Abstract
Wnt signaling is a major and highly conserved developmental pathway that guides many important events during embryonic and larval development. In adulthood, misregulation of Wnt signaling has been implicated in tumorigenesis and various age-related diseases. These effects occur through highly complicated cell-to-cell interactions mediated by multiple Wnt-secreted proteins. While they share a high degree of sequence similarity, their function is highly diversified. Although the role of Wnt ligands during development is well studied, very little is known about the possible actions of Wnt signaling in natural aging. In this study, Caenorhabditis elegans serves, for the first time, as a model system to determine the role of Wnt ligands in aging. Caenorhabditis elegans has five Wnt proteins, mom-2, egl-20, lin-44, cwn-1, and cwn-2. We show that all five Wnt ligands are expressed and active past the development stages. The ligand mom-2/Wnt plays a major detrimental role in longevity, whereas the function of lin-44/Wnt is beneficial for long life. Interestingly, no evidence was found for Wnt signaling being involved in cellular or oxidative stress responses during aging. Our results suggest that Wnt signaling regulates aging-intrinsic genetic pathways, opening a new research direction on the role of Wnt signaling in aging and age-related diseases.
Keywords: aging, antagonistic pleiotropy, C. elegans, development, life span, longevity regulation, oxidative stress, Wnt signalling
Introduction
Wnt signaling is one of the major developmental pathways, evolutionarily conserved in all animals (Logan & Nusse, 2004). Genomes of almost all metazoans contain several Wnt genes. In humans, there are 19 Wnt genes that encode Wnt ligands. All throughout the animal kingdom, most of these genes are structurally and functionally highly conserved, suggesting that Wnt signaling played an important role in the evolution of multicellular organisms (Clevers & Nusse, 2012). The Wnt signaling pathway participates in a multitude of developmental processes as well as tissue homeostasis and control of stem cell proliferation and differentiation in adulthood (Clevers & Nusse, 2012). It is therefore not surprising that deregulation of Wnt signaling pathway has been linked to many developmental defects in addition to many age-related diseases including osteoporosis and cancer (Clevers & Nusse, 2012).
Mutagenesis experiments on Wnt genes suggest that their primary function is transcriptional regulation through the canonical/β-catenin signaling pathway (Logan & Nusse, 2004). In the canonical Wnt/β-catenin signaling pathway, in the absence of the Wnt signal, β-catenin activity is inhibited by a cytosolic destruction complex consisting of Axin, GSK-3β (glycogen synthase kinase 3β), APC (adenomatous polyposis coli) and CK1 (casein kinase 1). This destruction complex enables GSK-3 β-dependent phosphorylation and subsequent proteosomal degradation of the β-catenin protein. This continual elimination prevents the β-catenin from migrating into the nucleus and activating the expression of target genes. When Wnt binds the co-receptors, frizzled (Fz) and low-density lipoprotein (LDL) receptor-related protein (LRP)5/6, disheveled (Dvl) is activated and inhibits the GSK-3 kinase. This, in turn, leads to the stabilization of cytosolic β-catenin, which can then accumulate and translocate to the nucleus. In the nucleus, β-catenin interacts with DNA-bound T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, leading to the activation of specific genes that control a wide array of processes during embryonic development (Logan & Nusse, 2004; MacDonald et al., 2009). In addition to their canonical signaling pathway, Wnts can act via noncanonical Wnt pathways that signal independently of β-catenins (Veeman et al., 2003). In these cases, Wnt signaling is transduced either via calcium flux, c-Jun N-terminal kinase (JNK), protein kinase C, or G proteins. A wide array of genes that are regulated by noncanonical Wnt signaling pathways have been implicated in control of gastrulation, as well as in the induction of the heart tissue, dorsoventral patterning, tissue development, and neuronal migration (Veeman et al., 2003).
Recent studies have also implicated Wnt signaling in the general aging process. Brack and colleagues have shown that serum from old mice, once perfused in young mice, increases myogenic-to-fibrogenic fate conversion in young muscle progenitors, a process seen in aged muscles. Moreover, they showed that this effect depends on Wnt3A activity and can be suppressed by injections of Dickkopf-1 (DKK-1), a Wnt antagonist that is able to enhance muscle regeneration in old mice as well (Brack et al., 2007). These data were corroborated by results from the Komuro group (Naito et al., 2012). They reported that Wnt signaling activity increases with age in serum and multiple tissues of wild-type mice, promoting age-associated decline in tissue regeneration. In another study by Liu et al., (2007) the accelerated aging observed in the Klotho mouse model has been ascribed to chronic Wnt stimulation (due to the absence of the Klotho protein, another Wnt antagonist similar to DKK-1) contributing to stem cell depletion and aging. In addition, several reports have shown that mutations that lead to overactivation of the Wnt signaling pathway contribute to the development of many age-related diseases, such as cancer, osteoporosis, and metabolic dysfunction (MacDonald et al., 2009). Based on these studies, one can propose that Wnt signaling is increased in old animals and that high levels of Wnt are detrimental to organism function. However, the role of Wnt in the aging process may be more complex, as down-regulation of this pathway can also be detrimental in terms of tissue maintenance. Miranda et al., (2012) provided evidence that neuronal progenitor cell proliferation decreases following the attenuation of Wnt signaling from astrocytes in the aging brain. To distinguish between these two contradictory roles of Wnt signaling during aging, one has to first identify the molecular mechanisms that are regulated by Wnt signaling during natural aging.
Here, we used the nematode Caenorhabditis elegans to determine the role of Wnt ligands in worm longevity. There are five genes that encode Wnt ligands in C. elegans, lin-44, egl-20, mom-2, cwn-1 and cwn-2 (Eisenmann, 2005). However, we know very little about the role of the Wnt signaling pathway after development is completed (Fig. 1A). Do Wnt ligands continue to be expressed? Is the Wnt signaling pathway active during aging? If it is active, how does it affect aging? Does it regulate the same targets as during development? To address these questions, we examined the role of individual Wnt ligands in the aging process.
