per2. The effects of both activation and repression are lost without the E-box. D, Per2 enhances Rorαa- or Rorαb-mediated bmal1b expression. Both Rorαa and Rorαb can activate bmal1b transcription. The activation efficacy of Rorαa is ∼2-fold that of Rorαb. Rev-erbα outcompetes Rorαa or Rorαb to repress bmal1b expression. Per2 reverses the inhibitory effects of Rev-erbα to enhance bmal1b expression. All effects are lost without the two RORE motifs. E, zebrafish Rev-erbα outcompetes Rorα to repress bmal1b in a dose-dependent manner. F, zebrafish enhances bmal1b expression in a dose-dependent manner. G, the inhibitory effect of Rev-erbα was not significant when RORE1 was mutated. H, the inhibitory effect of Rev-erbα still persisted when RORE2 was mutated. The Dual-Luciferase reporter system was used in the experiments. ***, p < 0.001, unpaired two-tailed Student's t test. Error bars represent S.E.
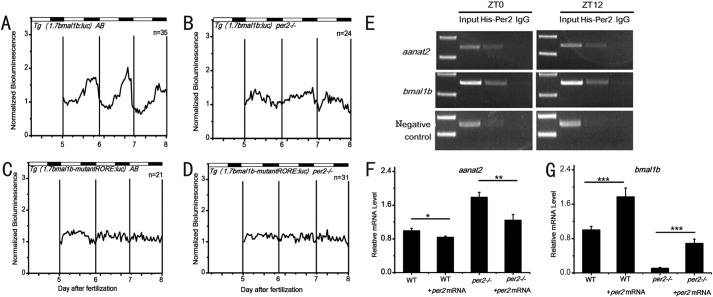
FIGURE 4.
Enhancement of bmal1b expression by Per2 in vivo. A, strong rhythmic expression of bioluminescence driven by the bmal1b promoter in the wild-type fish. B, weak but still rhythmic expression of bioluminescence driven by the bmal1b promoter in per2 mutant fish. C, no rhythmic expression of bioluminescence driven by the bmal1b promoter with two mutated RORE motifs in the wild-type fish. D, no rhythmic expression of bioluminescence driven by the bmal1b promoter with two mutated RORE motifs in per2 mutant fish. We generated four stable transgenic zebrafish lines, normal bmal1b-luc in wild-type fish, normal bmal1b-luc in per2 mutant fish, mutated bmal1b-luc in wild-type fish, and mutated bmal1b-luc in per2 mutant fish. The experiments were done with stable F1 transgenic fish lines. E, Per2 binds to the RORE motifs in the bmalb promoter and the E-box in the aanat2 promoter as shown by ChIP assays. Capped per2 mRNAs were microinjected into one-cell embryos, and ChIP assays were done with larvae at 96 (ZT0) and 108 h postfertilization (ZT12). F and G, rescue of annat2 and bmal1b expression by capped wild-type per2 mRNAs. Microinjection of capped wild-type per2 mRNAs rescues expression of annat2 (F) and bmal1b (G) in per2 mutant fish. Quantitative RT-PCR analysis was done with microinjected per2 mutant larvae and control larvae at 108 h postfertilization (ZT12). *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars represent S.E.
Similar to the two RORE boxes in the mouse Bmal1 promoter (68), we also identified the two RORE motifs in the zebrafish bmal1b promoter region (Fig. 3B). To determine the role of Per2 in zebrafish bmal1b expression, a 1.7-kb promoter of bmal1b harboring the two RORE motifs was isolated and cloned into the pGL4.17 vector. Due to the third round of teleost genome duplication (58, 62, 69), zebrafish have two copies of rorα genes, rorαa and rorαb (70). The full-length cDNAs of rev-erbα, rorαa, and rorαb were PCR-amplified and cloned into the pcDNA3.1-Myc/His vector, respectively. Cell transfection assays showed that these two rorα genes can significantly activate bmal1b expression, and in particular, the activation activity of rorαa is ∼2-fold higher than that of rorαb (Fig. 3D). In addition, Rev-erbα can outcompete Rorαa or Rorαb to inhibit bmal1b expression in a dosage-dependent manner (Fig. 4E). These results demonstrate that like what happens in mammals Rorα activates bmal1b expression, whereas Rev-erbα represses it in zebrafish.
To determine how Per2 regulates bmal1b expression, we also conducted co-transfection experiments with per2, rorαa, rorαb, or rev-erbα. Results showed that Per2 can enhance bmal1b expression via RORE motifs (Fig. 3D), and the enhancing effects of Per2 on bmal1b expression are also dosage-dependent (Fig. 3F). To examine the roles of the two RORE motifs in bmal1b expression, we mutagenized them in the bmal1b promoter. Luciferase assays showed that the inhibitory effect of Rev-erbα, the activating effect of Rorαa, and the enhancing effect of Per2 on bmal1b expression are all abolished (Fig. 3D) without the two RORE motifs (Fig. 3D). These results indicated that the two RORE motifs are required for Per2 to enhance bmal1b expression in zebrafish (Fig. 3D).
To further distinguish the roles of these two RORE motifs in bmal1b expression, we mutagenized them individually. Results showed that although both the individually mutated RORE motifs can result in the reduction of Rorα-mediated bmal1b expression RORE1 appears to be especially important for Rorα-mediated activation, whereas RORE2 is necessary for enhancement of the level of activation (Fig. 3, G and F).
Per2 Enhances bmal1b Expression in Vivo
To examine the positive role of Per2 in regulating bmal1b in vivo, we generated four stable transgenic zebrafish lines including 1) wild-type Tg (1.7bmal1b-luc) AB wherein luciferase is driven by the normal 1.7-kb bmal1b promoter in wild-type fish, 2) wild-type Tg (1.7bmal1b-ROREmut-luc) AB wherein luciferase is driven by the 1.7-kb bmal1b promoter with the two mutated RORE motifs in wild-type fish, 3) per2 mutant Tg (1.7bmal1b-luc) per2−/− wherein luciferase is driven by the normal 1.7-kb bmal1b promoter in per2 mutant fish, and 4) per2 mutant Tg (1.7bmal1b-ROREmut-luc) per2−/− wherein luciferase is driven by the 1.7-kb bmal1b promoter with the mutated RORE motifs in per2 mutant fish. Bioluminescence assays showed that although in wild-type Tg (1.7bmal1b-luc) AB transgenic fish bmal1b displays robust oscillation and in per2 mutant Tg (1.7bmal1b-luc) per2−/− transgenic fish bmal1b exhibits much dampened oscillation (Fig. 4, A and B) in both wild-type Tg (1.7bmal1b-ROREmut-luc) AB and per2 mutant Tg (1.7bmal1b-ROREmut:luc) per2−/− transgenic fish bmal1b shows no oscillation (Fig. 4, C and D). These results indicate that Per2 and RORE motifs are crucial for bmal1b rhythmic expression in vivo.
To determine whether Per2 binds to the RORE motifs in the bmal1b promoter region, capped mRNAs of per2 were microinjected into one-cell embryos for ChIP assays. The results showed that Per2 can bind to RORE motifs in the bmal1b promoter at ZT0 and ZT12 in vivo (Fig. 4E), demonstrating that the enhancing effect of Per2 on bmal1b expression depends upon the RORE motifs in the bmal1b promoter.
We also performed rescue experiments by microinjecting capped wild-type per2 mRNAs into per2 mutant embryos. qRT-PCR results showed that wild-type per2 mRNAs indeed can down-regulate aanat2 but up-regulate bmal1b in per2 mutant fish (Fig. 4, F and G), indicating that the TALEN-induced mutation is responsible for its phenotypes.
Per2 Enhances bmal1b Expression through Rorα Rather than Rev-erbα in Zebrafish
To determine whether Per2 enhances bmal1b expression through Rorαa, Rorαb, or Rev-erbα in zebrafish, we conducted cell transfection experiments. The results showed that Per2 alone cannot reverse the inhibitory effect of Rev-erbα on bmal1b expression in the absence of Rorα (Fig. 5A) and that Per2 can enhance Rorαa- or Rorαb-mediated bmal1b expression (Fig. 5B). We also performed co-immunoprecipitation (co-IP) assays. The results showed that Per2 can directly bind to Rorαa rather than to Rev-erbα (Fig. 4, C and D). Taken together, these results showed that Per2 can enhance bmal1b expression through Rorαa or Rorαb rather than through Rev-erbα in zebrafish.
FIGURE 5.
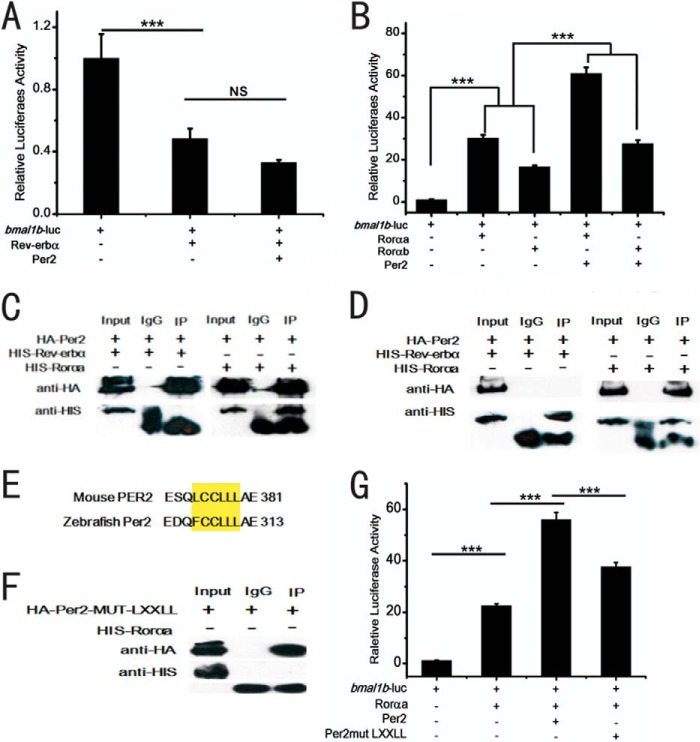
The positive role of zebrafish Per2 in regulating bmal1b through its binding to Rorα rather than Rev-erbα. A, zebrafish Per2 alone cannot reverse the inhibitory effect of Rev-erbα on bmal1b without Rorαa or Rorαb. B, Per2 enhances Rorαa- or Rorαb-mediated bmal1b transcription. C and D, Per2 binds to Rorαa rather than Rev-erbα in zebrafish as shown by co-IP assays. C, the left panel shows that HA-tagged Per2 could not pull down His-tagged Rev-erbα, and the right panel shows that HA-tagged Per2 did pull down His-tagged Rorαa. D, the left panel shows that His-tagged Rev-erbα could not pull down HA-tagged Per2, and the right panel shows that His-tagged Rorαa did pull down HA-tagged Per2. E, the conserved protein-protein interaction motif LXXLL (yellow) between zebrafish Per2 and mouse PER2. F, Per2 with mutated LXXLL motif cannot bind to Rorαa as shown by co-IP assays. G, Per2 with mutated LXXLL motif cannot enhance Rorαa-mediated transcription of bmal1b as shown by in vitro cell transfection. ***, p < 0.001; NS, not significant. Error bars represent S.E.
In mice, the N-terminal LXXLL motif of PER2 was shown to be able to bind to REV-ERBα (24). We observed that zebrafish Per2 also possesses the same LXXLL motif at its N terminus (Fig. 5E). Does zebrafish Per2 bind to Rorαa through its N-terminal LXXLL motif? To address this question, we mutagenized the Per2 LXXLL motif and performed co-IP and transfection assays. The results showed that Per2 with the mutated LXXLL motif neither binds to Rorαa (Fig. 5F) nor enhances Rorαa-mediated bmal1b transcription (Fig. 5G), further supporting the notion that the positive role of zebrafish Per2 in bmal1b expression is fulfilled through its binding to Rorαa rather than to Rev-erbα.
Enhancement of Bmal1 Expression by PER2 Is Evolutionally Conserved
Our results showed that Per2 enhances bmal1b expression through binding to Rorα in zebrafish. Although previous studies have shown that Bmal1 expression also was down-regulated in the Per2 knock-out mouse (21, 40, 43), PER2 directly interacts with the REV-ERBα in mice, and overexpression of Per2 can increase Bmal1 expression in cultured cells (24), the mechanisms underlying how mouse PER2 regulates BMAL1 have not been carefully examined. To delineate how PER2 enhances Bmal1 expression in mice, we performed cell transfection experiments. Indeed, the results showed that mouse RORα can activate Bmal1 expression, whereas mouse REV-ERBα can repress RORα-mediated Bmal1 expression, and PER2 can enhance RORα-mediated Bmal1 expression (Fig. 6A). In addition, in the case of the mutated RORE motifs, all these effects were abolished (Fig. 6A). Moreover, PER2 can significantly enhance Bmal1 transcription with the presence of RORα (Fig. 6B); however, PER2 cannot reverse the inhibitory effect of REV-ERBα on Bmal1 transcription without RORα (Fig. 6C), suggesting that although PER2 interacts with REV-ERBα the enhancing activity of PER2 on Bmal1 expression is still mediated by RORα in mice, which is different from the notion that mouse PER2 binds to REV-ERBα to enhance Bmal1 expression as implicated previously (24).