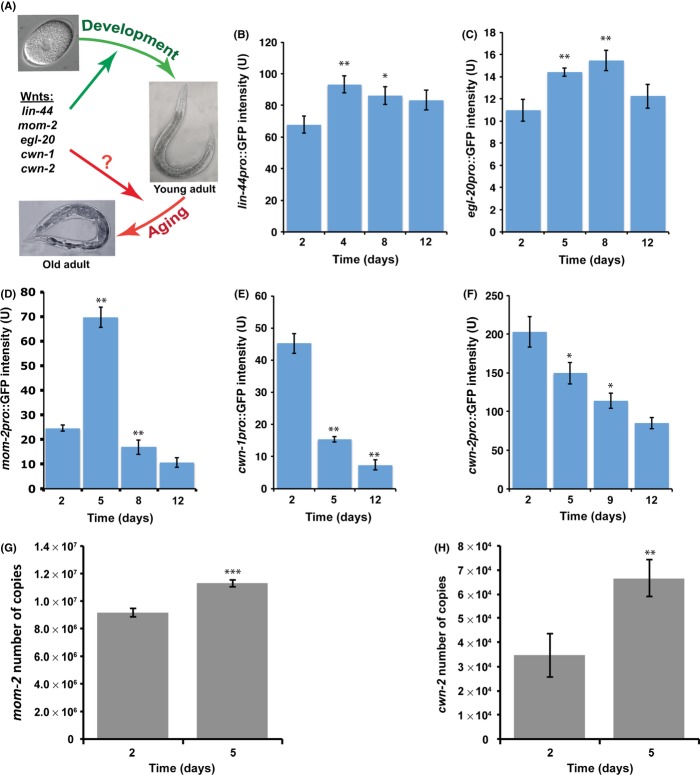
Figure 1.
Expression of Caenorhabditis elegans Wnts regulated by age. Model for role of Wnt signaling pathway in aging in C. elegans (A). We hypothesize that all five Wnt ligands continue to be expressed past development to regulate C. elegans longevity. Expression of lin-44pro::4xNLSGFP (B), egl-20pro::4xNLSGFP (C), and mom-2pro::4xNLSGFP (D) increases with age. Expression of cwn- 1pro::4xNLSGFP (E) and cwn-2pro::4xNLSGFP in the muscle (F) decreases with age. Expression levels of various Wnts were calculated by measuring pixel intensity from GFP images using Image J. The y-axis denotes GFP expression (arbitrary units) and the x-axis denotes days of adulthood. Average expression and SD from 20 animals are shown. (G and H) The histogram shows the level of expression (expressed in number of copies) of mom-2 and cwn-2 Wnt ligands, measured by RTqPCR in wild type (N2) worms on day 2 and 5 (n = 100). The general mRNA levels of both ligands significantly (P < 0.001) increased.
Results
All five Wnts are expressed in adulthood
We wanted to examine whether any of the C. elegans Wnt ligands are expressed during aging. To do that, we took advantage of previously generated transcriptional GFP reporter constructs for all five Wnts (Gleason et al., 2006). Using these transgenic lines, we were able to observe temporal and spatial expression from these five Wnt reporters throughout the worm’s lifespan, from day 2 (young) to day 12 (old) of adulthood.
We found that expression of lin-44 and egl-20 is high throughout the entire lifespan of the worm and increases during aging (Fig. 1B,C). As in development, lin-44 and egl-20 expression was observed only in the posterior of the animal. Expression of lin-44 is present in tail hypodermis, hyp8, hyp9, hyp10, and hyp11 cells (Fig. S1A). These are exactly the same expression patterns as previously described during larval development (Herman et al., 1995; Gleason et al., 2006). The egl-20/Wnt is expressed in the anal depressor muscle and in the postembryonic rectal epithelial cells (B, F, K, and U) during aging (Fig. S1B). These cells also express egl-20/Wnt during larval development (Gleason et al., 2006). However, we did not observe any expression in hypodermal or muscle cells, as previously reported (Gleason et al., 2006).
The expression dynamics of mom-2/Wnt are quite different throughout the worm lifespan. Expression of mom-2/Wnt increases 3-fold during the first 5 days of adulthood and then decreases 4-fold by day 8 of adulthood, eventually showing little or no expression in old worms (Fig. 1D). In contrast to lin-44 and egl-20, expression localization of mom-2 differs slightly between aging and development. During development, mom-2 is expressed throughout the whole body of the worm, in muscles, hypodermal and intestinal cells, vulva precursor cells, as well as in ventral cord motor neurons (Gleason et al., 2006). In young (day1 and 2) and middle-aged (day 5) adults, mom-2/Wnt expression was observed only in posterior intestinal and intestinal–rectal valve cells (Fig. S1C). We were not able to detect any mom-2 expression in any other tissues.
Expression of both cwn-1 and cwn-2 Wnt ligands decreases during aging (Fig. 1E,F). In very young adult worms, day 1 of adulthood, cwn-1/Wnt is expressed in body-wall muscles and ventral cord motor neurons in the posterior part of the worm (Fig. S2A). However, cwn-1/Wnt expression in body-wall muscles almost entirely disappears in the following 24 h. By day 2 of adulthood, we were able to observe only cwn-1 expression in ventral cord motor neurons. This expression is low in middle-age (day 5) worms and barely detectable in old (day 12) animals (Fig. 1E). Overall, the cwn-1/Wnt expression pattern is similar to that previously observed during development. In addition to body-wall muscles and ventral cord motor neurons, cwn-1/Wnt was detected in intestinal cells, vulval muscle, and fused seam cells during larvae development (Gleason et al., 2006), although this expression pattern is absent in adulthood.