FIGURE 6.
Enhancement of bmal1b expression by Per2 is evolutionally conserved between zebrafish and mice. A, roles of RORα, REV-ERBα, PER2, and RORE in Bmal1 transcription. RORα activates Bmal1 transcription, whereas REV-ERBα competes with RORα to repress Bmal1 expression. Mouse PER2 reverses the inhibitory effect of REV-ERBα on RORα-mediated Bmal1 transcription. All effects are lost without the two RORE motifs. B, mouse PER2 alone cannot reverse the repressive effect of REV-ERBα on Bmal1 expression. C, mouse PER2 enhances RORα-mediated Bmal1 transcription with the presence of RORα. D, mouse PER2 enhances zebrafish Rorαa- or zebrafish Rorαb-mediated zebrafish bmal1b transcription. E, zebrafish Per2 enhances mouse RORα-mediated mouse Bmal1 transcription. F, zebrafish Per2 cannot reverse the inhibitory effect of mouse REV-ERBα on RORα-mediated Bmal1 transcription. Zebrafish Rev-erbα cannot outcompete mouse RORα nor can zebrafish Rev-erbα repress mouse Bmal1 transcription. G, mouse PER2 cannot reverse the inhibitory effect of zebrafish Rev-erbα on Rorαa-mediated bmal1b transcription; however, mouse REV-ERBα can outcompete zebrafish Rorαa to repress zebrafish bmal1b transcription. H, cross-transfection experiments showing that zebrafish Rev-erbα significantly inhibits mouse Bmal1 expression without the presence of RORα. ***, p < 0.001; NS, not significant. Error bars represent S.E.
To elucidate whether the roles of zebrafish Per2/mouse PER2 in regulation of bmal1b or Bmal1 are conserved between mice and zebrafish, zebrafish Per2 was co-transfected with mouse Bmal1-luc and RORα. The results showed that mouse PER2 can enhance zebrafish Rorαa- or Rorαb-mediated bmal1b expression (Fig. 6D). Similarly, zebrafish Per2 can enhance mouse RORα-mediated Bmal1 expression (Fig. 6E). These results supported the conservative function of the Per2/PER2 proteins in enhancing expression of bmal1b/Bmal1 in zebrafish and mice.
We also examined the role of Rev-erbα in this system by co-transfection experiments. The results showed that neither can zebrafish Per2 enhance mouse RORα-mediated mouse Bmal1 expression nor can mouse PER2 enhance zebrafish Rorα-mediated zebrafish bmal1b expression (Fig. 6, F and G). The cell transfection experiments also showed that even though zebrafish Rev-erbα can significantly inhibit mouse Bmal1 expression (Fig. 6H) without the presence of RORα and mouse REV-ERBα also can significantly inhibit zebrafish bmal1b expression without the presence of Rorα (data not show) zebrafish Rev-erbα cannot outcompete mouse RORα to repress Bmal1 expression. However, the mouse REV-ERBα can outcompete zebrafish Rorαa or Rorαb to repress bmal1b expression (Fig. 6, F and H), suggesting that mouse REV-ERBα has evolved stronger inhibitory abilities than zebrafish Rev-erbα.
Tissue-specific Circadian Regulation by Zebrafish Per2
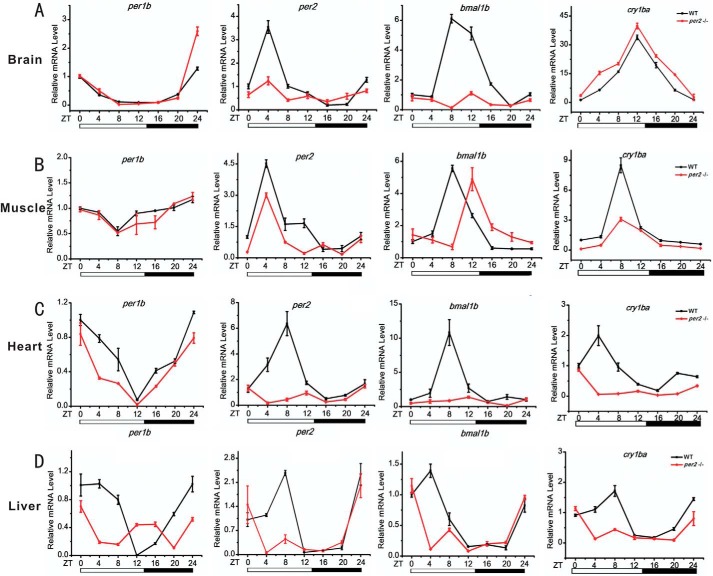
Zebrafish per2 is expressed extensively in numerous tissues/organs (32, 71). To investigate the per2 functions in different tissues/organs, we compared expression patterns of several canonical circadian clock genes including per2 itself in different organs of wild-type and per2 mutant zebrafish including the brain, muscle, heart, and liver. Results showed marked down-regulation of per1b and per2 in all four organs of the per2 mutant fish (Fig. 7). In contrast, the expression of cry1ba was significantly up-regulated in the brain but down-regulated in the muscle, heart, and liver of per2 mutant fish (Fig. 7). The expression of bmal1b exhibited a significant phase delay in the muscle and was down-regulated in the brain, heart, and liver of per2 mutant fish. Intriguingly, the four circadian clock genes per1b, per2, cry1ba, and bmal1b were all down-regulated in the heart and liver of per2 mutant fish (Fig. 7, C and D), implicating that Per2 is critical for maintaining circadian regulation in the heart and liver and in turn impacts functions of these two important peripheral organs. These results showed that although different circadian clock genes display distinct rhythmic expression patterns in the same organ the same circadian clock gene exhibits distinct rhythmic expression patterns in a different organ, and these genes were all significantly disrupted in the per2 mutant fish (Fig. 7), thereby implicating that Per2 may play distinct regulatory roles in different zebrafish peripheral organs/tissues.
FIGURE 7.
Tissue-specific regulatory roles of Per2 in zebrafish peripheral tissues/organs. Wild-type and per2 mutant fish were sacrificed at 120 days postfertilization, and the organs/tissues of the brain, muscle, heart, and liver were dissected out and collected for RNA extraction at 4-h intervals under LD for a total of consecutive 24 h. Each sample contains tissues/organs from at least two female fish and two male fish. Shown is qRT-PCR analysis of expression levels of four circadian clock genes (per1b, per2, cry1ba, and bmal1b) in the brain (A), muscle (B), heart (C), and liver (D) of wild-type and per2 mutant adult fish. Data represent mean ± S.E. (error bars) (n = 2–3).
DISCUSSION
Per2 Is Essential for the Zebrafish Circadian Clock
Zebrafish have recently figured as an excellent circadian model and contributed to our understanding of vertebrate circadian rhythmicity (30–32). Genetic analysis of the zebrafish circadian clock, however, has lagged behind largely due to difficulties in obtaining stable genetic circadian mutants (14, 31, 72). Here we used TALEN, a fast and convenient genome-editing tool, to generate null mutants for zebrafish per2. Analysis of per2 mutant zebrafish helps us to ascertain the roles of Per2 in the zebrafish circadian clock. Per2−/− knock-out mice display a short circadian period and arrhythmicity under DD (21), and a human PER2 mutation results in ∼4-h phase advance under LD (45). Our per2 mutant zebrafish displayed reduced activities under LD (Fig. 2, A and B) and a 2-h phase delay and a 1.1-h prolonged period under DD (Fig. 2, C and D), which are completely different from the phenotypes of the zebrafish per1b insertional null mutant that displays hyperactivities under LD and a 2-h phase advance under DD (73). Hence, like mammalian Per2, zebrafish per2 also is critical for maintaining fish locomotor rhythmicity. The fact that both zebrafish per2 and per1b mutant fish still are not completely arrhythmic under DD strongly suggests that zebrafish per genes resemble mouse Per genes in that they are partially redundant and can compensate for the other's loss for maintaining locomotor rhythmicity (74). However, the intricate roles of Per2 in regulating activity rhythms differ between fish and mammals and likely represent different stages of Per2 functions during evolution from fish to mammals.
We also found that rhythmic expression patterns as well as phases of key circadian genes are disrupted in per2 mutant fish (Fig. 2, E and F). For instance, both per1b and per3 were significantly down-regulated in per2 mutant fish under both LD and DD, suggesting that Per2 plays a positive role in regulating these two per genes (Fig. 2, E and F), which diametrically differs from the repressive role of Per1b in regulating other per genes; i.e. all three other per genes are significantly up-regulated in the per1b-null mutant fish (73). Although Per1 knock-out mice have no effects on rhythmic expression of Per1 or Per2, both Per1 and Per2 are significantly down-regulated in Per2 knock-out mice (21, 40). Thus both zebrafish Per2 and mouse PER2 share the conservative function of positively regulating per genes. Together, the disrupted locomotor behaviors and altered expression of circadian clock genes in per2 mutant indicate that per2 is an essential component in the zebrafish circadian clock.
Dual Roles of Per2 in the Zebrafish Circadian Clock
In the Drosophila negative feedback loop, per and tim act as negative regulators (75). Similarly, it has long been thought that mammalian Per1, Per2, Cry1, and Cry2 proteins as negative factors form heterodimers to repress CLOCK-BMAL1-mediated transcription (2, 59, 76). However, there is evidence that PER2 might also play a positive role in mammalian circadian regulation (21, 40, 77). Although PER2 is not a transcriptional factor, it can directly bind to numerous nuclear receptors to control mammalian physiological processes (24, 78). Our results showed that zebrafish Per2 not only represses aanat2 expression through E-box but also enhances bmal1b expression through RORE. The repressive role of Per2 is in line with the traditional notion that Per2 and Cry form a heterodimer that represses Clock-Bmal heterodimer-mediated transcription (Fig. 3C) (2, 59), whereas the enhancing role of Per2 is fulfilled by its binding to Rorα nuclear receptor (Fig. 3D).
The Positive Role of Zebrafish Per2 and Mouse PER2 in Circadian Regulation Is Fulfilled through Mediation of RORα or Rorα
Even though previous studies have shown that mouse PER2 can increase Bmal1expression (21, 24, 43), the exact mechanisms are not clear. Our detailed cell transfection assays showed that although mouse PER2 binds to REV-ERBα (24) it still requires RORα mediation to enhance BMAL1 expression (Fig. 6, A–C). Our studies also showed down-regulation of RORE-containing bmal1b in per2 mutant zebrafish (Fig. 2, E and F), and zebrafish Per2 positively regulated bmal1b expression through RORE (Fig. 3D). In particular, co-IP experiments showed that Per2 directly interacts with Rorαa through the LXXLL motif conserved between zebrafish Per2 and mouse PER2 (Fig. 5E).
The roles of RORα, REV-ERBα, and PER2 in regulation of Bmal1 are highly conserved between zebrafish and mice but with a difference. Although zebrafish Rorαa/Rorαb or mouse RORα can bind RORE to activate bmal1b or Bmal1 expression (Fig. 8, A, panel 1, and B, panel 1), zebrafish Rev-erbα or mouse REV-ERBα can outcompete Rorα or RORα to bind to RORE to repress bmal1b or Bmal1 expression (Fig. 8, A, panel 2, and B, panel 2), and zebrafish Per2 or mouse PER2 alone cannot reverse the repressive effects of Rev-erbα or REV-ERBα on bmal1b or Bmal1 expression without the presence of zebrafish Rorα or mouse RORα (Fig. 8, A, panel 3, and B, panel 3), zebrafish Per2 or mouse PER2 alone can significantly enhance Rorα-mediated bmal1b expression or RORα-mediated Bmal1 expression without the presence of Rev-erbα or REV-ERBα (Fig. 8, A, panel 4, and B, panel 4), which are all conserved in zebrafish and mice. The difference is that zebrafish Per2 binds to Rorα rather than Rev-erbα to enhance bmal1b expression (Fig 8A, panel 4), whereas mouse PER2 enhances Bmal1 expression still through RORα mediation, but it binds to REV-ERBα rather than RORα (Fig. 8B, panel 5). These results revealed that zebrafish Per2 or mouse PER2 enhances bmal1b or Bmal1 expression through mediation of Rorα or RORα. A recent study showed that mouse PER1 and PER2 but not PER3 can inhibit CRY-mediated transcriptional repression by preventing CRY from being recruited to the CLOCK-BMAL1 complex, providing evidence that PER2 and PER1 have positive roles in the mammalian circadian clock (79), which complements our promoter analysis of zebrafish per2 and mouse Per2 in support of PER2 as a positive factor in vertebrate circadian regulation. Hence, the positive role of PER2 in regulation of Bmal1 expression is conserved from zebrafish to mice, which is at odds with the prevailing notion of PER2 as a negative circadian factor (1, 2, 59).
FIGURE 8.