The cwn-2/Wnt expression was observed all along the anterior–posterior body axis of the animal in body-wall muscles (Fig. S2B). We did not detect any expression in ventral cord motor neurons or intestinal cells as was previously observed during worm development (Gleason et al., 2006). This observation was particularly troubling as it has been previously reported, using similar genetic constructs, that cwn-2/Wnt is expressed at high levels in pharynx and SMD neurons in adult worms (Song et al., 2010). These discrepancies could potentially affect our conclusion that cwn-2/Wnt expression decreases with age. To resolve this issue, we collected total RNA from day 2 (young adult) and day 5 (mid-age adult) worms and performed qPCR for the cwn-2/Wnt and mom-2/Wnt (as it also shows a small discrepancy in tissue specific gene expression compared with previous observations). We found that total expression levels for both Wnt ligands significantly increased between day 2 and 5 of adulthood (Fig. 1G,H). It is possible that expression of cwn-2/Wnt decreases in muscle cells, as indicated by analysis using transcriptional fusion, but increases in the pharynx and SMD neurons, where this reporter is silenced. In addition, expression of mom-2/Wnt is highly increased in posterior intestinal cells, but remains unchanged or may be even decreased in other tissues, where we failed to detect any expression using our transcriptional reporters.
Taken together, these data show that that all five Wnt ligands continue to be expressed after development is completed, and that the Wnt signaling pathway might be active not only in development, but also in aging. However, the expression pattern of most Wnt ligands changes over time, which could imply that Wnt signaling function during aging could be somewhat different than in development.
Effect of Wnt signaling on elt-5/elt-3 GATA transcriptional circuit
The standard readout for the activity of the Wnt signaling pathway is the activation of reporter genes. However, very few targets of the Wnt signaling pathway in C. elegans development have been identified. We know even less about potential targets of Wnt signaling during worm aging. We consulted Wormbase (www.wormabase.org) for a list of potential targets that are regulated by the Wnt ligands and POP-1, the only TCF/LEF factor in C. elegans and a common component in both canonical and noncanonical Wnt signaling pathways. Out of 15 potential targets of the Wnt signaling pathway in C. elegans, we were able to detect expression for 11 genes starting at day 2 of adulthood (Table 1). We examined expression of these genes at different ages (day 2, 5, 7, and 11 of adulthood). We found that expression of end-3 (GATA transcription factor) and sdz-26 (SKN-1-dependent Zygotic transcript) decreases, and expression of elt-5 (GATA transcription factor) is increased with age but that expression of the other 8 genes is steady between young and old worms. Hence, expression changes in end-3, sdz-26, and elt-5 during aging can give us the first insight into Wnt signaling function in adult worms.
Table 1.
Relative quantification by RT-qPCR of mRNA expression levels of 11 known targets of Wnt signaling pathway during aging (between days 2 and 11 of adulthood)
| Gene | Function | Age regulation | Fold change | P-value |
|---|---|---|---|---|
| elt-5/F55A8.1* | GATA transcription factor | Up-regulated | 0.33 | 0.03 |
| end-3/F58A3.5 | GATA transcription factor | Down-regulated | 0.6 | 0.0001 |
| sdz-26/R06A4.6 | SKN-1-dependent zygotic transcript | Down-regulated | 3 | 0.0001 |
| lin-39/C07H6.7 | Homeodomain protein | No change | N/A | N/A |
| glr-1/C06E1.4 | Glutamate receptor subunit | No change | N/A | N/A |
| end-1/F58A3.2 | GATA transcription factor | No change | N/A | N/A |
| sdz-23/F58G4.4 | Putatively secreted protein with a single EGF domain | No change | N/A | N/A |
| wrm-1/B0336.1 | β-catenin | No change | N/A | N/A |
| psa-3/F39D8.2 | Axin family | No change | N/A | N/A |
| ceh-10/C09G12.8 | GTPase orthologous to human RAC1 | No change | N/A | N/A |
| ceh-22/F29F11.5 | Homeodomain protein | No change | N/A | N/A |
Also published in Budovskaya et al., (2008).
N/A, no differences detected.
It has previously been shown that expression of both end-3 and sdz-26 are negatively regulated by POP-1/TCF during endoderm specification (Maduro et al., 2005a; Shetty et al., 2005). Our results suggest that Wnt signaling continues to function and represses expression of end-3 and sdz-26 in adulthood. Unfortunately, expression of both end-3 and sdz-26 is quite low even at day 2 of adulthood, which makes them very poor markers to study the function of Wnt signaling activity all throughout aging process. Therefore, we used elt-5 GATA as a biomarker to access how Wnt signaling activity changes as the worms grow old.
Wnt signaling pathways are known to activate the expression of elt-5 GATA transcription factor during embryonic and larval development (Koh et al., 2002; Cassata et al., 2005). Previously, we have shown that the elt-3/elt-5/elt-6 GATA transcriptional circuit is, at least in part, responsible for driving the aging process in C. elegans (Budovskaya et al., 2008). We have shown that expression of repressor elt-5 GATA increases during aging. This leads to a decreased expression of the elt-3 GATA transcription factor in old age that causes down-regulation of many downstream age-regulated genes. In addition, close examination of the promoter region of the elt-5 gene have revealed that it contains seven potential TCF/LEF (CTTTGWW) binding sites (Maduro et al., 2005b). Therefore, we hypothesized that elt-5 GATA is one of the targets of the Wnt signaling pathway during aging. The Wnt signaling pathway continues to activate expression of elt-5 GATA, which leads to down-regulation of the elt-3 GATA transcription factor, which has detrimental effects on longevity.