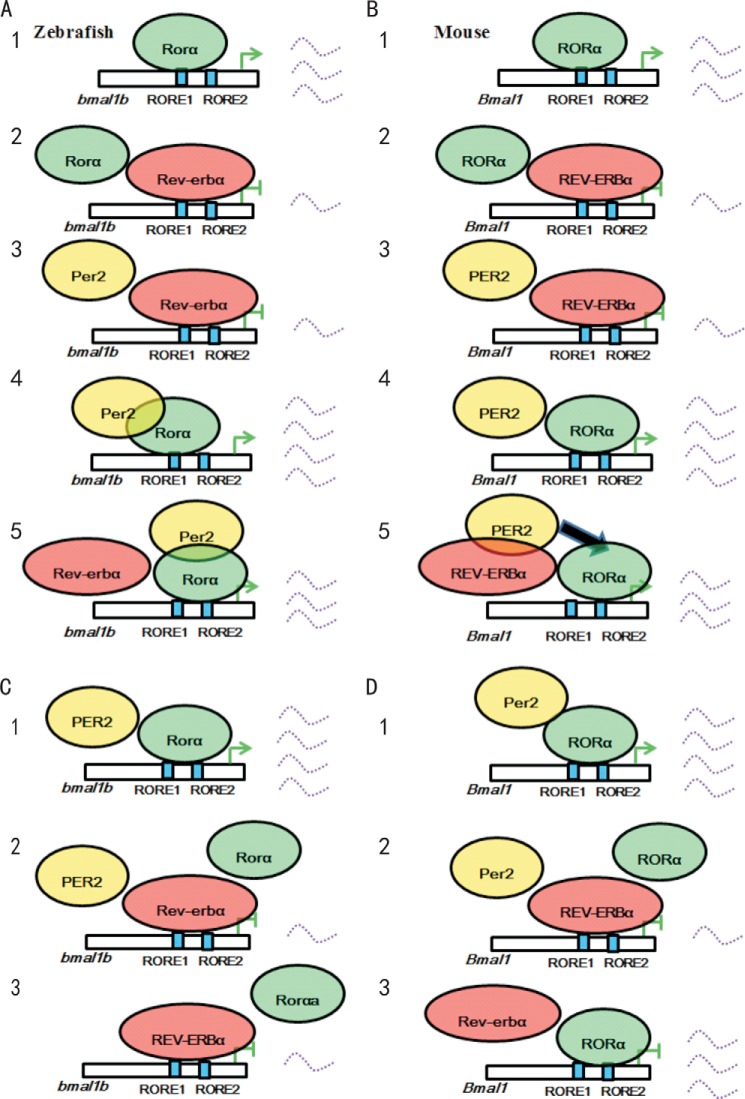
A model for regulation of bmal1b/Bmal1 by Per2/PER2 in zebrafish and mice. A, panel 1, in zebrafish, Rorα binds to RORE to activate bmal1b expression; panel 2, Rev-erbα outcompetes Rorα to repress bmal1b; panel 3, Per2 alone cannot reverse the inhibitory effect of Rev-erbα on bmal1b expression without the presence of Rorα; panel 4, Per2 alone can significantly enhance Rorα-mediated bmal1b expression; panel 5, Per2 enhances Rorα-mediated bmal1b transcription through binding to Rorα rather than Rev-erbα. B, panel 1, in mice, RORα binds to RORE to activate Bmal1 expression; panel 2, REV-ERBα outcompetes RORα to repress Bmal1; panel 3, PER2 alone cannot reverse the inhibitory effect of REV-ERBα on Bmal1 expression without the presence of RORα; panel 4, PER2 alone can significantly enhance RORα-mediated Bmal1 expression; panel 5, PER2 binds to REV-ERBα rather than RORα but enhances Bmal1 transcription still through RORα (arrow). C, panel 1, mouse PER2 alone enhances zebrafish Rorαa-mediated bmal1b transcription; panel 2, mouse PER2 cannot reverse the inhibitory effect of zebrafish Rev-erbα on bmal1b expression in the presence of Rorαa; panel 3, mouse REV-ERBα outcompetes zebrafish Rorαa to repress bmal1b. D, panel 1, zebrafish Per2 alone enhances mouse RORαa-mediated Bmal1 transcription; panel 2, zebrafish Per2 cannot reverse the inhibitory effect of mouse REV ERBα on Bmal1 expression in the presence of RORα; panel 3, zebrafish Rev-erbα cannot outcompete mouse RORαa to repress Bmal1.
Alignment of the amino acid sequence revealed that numerous functional motifs are highly conserved in zebrafish Per2 and mouse PER2 (data not shown), thereby supporting the notion that their roles in regulation of bmal1b or Bmal1 are conserved. We also examined the possible roles of zebrafish Per2 and Rev-erbα in mice and vice versa and found that although mouse PER2 can enhance zebrafish Rorα-mediated bmal1b expression (Fig. 8C, panel 1) so can zebrafish Per2 enhance mouse RORα-mediated Bmal1 transcription (Fig. 8D, panel 1); mouse PER2 cannot reverse the inhibitory effect of zebrafish Rev-erbα on bmal1b expression in the presence of Rorαa, nor can zebrafish Per2 reverse the inhibitory effect of mouse REV-ERBα on Bmal1 expression in the presence of RORα (Fig. 8, C, panel 2, and D, panel 2). The difference is that although mouse REV-ERBα can outcompete zebrafish Rorα to bind to RORE to repress zebrafish bmal1b expression (Fig. 8C, panel 3) zebrafish Rev-erbα cannot (Fig. 8D, panel 3).
Because zebrafish and tetrapods including mammals shared a common ancestor more than 300 million years ago (58, 80), it is fascinating that most aspects of the functions of zebrafish Per2 and mouse PER2 are still highly conserved (for instance, both can positively regulate expression of bmal1b or Bmal1). However, it is not unexpected that zebrafish Per2 and mouse PER2 have evolved divergent functions; i.e. zebrafish Per2 binds to Rorα to exert its enhancing effects on bmal1b expression, whereas mouse PER2 has evolved to bind to REV-ERBα instead but still requires RORα mediation to exert its enhancing effects on Bmal1 expression (Fig. 8). The functions of zebrafish Rev-erbα and mouse REV-ERBα are also mostly conserved but show divergence in that mouse REV-ERBα has evolved a stronger ability to outcompete zebrafish Rorα to repress zebrafish bmal1b expression (Fig. 8). Therefore, it is necessary and important to study the functions of zebrafish circadian clock genes because it provides comparative perspectives for how the functions of these key circadian clock genes have evolved.