To test this hypothesis, we compared the expression of elt-3 and elt-5 GATA in control and Wnt (RNAi) mutant backgrounds (Fig. 2). To avoid potentially detrimental effects of reduced Wnt ligands expression on development, we used RNAi to reduce egl-20, cwn-1, cwn-2, mom-2, and lin-44 expression starting on day 2 of adulthood and then observed the effects of down-regulation of the Wnt signaling pathway on the expression of the elt-3pro::GFP and elt-5pro::Cherry reporters on day 5 of adulthood. We found that egl-20(RNAi), cwn-2(RNAi), and lin-44(RNAi) treatments resulted in decreased expression of elt-5pro::Cherry and increased expression of elt-3pro::GFP in the head hypodermis (Fig. 2A,C), whereas RNAi against each of the five Wnt ligands resulted in decreased expression of elt-5pro::Cherry and subsequently increased expression of elt-3pro::GFP in the trunk hypodermis (Fig. 2B,D). Similar results were obtained from analyzing the effect of RNAi treatment against each of the five Wnt ligands on ELT-3 protein expression (Fig. S3). This suggests that even post-translational stability of ELT-3 GATA is not affected in Wnt signaling mutants. Overall, these results suggest that the elt-3 and elt-5 GATA transcriptional factors are still under control of the Wnt signaling pathway during aging and all five Wnts play a role in their regulation in a tissue specific manner. Whether this control of elt-5 expression is direct or indirect will require further investigation.
Figure 2.
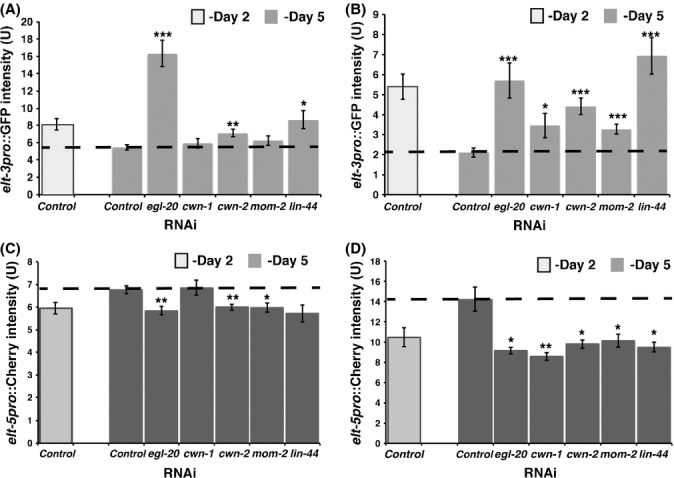
Effect of Wnt ligands down-regulation (RNAi) on elt-3 and elt-5 expression. (A) elt-3pro::GFP expression in the head hypodermis is increased in egl- 20(RNAi), cwn-2(RNAi), or lin-44(RNAi) animals. (B) elt-3pro::GFP expression in the trunk hypodermis is increased in egl-20(RNAi), cwn-1(RNAi), cwn- 2(RNAi), mom-2(RNAi), or lin-44(RNAi) animals. (C) elt-5pro:: mCherry expression in the head hypodermis is decreased in egl-20(RNAi), cwn- 2(RNAi), mom-2(RNAi), or lin-44(RNAi) animals. (D) elt-5pro:: mCherry expression in the trunk hypodermis is increased in egl-20(RNAi), cwn- 1(RNAi), cwn-2(RNAi), mom-2(RNAi), or lin-44(RNAi) animals. RNAi was induced starting at day two of adulthood by feeding worms bacteria expressing dsRNA. elt-3pro::GFP and elt-5pro::mCherry expression was measured starting at day 5. The y-axis denotes reporters expression (arbitrary units). Average expression and SE from 20 animals are shown.
Wnt signaling activity in aging
Measuring the expression of a few specific target genes could create a bias in our analysis. Therefore, instead of concentrating on one target or group of targets at a time, we wanted to analyze the global role of Wnt signaling activity during aging. In mammals, general Wnt signaling activity is also measured in vitro with the TOPFLASH reporter that consists of multiple TCF binding sites driving expression of luciferase (van de Wetering et al., 1997). For our analysis, we used a previously described homologs reporter for assessing C. elegans Wnt signaling activity (Green et al., 2008). The POPTOP (POP-1 and TCF Optimal Promoter) reporter consists of seven copies of POP-1/TCF binding sites, together with the pes-10 minimal promoter, driving expression of the red fluorescent protein, mCherry (Fig. 3A). This construct exhibits some background expression probably due to low activity of the minimal pes-10 promoter. Therefore, expression levels from the POPTOP reporter must be compared to expression from the control reporter, POPFOP (POP-1 Far from Optimal Promoter), which contains a mutated POP-1/TCF binding sites and cannot be activated by the Wnt signaling pathway.
Figure 3.
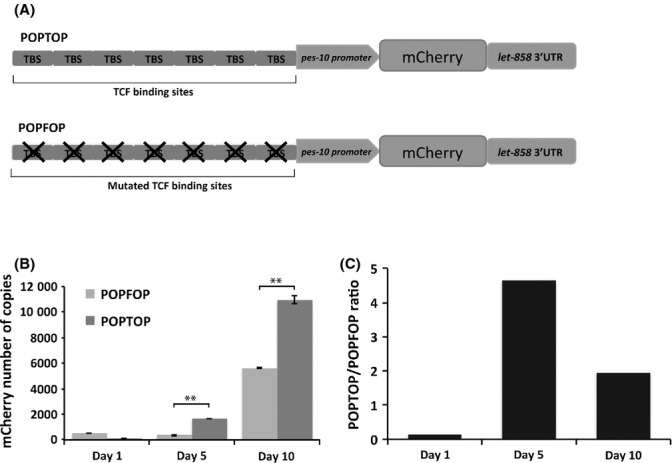
Wnt signaling activity during aging. (A) Schematic representation of the constructs used to measure Wnt signaling activity in vivo. On top, POPTOP (POP-1 and TCF Optimal Promoter) construct which contains seven copies of POP-1/TCF binding sites upstream of a minimal promoter driving the expression of the fluorescent protein mCherry. Below, POPFOP (POP-1 and Far from Optimal Promoter) control construct is represented, which contains mutated TCF/POP-1 binding sites, used to measure background expression. (B) Number of copies of mCrerry transcript derived from the two constructs was measured by quantitative RT-PCR, using pD4H1-mCherry vector to build the standard curve. (C) POPTOP/POPFOP mCherry expression ratio.