In conclusion, our analysis of the per2-null mutants generated by TALEN showed that the rhythms of locomotor behaviors and expression of core circadian genes and one circadian clock controlled-gene expression are disrupted in per2 mutant fish, indicating that per2 is essential for the zebrafish circadian clock. We determined that zebrafish per2 plays both positive and negative roles in circadian regulation, and in particular, Per2 positively regulates bmal1b expression by directly binding to Rorα. Mouse PER2 enhances Bmal1 expression still through RORα mediation even though it has evolved to bind to REV-ERBα instead. Moreover, zebrafish Per2 appears to have tissue-specific functions in the peripheral circadian clocks. These results help define the Per2 functions in the zebrafish circadian clock and provide invaluable evidence for a positive role of PER2 in the vertebrate circadian system.
Acknowledgments
We thank Bo Zhang for providing the TALEN “unit assembly” system; Yilin Yan for technical support; Ying Xu and Chang Liu for providing mouse Per2, Rev-erbα, Rorα, and Bmal1-luc vectors; and members of the Wang laboratory for helpful discussion.
This work was supported by National Basic Research Program of China (973 Program) Grant 2012CB947600, National Natural Science Foundation of China Grants 31030062 and 81070455, National High Technology Research and Development Program of China (863 Program) Grant 2011AA100402-2, Jiangsu Distinguished Professorship Program Grant SR13400111, Natural Science Foundation of Jiangsu Province Grant BK2012052, Priority Academic Program Development of Jiangsu Higher Education Institutions Grant YX13400214, the High-Level Innovative Team of Jiangsu Province, and Soochow University Startup Fund Grant Q4134918.
- per
- period
- tim
- timeless
- Cry
- cryptochrome
- TALEN
- transcription activator-like effector nuclease
- LD
- light/dark
- DD
- constant darkness
- Rorα
- retinoic acid receptor (RAR)-related orphan receptor α/Nr1f1, nuclear receptor subfamily 1, group F, member 1
- Rev-erbα/Nr1d1
- nuclear receptor subfamily 1, group D, member 1
- RORE
- Ror/Rev-erb response element
- aanat
- arylalkylamine N-acetyltransferase
- co-IP
- co-immunoprecipitation
- qRT-PCR
- quantitative real time PCR
- CRISPR
- clustered regularly interspaced short palindromic repeats
- Cas9
- CRISPR-associated protein 9.
REFERENCES
- 1. Dunlap J. C. (1999) Molecular bases for circadian clocks. Cell 96, 271–290 [DOI] [PubMed] [Google Scholar]
- 2. Ko C. H., Takahashi J. S. (2006) Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 15, R271–R277 [DOI] [PubMed] [Google Scholar]
- 3. Panda S., Hogenesch J. B., Kay S. A. (2002) Circadian rhythms from flies to human. Nature 417, 329–335 [DOI] [PubMed] [Google Scholar]
- 4. Myers M. P., Wager-Smith K., Rothenfluh-Hilfiker A., Young M. W. (1996) Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science 271, 1736–1740 [DOI] [PubMed] [Google Scholar]
- 5. Ben-Shlomo R., Kyriacou C. P. (2002) Circadian rhythm entrainment in flies and mammals. Cell Biochem. Biophys. 37, 141–156 [DOI] [PubMed] [Google Scholar]
- 6. Low-Zeddies S. S., Takahashi J. S. (2001) Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell 105, 25–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu K., DiAngelo J. R., Hughes M. E., Hogenesch J. B., Sehgal A. (2011) The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell Metab. 13, 639–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morin L. P., Shivers K. Y., Blanchard J. H., Muscat L. (2006) Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience 137, 1285–1297 [DOI] [PubMed] [Google Scholar]
- 9. Kitayama Y., Iwasaki H., Nishiwaki T., Kondo T. (2003) KaiB functions as an attenuator of KaiC phosphorylation in the cyanobacterial circadian clock system. EMBO J. 22, 2127–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menet J. S., Abruzzi K. C., Desrochers J., Rodriguez J., Rosbash M. (2010) Dynamic PER repression mechanisms in the Drosophila circadian clock: from on-DNA to off-DNA. Genes Dev. 24, 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gardner G. F., Feldman J. F. (1980) The frq locus in Neurospora crassa: a key element in circadian clock organization. Genetics 96, 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hardin P. E., Hall J. C., Rosbash M. (1992) Behavioral and molecular analyses suggest that circadian output is disrupted by disconnected mutants in D. melanogaster. EMBO J. 11, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Subramanian P., Balamurugan E., Suthakar G. (2003) Circadian clock genes in Drosophila: recent developments. Indian J. Exp. Biol. 41, 797–804 [PubMed] [Google Scholar]
- 14. Debruyne J. P., Noton E., Lambert C. M., Maywood E. S., Weaver D. R., Reppert S. M. (2006) A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50, 465–477 [DOI] [PubMed] [Google Scholar]
- 15. Vitaterna M. H., Ko C. H., Chang A. M., Buhr E. D., Fruechte E. M., Schook A., Antoch M. P., Turek F. W., Takahashi J. S. (2006) The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc. Natl. Acad. Sci. U.S.A. 103, 9327–9332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoo S. H., Yamazaki S., Lowrey P. L., Shimomura K., Ko C. H., Buhr E. D., Siepka S. M., Hong H. K., Oh W. J., Yoo O. J., Menaker M., Takahashi J. S. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 101, 5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blau J. (2001) The Drosophila circadian clock: what we know and what we don't know. Semin. Cell Dev. Biol. 12, 287–293 [DOI] [PubMed] [Google Scholar]
- 18. Vanselow K., Kramer A. (2007) Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb. Symp. Quant. Biol. 72, 167–176 [DOI] [PubMed] [Google Scholar]
- 19. Zhao J., Kilman V. L., Keegan K. P., Peng Y., Emery P., Rosbash M., Allada R. (2003) Drosophila clock can generate ectopic circadian clocks. Cell 113, 755–766 [DOI] [PubMed] [Google Scholar]
- 20. King D. P., Zhao Y., Sangoram A. M., Wilsbacher L. D., Tanaka M., Antoch M. P., Steeves T. D., Vitaterna M. H., Kornhauser J. M., Lowrey P. L., Turek F. W., Takahashi J. S. (1997) Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng B., Larkin D. W., Albrecht U., Sun Z. S., Sage M., Eichele G., Lee C. C., Bradley A. (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400, 169–173 [DOI] [PubMed] [Google Scholar]