Although POPTOP expression is visible at all larval stages during worm development, we were unfortunately not able to measure POPTOP expression in vivo in adult worms due to the weak signal of mCherry and high levels of gut autofluorescence. Therefore, we decided to examine levels of POPTOP expression in vitro using quantitative RT-PCR. We found that POPTOP expression increased about fivefold in the first 5 days of adulthood (Fig. 3B,C). The POPTOP expression decreased slightly by day 10 of adulthood, but still remained twofold higher then in young animals. These results indicate a general increase in Wnt signaling activity during aging in C. elegans.
Wnt influences Caenorhabditis elegans longevity
We hypothesize that increased levels of Wnt ligands in young adult worms lead to increased levels of Wnt signaling that promotes aging (developmental drift). Therefore, inactivation of either one or all Wnts by either RNAi or mutations should increase the lifespan of the worm. Another possibility is that high levels of Wnt expression are protective for the organism; for example, they activate genes that are responsible for damage repair and protection from pathogenic infections (damage accumulation). If that were the case, then inactivation of these Wnts would decrease the worm’s lifespan. Our analysis of elt-3 and elt-5 GATA expression shows that all five Wnts function in the same manner: they increase expression of elt-5 GATA and consequently repress expression of elt-3, which at least in part causes aging in C. elegans (Budovskaya et al., 2008). This would indicate that mutations in any of the Wnt ligands should prolong the lifespan.
To test this hypothesis, we used RNAi treatment against each Wnt ligand to investigate their effect on longevity. We found that only mom-2(RNAi) treatment significantly increases the lifespan of wild-type, N2 animals by about 33%. Unexpectedly, lin-44(RNAi) treatments significantly shorten (by about 15%) the lifespan of wild-type, N2 animals (Table 2). This result implies that in C. elegans, the Wnt signaling pathway plays a different role in longevity than in development.
Table 2.
Median lifespan changes in Wnt ligands mutants
| Mutation | Strain | Median lifespan | ||
|---|---|---|---|---|
| N2 | N2 | 19.5 | 21 | 20 |
| mom-2(or77) | EU1424 | 31d | 30.5d | 28c |
| egl-20(n585) | MT1215 | 17d | 14d | 14.5d |
| lin-44(n1792) | MT5383 | 10.7d | 11d | 10.5d |
| cwn-1(ok546) | RB763 | 18 | 23 | 21 |
| cwn-2(ok895) | VC636 | 23d | 28d | 21 |
| RNAi treatment | ||||
| Control* | N2 | 14.3 | 15 | |
| mom-2(RNAi)* | N2 | 19.1d | 20.2d | |
| Control | N2 | 25 | 24 | 25 |
| egl-20(RNAi) | N2 | 28a | 21 | 26a |
| cwn-1(RNAi) | N2 | 24.5 | 25 | 24 |
| cwn-2(RNAi) | N2 | 24.1 | 23.3 | 26.2 |
| lin-44(RNAi) | N2 | 21.1b | 20.5b | 21.4b |
P-values relative to control for each experiment were calculated using log-rank (Mantel-Cox) method (Lawless, 1982) in Prism 6 software.
aP < 0.1, bP < 0.05, cP < 0.005, dP < 0.0001.
Experiments were conducted at 20 °C. All the other experiments were conducted at 15 °C. Each column represents individual experiments; n > 80 worms.
It has been previously shown by the smFISH technique that mom-2/Wnt is expressed in germ cell precursors (Z2 and Z3) during embryonic development and continues to be expressed in the germline throughout larval development (Harterink et al., 2011). Mutations in mom-2/Wnt influence germline function have been shown to induce sterility (Ceron et al., 2007). In addition, it has been well established that germline signals act through insulin/IGF-1 signaling cascade to promote aging in C. elegans (Hsin & Kenyon, 1999). We have not observed any mom-2/Wnt expression in the germline. However, we cannot exclude the possibility that mom-2/Wnt continues to be expressed in the germline at low levels and affects germline function and, as consequence, the worm’s longevity. To assess this possibility, we analyzed the rate of reproduction and brood size of worms treated with mom-2(RNAi) at day 1 of adulthood compared to animals feed with control RNAi (Fig. S4). We observed no difference in germline function in these animals. mom-2(RNAi) did not cause any change in the general brood size or in the number of eggs that did not hatch. Therefore, we can conclude that mom-2/Wnt acts to promote aging independently of germline function.
The RNAi treatments against egl-20, cwn-1, and cwn-2 Wnts did not have any effect on longevity of N2 animals. It is possible that the RNAi treatment did not reduce levels of Wnt ligands enough to have a significant effect on longevity. To overcome this problem, we used genetic mutants that affect function of various Wnts.
There are many mutant alleles for different Wnt ligands. Unfortunately, many mutants cause severe developmental defects and are not suitable for longevity analysis as they produce sick individuals. For our purposes, we picked only mutations that do not compromise worm development (no delay in developmental timing) and produce normal, properly developed, individuals.
The mom-2/Wnt plays a very important role in the noncanonical, or ‘Wnt/β-catenin asymmetry’, signaling pathway that is required during early embryogenesis to insure proper endoderm induction (Eisenmann, 2005). Most of the mom-2 mutants are temperature sensitive and exhibit a strong embryonic lethal phenotype. For our analysis, we used the mom-2(or77) mutation. This mutation is a weak allele of mom-2/Wnt that affects a splice acceptor site at the last exon. The mom-2(or77) mutants develop normally (only 8% of embryos lack a gut at 15 °C) (Thorpe et al., 1997). After development, a small percentage (~10%) of the adult hermaphrodites retains eggs (bag of worms phenotype). We performed our lifespan analysis on the mom-2(or77) mutants and observed, as in the case of RNAi treatment, a significant increase in longevity of the mom-2(or77) mutant compared to wild-type controls (Fig. 4A).