- 22. Brager A. J., Stowie A. C., Prosser R. A., Glass J. D. (2013) The mPer2 clock gene modulates cocaine actions in the mouse circadian system. Behav. Brain Res. 243, 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maywood E. S., O'Brien J. A., Hastings M. H. (2003) Expression of mCLOCK and other circadian clock-relevant proteins in the mouse suprachiasmatic nuclei. J. Neuroendocrinol. 15, 329–334 [DOI] [PubMed] [Google Scholar]
- 24. Schmutz I., Ripperger J. A., Baeriswyl-Aebischer S., Albrecht U. (2010) The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 24, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., Schibler U. (2002) The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 [DOI] [PubMed] [Google Scholar]
- 26. Triqueneaux G., Thenot S., Kakizawa T., Antoch M. P., Safi R., Takahashi J. S., Delaunay F., Laudet V. (2004) The orphan receptor Rev-erbα gene is a target of the circadian clock pacemaker. J. Mol. Endocrinol. 33, 585–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akashi M., Takumi T. (2005) The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat. Struct. Mol. Biol. 12, 441–448 [DOI] [PubMed] [Google Scholar]
- 28. Whitmore D., Foulkes N. S., Strähle U., Sassone-Corsi P. (1998) Zebrafish Clock rhythmic expression reveals independent peripheral circadian oscillators. Nat. Neurosci. 1, 701–707 [DOI] [PubMed] [Google Scholar]
- 29. Vatine G., Vallone D., Gothilf Y., Foulkes N. S. (2011) It's time to swim! Zebrafish and the circadian clock. FEBS Lett. 585, 1485–1494 [DOI] [PubMed] [Google Scholar]
- 30. Cahill G. M. (2002) Clock mechanisms in zebrafish. Cell Tissue Res. 309, 27–34 [DOI] [PubMed] [Google Scholar]
- 31. Wang M. Y., Huang G. D., Wang H. (2012) Advances in the zebrafish circadian clock mechanisms. Yi Chuan 34, 1133–1143 [PubMed] [Google Scholar]
- 32. Vatine G., Vallone D., Appelbaum L., Mracek P., Ben-Moshe Z., Lahiri K., Gothilf Y., Foulkes N. S. (2009) Light directs zebrafish period2 expression via conserved D and E boxes. PLoS Biol. 7, e1000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gavriouchkina D., Fischer S., Ivacevic T., Stolte J., Benes V., Dekens M. P. (2010) Thyrotroph embryonic factor regulates light-induced transcription of repair genes in zebrafish embryonic cells. PLoS One 5, e12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dekens M. P., Whitmore D. (2008) Autonomous onset of the circadian clock in the zebrafish embryo. EMBO J. 27, 2757–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang J., Liu C., Ma F., Chen W., Liu J., Hu B., Zheng L. (2014) Circadian clock mediates light/dark preference in zebrafish (Danio rerio). Zebrafish 11, 115–121 [DOI] [PubMed] [Google Scholar]
- 36. Tamai T. K., Young L. C., Cox C. A., Whitmore D. (2012) Light acts on the zebrafish circadian clock to suppress rhythmic mitosis and cell proliferation. J. Biol. Rhythms 27, 226–236 [DOI] [PubMed] [Google Scholar]
- 37. Cavallari N., Frigato E., Vallone D., Fröhlich N., Lopez-Olmeda J. F., Foà A., Berti R., Sánchez-Vázquez F. J., Bertolucci C., Foulkes N. S. (2011) A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol. 9, e1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cahill G. M. (1996) Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 708, 177–181 [DOI] [PubMed] [Google Scholar]
- 39. Smadja Storz S., Tovin A., Mracek P., Alon S., Foulkes N. S., Gothilf Y. (2013) Casein kinase 1delta activity: a key element in the zebrafish circadian timing system. PLoS One 8, e54189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z. S., Eichele G., Bradley A., Lee C. C. (2001) Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 [DOI] [PubMed] [Google Scholar]
- 41. Vanselow K., Vanselow J. T., Westermark P. O., Reischl S., Maier B., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A. (2006) Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 20, 2660–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grimaldi B., Bellet M. M., Katada S., Astarita G., Hirayama J., Amin R. H., Granneman J. G., Piomelli D., Leff T., Sassone-Corsi P. (2010) PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 12, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu L., Pelicano H., Liu J., Huang P., Lee C. (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 [DOI] [PubMed] [Google Scholar]
- 44. Xu Y., Toh K. L., Jones C. R., Shin J. Y., Fu Y. H., Ptácek L. J. (2007) Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toh K. L., Jones C. R., He Y., Eide E. J., Hinz W. A., Virshup D. M., Ptácek L. J., Fu Y. H. (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291, 1040–1043 [DOI] [PubMed] [Google Scholar]
- 46. Field M. D., Maywood E. S., O'Brien J. A., Weaver D. R., Reppert S. M., Hastings M. H. (2000) Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 25, 437–447 [DOI] [PubMed] [Google Scholar]
- 47. Ziv L., Levkovitz S., Toyama R., Falcon J., Gothilf Y. (2005) Functional development of the zebrafish pineal gland: light-induced expression of period2 is required for onset of the circadian clock. J. Neuroendocrinol. 17, 314–320 [DOI] [PubMed] [Google Scholar]
- 48. Ziv L., Gothilf Y. (2006) Period2 expression pattern and its role in the development of the pineal circadian clock in zebrafish. Chronobiol. Int. 23, 101–112 [DOI] [PubMed] [Google Scholar]
- 49. Huang P., Xiao A., Zhou M., Zhu Z., Lin S., Zhang B. (2011) Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 29, 699–700 [DOI] [PubMed] [Google Scholar]
- 50. Sander J. D., Dahlborg E. J., Goodwin M. J., Cade L., Zhang F., Cifuentes D., Curtin S. J., Blackburn J. S., Thibodeau-Beganny S., Qi Y., Pierick C. J., Hoffman E., Maeder M. L., Khayter C., Reyon D., Dobbs D., Langenau D. M., Stupar R. M., Giraldez A. J., Voytas D. F., Peterson R. T., Yeh J. R., Joung J. K. (2011) Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat. Methods 8, 67–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., Sander J. D., Peterson R. T., Yeh J. R., Joung J. K. (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Westerfield M. (1995) The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish (Danio rerio), pp. 1.1–1.27, Institute of Neuroscience, University of Oregon, Eugene, OR [Google Scholar]