Figure 4.
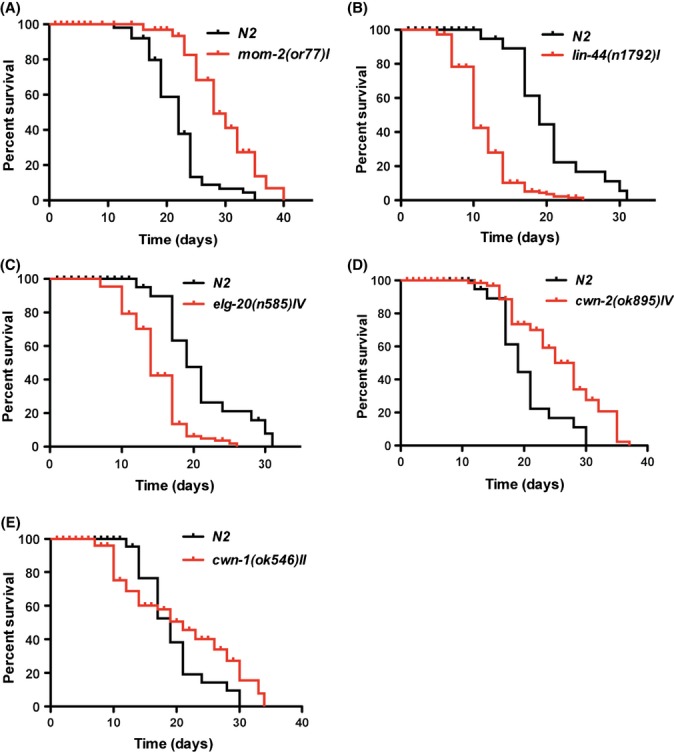
Effect of mutations in various Wnt ligands on longevity. Weak mutation in mom-2/Wnt, mom-2(or77) (A), or deletion of cwn-2/Wnt, cwn- 2(ok895) (D), leads to significantly increased life span of the worms compared to N2 controls (P-value <0.0001). The mutation in lin-44/Wnt, lin-44(n1792) (B), or egl- 20/Wnt, elt-20(n585) (C) shows significantly decreased life span compared to N2 worms (P-value <0.0001). Deletion of cwn-1/Wnt, cwn-1(ok546) (E), does not affect longevity of mutant worms compared to wild type control.
Next, we tested the effect of the lin-44(n1792) mutant on longevity. In this mutant, lin-44 gene contains a nonsense mutation in the second exon that results in premature termination of transcription. These worms display reverse polarity and loss of asymmetry during B- and T-cell divisions (Rocheleau et al., 1999; Sawa et al., 2000). Despite the reversed polarity, lin-44(n1792) mutants are generally healthy and do not display severe morphological or physiological defects. The only side effect of lin-44(n1792) is an egg-laying defect. At 20 °C, about 50% of the adult hermaphrodites retain eggs. We performed lifespan analysis of the lin-44(n1792) mutation at 15 °C and observed that only 20% of the population was egg-laying defective. These individuals were censored and not counted in our longevity analysis. As in the case of RNAi treatment, the lin-44(n1792) mutation significantly decreases longevity of the worms compared to wild-type controls (Fig. 4B).
For egl-20/Wnt, we chose the egl-20(n585) mutation to analyze its effect on longevity. The egl-20(n585) mutation has a single-nucleotide substitution that causes replacement of the conserved cysteine with a serine (Harris et al., 1996; Maloof et al., 1999). This mutation was picked for our analysis because it has been shown that it significantly lowers gene and protein expression levels (Pan et al., 2008). However, egl-20(n585) produces several severe developmental defects at a low frequency, such as a worm carcass that gets filled with retained eggs that hatch inside (bag of worms phenotype) (Trent et al., 1983), a delay in egg laying (Desai et al., 1988), reversion of cell polarity, defects in cell migration (particularly in HSN motor neurons and Q neuroblast migration), and very mild defects in hypodermal cell development (Harris et al., 1996; Pan et al., 2008). It has been reported that these egl-20 mutants are slightly temperature sensitive; therefore, we performed our longevity assays at 15 °C to minimize these effects. Individuals that displayed severe developmental defects were censored and not counted. The elg-20(n585) mutation significantly decreased lifespan of the worms compared to wild-type controls (Fig. 4C).
The fourth Wnt ligand, cwn-2/Wnt, plays an important role in regulating nerve ring placement. Mutations that affect cwn-2/Wnt function are not embryonically lethal and do not cause any morphological or physiological defects. We used the cwn-2(ok895) mutant for our analysis. We found that the cwn-2(ok895) mutant lives significantly longer than wild-type worms, implying that cwn-2 function is detrimental for longevity (Fig. 4D).
To test the effect of cwn-1/Wnt on longevity, we used a deletion allele, cwn-1(ok546). This mutation has been created by the International C. elegans Gene Knockout Consortium. During embryogenesis, cwn-1/Wnt is required for cell fate specification in the C-lineage that gives rise to ectodermal and muscles tissue (Baugh et al., 2005). It has been shown that cwn-1(RNAi) treatment results in abnormal tail morphogenesis of C. elegans larvae. Although the cwn-1(ok546) mutant is a null allele of cwn-1, it displays very mild vulva-less phenotypes. About 40% of the animals lack a vulva at 20 °C and have the bag of worm phenotype. This defect is significantly reduced at 15 °C. Therefore, we performed our lifespan analysis of the cwn-1(ok546) mutation at 15 °C and observed that only 15–20% of the population was egg-laying defective. These individuals were censored and not counted in our longevity analysis. The cwn-1(ok546) mutant did not affect median lifespan of the worm, but caused significant extension of the maximal lifespan compared to wild-type controls (Fig. 4E).
These results indicate that even through all five Wnt ligands act in the same manner to activate expression of the elt-5 GATA transcription factor during aging, their general activity in adulthood is quite different. Activities of mom-2/Wnt and cwn-2/Wnt are detrimental for longevity, whereas activities of lin-44/Wnt and egl-20/Wnt are beneficial for long life.
Wnt signaling does not affect stress resistance during aging
There are two possibilities about how Wnt signaling regulates longevity. First, Wnt signaling continues to function after development and regulates the same set of genes during aging, which have detrimental effects on longevity (developmental drift theory). Indeed, we found evidence that in the case of elt-5 and elt-3 GATA transcription factors, Wnt signaling regulates their expression in both development and aging. Our results suggest that all five Wnts function to induce expression of elt-5 GATA, to repress expression of elt-3 and to drive aging.
The second possibility is that Wnt signaling changes function during aging, for example, from development to maintenance and stress response (damage accumulation theory). We hypothesized that Wnt signaling activity is increased as a result of stress and damage accumulation.
In test of this theory concerning Wnt function, we first analyzed whether the increased expression of Wnt ligands is influenced by oxidative damage, stress, impaired mitochondrial function, or pathogenic infections. To do this, we used a previously published microarray analysis of heat shock (McCarroll et al., 2004), hypoxia response (Shen et al., 2005), mitochondria dysfunction (Falk et al., 2008), and response to Pseudomonas aeruginosa infection (Shapira et al., 2006) to determine how these stresses affect Wnt ligands expression. Interestingly, we found that none of these stresses changes expression of Wnt ligands during aging suggesting an intrinsic mechanism of regulation.
Next, we tested the hypothesis that Wnt ligands switched their function from development to maintenance. If Wnt ligands function to promote aging (cwn-2 and mom-2) by repressing expression of stress response genes, then cwn-2(ok895) and mom-2(or77) mutants would have higher resistance to stress and cellular damage. On the other hand, if Wnt ligands have an anti-aging role (egl-20 and lin-44) by activating stress response genes and protection form oxidative stress and cellular damage, then egl-20(n585) and lin-44(n1792) mutants would be even more susceptible to stress and cellular damage than wild-type animals. We measured the survival of 1-day-old worms exposed to two stress conditions that most likely linked to aging, heat shock, and oxidative stress. Interestingly, none of the mutants showed significant resistance or sensitivity to heat shock (Fig. 5A–B) or oxidative stress (Fig. 5C–D) (see also Table S1). These data suggest that the mechanism by which Wnt signaling regulates aging might be different than just regulation of its ability to respond to stress and damage. Thus, a comprehensive analysis of targets of Wnt signaling during aging is crucial to this endeavor.
Figure 5.
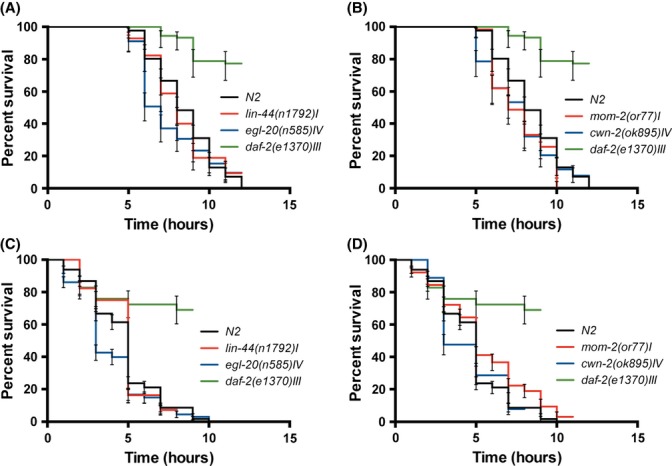
Wnt signaling does not affect worm’s thermo-tolerance and resistance to oxidative stress during aging. None of the mutants show resistance or sensitivity to heat shock stress (A, B) or to oxidative stress (C, D). As a positive control daf-2(e1370) was included in both assays. The graphs depict the averages from three independent experiments.
Discussion
This work characterizes the role of Wnt proteins in the natural aging process. We used previously described transcriptional GFP reporters for all five Wnt genes (Gleason et al., 2006) to determine the level and source of expression of Wnt ligands during aging. We showed that Wnt ligands are expressed past development and Wnt signaling activity is increased as the worm ages. However, not all the cells that express Wnt ligands during development continue to express the same Wnt ligands in adulthood. In fact, only lin-44 preserves the same expression pattern during development and adulthood. The most striking change in expression pattern was observed for mom-2/Wnt. During development, mom-2/Wnt was reported to be expressed all throughout the anterior–posterior axis (Gleason et al., 2006), whereas our analysis of mom-2 expression in adult worms revealed predominantly posterior pattern of expression. This observation has been corroborated by the study of Wnt gene expression using single molecule FISH (smFISH) in C. elegans (Harterink et al., 2011). Harterink et al., have observed mom-2 expression only in the germ line precursor cells and in the few unidentified cells in the tail. Unfortunately, due to technical limitations in visualization of the smFISH probes in adult worms (personal communication), the analysis of Wnt expression could only be performed in embryos and L1 staged worms. It is possible then that mom-2 expression increases during development all along the anterior–posterior body axis as described in the study of Gleason et al. (2006). The question remains however, what forces mom-2 expression to be relocated to the posterior intestinal and intestinal-rectal valve cells during aging?