- 53. Doyle E. L., Booher N. J., Standage D. S., Voytas D. F., Brendel V. P., Vandyk J. K., Bogdanove A. J. (2012) TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 40, W117–W122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang P., Xiao A., Tong X., Zu Y., Wang Z., Zhang B. (2014) TALEN construction via “unit assembly” method and targeted genome modifications in zebrafish. Methods 69, 67–75 [DOI] [PubMed] [Google Scholar]
- 55. Weger M., Weger B. D., Diotel N., Rastegar S., Hirota T., Kay S. A., Strähle U., Dickmeis T. (2013) Real-time in vivo monitoring of circadian E-box enhancer activity: a robust and sensitive zebrafish reporter line for developmental, chemical and neural biology of the circadian clock. Dev. Biol. 380, 259–273 [DOI] [PubMed] [Google Scholar]
- 56. Liu C., Hu J., Qu C., Wang L., Huang G., Niu P., Zhong Z., Hong F., Wang G., Postlethwait J. H., Wang H. (2015) Molecular evolution and functional divergence of zebrafish (Danio rerio) cryptochrome genes. Sci. Rep., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hurd M. W., Debruyne J., Straume M., Cahill G. M. (1998) Circadian rhythms of locomotor activity in zebrafish. Physiol. Behav. 65, 465–472 [DOI] [PubMed] [Google Scholar]
- 58. Wang H. (2008) Comparative analysis of period genes in teleost fish genomes. J. Mol. Evol. 67, 29–40 [DOI] [PubMed] [Google Scholar]
- 59. Reppert S. M., Weaver D. R. (2002) Coordination of circadian timing in mammals. Nature 418, 935–941 [DOI] [PubMed] [Google Scholar]
- 60. Ishikawa T., Hirayama J., Kobayashi Y., Todo T. (2002) Zebrafish CRY represses transcription mediated by CLOCK-BMAL heterodimer without inhibiting its binding to DNA. Genes Cells 7, 1073–1086 [DOI] [PubMed] [Google Scholar]
- 61. Kobayashi Y., Ishikawa T., Hirayama J., Daiyasu H., Kanai S., Toh H., Fukuda I., Tsujimura T., Terada N., Kamei Y. (2000) Molecular analysis of zebrafish photolyase/cryptochrome family: two types of cryptochromes present in zebrafish. Genes Cells 5, 725–738 [DOI] [PubMed] [Google Scholar]
- 62. Wang H. (2009) Comparative genomic analysis of teleost fish bmal genes. Genetica 136, 149–161 [DOI] [PubMed] [Google Scholar]
- 63. Ziv L., Gothilf Y. (2006) Circadian time-keeping during early stages of development. Proc. Natl. Acad. Sci. U.S.A. 103, 4146–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gothilf Y., Coon S. L., Toyama R., Chitnis A., Namboodiri M. A., Klein D. C. (1999) Zebrafish serotonin N-acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology 140, 4895–4903 [DOI] [PubMed] [Google Scholar]
- 65. Uz T., Manev H. (2001) Prolonged swim-test immobility of serotonin N-acetyltransferase (AANAT)-mutant mice. J. Pineal Res. 30, 166–170 [DOI] [PubMed] [Google Scholar]
- 66. Appelbaum L., Vallone D., Anzulovich A., Ziv L., Tom M., Foulkes N. S., Gothilf Y. (2006) Zebrafish arylalkylamine-N-acetyltransferase genes—targets for regulation of the circadian clock. J. Mol. Endocrinol. 36, 337–347 [DOI] [PubMed] [Google Scholar]
- 67. Reppert S. M., Weaver D. R. (2001) Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 63, 647–676 [DOI] [PubMed] [Google Scholar]
- 68. Sato T. K., Panda S., Miraglia L. J., Reyes T. M., Rudic R. D., McNamara P., Naik K. A., FitzGerald G. A., Kay S. A., Hogenesch J. B. (2004) A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43, 527–537 [DOI] [PubMed] [Google Scholar]
- 69. Postlethwait J. H., Yan Y. L., Gates M. A., Horne S., Amores A., Brownlie A., Donovan A., Egan E. S., Force A., Gong Z., Goutel C., Fritz A., Kelsh R., Knapik E., Liao E., Paw B., Ransom D., Singer A., Thomson M., Abduljabbar T. S., Yelick P., Beier D., Joly J. S., Larhammar D., Rosa F., Westerfield M., Zon L. I., Johnson S. L., Talbot W. S. (1998) Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 18, 345–349 [DOI] [PubMed] [Google Scholar]
- 70. Flores M. V., Hall C., Jury A., Crosier K., Crosier P. (2007) The zebrafish retinoid-related orphan receptor (ror) gene family. Gene Expr. Patterns 7, 535–543 [DOI] [PubMed] [Google Scholar]
- 71. Ben-Moshe Z., Alon S., Mracek P., Faigenbloom L., Tovin A., Vatine G. D., Eisenberg E., Foulkes N. S., Gothilf Y. (2014) The light-induced transcriptome of the zebrafish pineal gland reveals complex regulation of the circadian clockwork by light. Nucleic Acids Res. 42, 3750–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tan Y., DeBruyne J., Cahill G. M., Wells D. E. (2008) Identification of a mutation in the Clock1 gene affecting zebrafish circadian rhythms. J. Neurogenet. 22, 149–166 [DOI] [PubMed] [Google Scholar]
- 73. Huang J., Zhong Z., Wang M., Chen X., Tan Y., Zhang S., He W., He X., Huang G., Lu H., Wu P., Che Y., Yan Y., Postlethwait J. H., Chen W., Wang H. (2015) Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention-deficit hyperactivity disorder (ADHD). J. Neurosci., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cermakian N., Monaco L., Pando M. P., Dierich A., Sassone-Corsi P. (2001) Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 20, 3967–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hardin P. E., Hall J. C., Rosbash M. (1990) Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 [DOI] [PubMed] [Google Scholar]
- 76. Ukai H., Ueda H. R. (2010) Systems biology of mammalian circadian clocks. Annu. Rev. Physiol. 72, 579–603 [DOI] [PubMed] [Google Scholar]
- 77. Camacho F., Cilio M., Guo Y., Virshup D. M., Patel K., Khorkova O., Styren S., Morse B., Yao Z., Keesler G. A. (2001) Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 489, 159–165 [DOI] [PubMed] [Google Scholar]
- 78. Zhang E. E., Liu Y., Dentin R., Pongsawakul P. Y., Liu A. C., Hirota T., Nusinow D. A., Sun X., Landais S., Kodama Y., Brenner D. A., Montminy M., Kay S. A. (2010) Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 16, 1152–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Akashi M., Okamoto A., Tsuchiya Y., Todo T., Nishida E., Node K. (2014) A positive role for PERIOD in mammalian circadian gene expression. Cell Rep. 7, 1056–1064 [DOI] [PubMed] [Google Scholar]
- 80. Postlethwait J., Amores A., Cresko W., Singer A., Yan Y.-L. (2004) Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 20, 481–490 [DOI] [PubMed] [Google Scholar]