Next, we determined that Wnt signaling during aging also regulates the elt-3/elt-5 transcriptional circuit. In particular, all five Wnts act as activators of elt-5 GATA expression and down-regulation of any of the Wnts causes down-regulation of elt-5 and subsequent up-regulation of elt-3 GATA transcription factors. These results suggest redundant negative function of Wnt signaling during aging. However, this is not the case. In this study, we reveal that function of all five Wnts is only redundant in regulation of the elt-3/elt-5 transcriptional circuit and not in longevity as a whole. In mom-2/Wnt and cwn-2/Wnt mutants, elt-3 expression is increased (a biomarker of young age) and worms live ~35% and ~18% longer compared to wild-type controls. In contrast, in lin-44/Wnt and egl-20/Wnt mutants, elt-3 expression is also increased, but worms live ~30% and ~25% shorter than their wild-type controls. Clearly, the elt-3/elt-5 transcriptional circuit is not the only genetic circuit regulated by Wnts. It seems that continued activity of mom-2/Wnt and cwn-2/Wnt in adult worms is detrimental for longevity, while lin-44/Wnt egl-20/Wnt is probably regulating many genetic programs that are beneficial for worm longevity. That is why mutation in lin-44/Wnt and egl-20/Wnt decreases lifespan of the worms despite its advantageous effect on the elt-3/elt-5 transcriptional circuit (Fig. 6). To better understand the dual role of the Wnt signaling pathway in age regulation, extensive analysis of transcriptional outcomes of the mom-2/cwn-2 and lin-44/egl-20 Wnts signaling is required.
Figure 6.
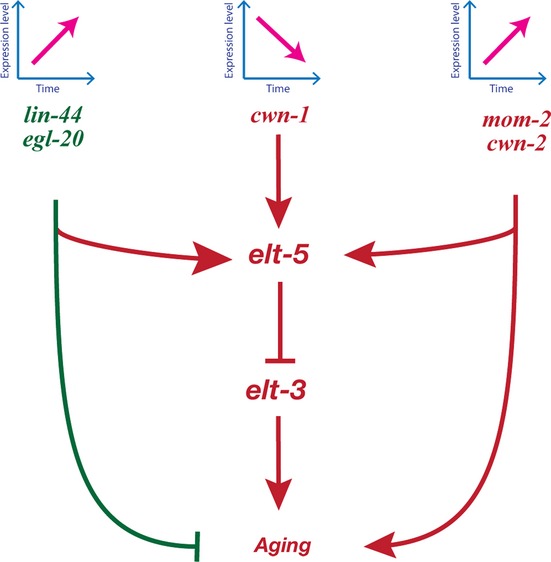
Model for role of the Wnt signaling pathways in age-regulation. The activity of the Wnt signaling pathway increases as worms age, leading to increased expression of elt-5 GATA transcription factor. This GATA transcription factor represses the expression of elt-3 in old worms. Changes in the elt-3 GATA transcription factor activate a cascade of downstream effects, which causes aging. The elt-3/elt-5 GATA transcriptional circuit could be one of a number of genetic programs that are under control of the Wnt signaling pathway during aging. In addition to developmental programs for aging, Wnt signaling might regulate genes responsible for cellular stress response, but not for oxidative damage.
Some unanswered key questions are the following: Why does Wnt signaling activity increase with age and what post-developmental function does Wnt signaling play in post mitotic organisms? There are at least two major theories to explain aging at the molecular level. During the last several decades, the ‘free-radical theory of aging’ offered a credible explanation for the molecular mechanisms underlying the aging process. This theory proposes that aging is a result of cellular and environmental damage (extrinsic factors) that accumulate over time. Indeed, damage accumulation explains some of the molecular changes observed with age, such as increased levels of protein oxidation and age pigments (Ishii et al., 2002; Gerstbrein et al., 2005). Furthermore, genetic studies have revealed that most mutations that have extended lifespan have either increased resistance to stress (e.g., daf-2) or decreased metabolic rate and concomitant decrease in the production of free radicals (e.g., clk-1) (Ewbank et al., 1997). However, we found that none of the stresses that are known to contribute to aging affect expression of Wnt ligands. In addition, in contrast to daf-2, mutation of neither mom-2 or cwn-2 nor lin-44 or egl-20 Wnts brings increased resistance or sensitivity to heat stress or oxidative damage.
Another view is that aging finds origin in genetic determinants whose effects are beneficial early in life (development), but become detrimental in later stages (antagonistic pleiotropy theory) (Williams, 1957; Kirkwood & Rose, 1991). There are many cases of antagonistic pleiotropy in mammals, often showing how developmental pathways, important effectors of corporeal organization and structure formation, contribute to aging later in life (de Magalhães, 2012). Thus, we hypothesized that an age-related increase in Wnt ligands expression and activity is due to passive drift within intrinsic process. In the case of mom-2/Wnt- and cwn-2/Wnt-regulated Wnt signaling pathways, we find a perfectly clear example of antagonistic pleiotropy. However, we have found that other Wnt ligands (lin-44 and egl-20) have pleiotropic function during development and aging, but their function is not antagonistic to, but rather beneficial for life.
Exploring the molecular mechanisms controlled by Wnt signaling pathways during aging will help us to elucidate how developmental pathways function past development. Mutations or misregulation of Wnt signaling pathways in mice and humans have been linked to various age-related diseases, such as osteoporosis, metabolic disorders, and cancer (Clevers & Nusse, 2012). This study presents the first successful attempt to develop C. elegans as a model system to study the role of Wnt signaling pathways in aging. In the future, we will use the vast knowledge and resources that have been built in studying the role of Wnt signaling in development to answer the most important question: do Wnt-regulated developmental pathways, which create young tissue, continue to work as the animals grow old, instead contributing to aging and death? If we understand this aspect of Wnt signaling, we can potentially apply this knowledge in higher organisms to develop therapies to prevent the occurrence of many age-related diseases.
Materials and methods
Strains
All C. elegans strains (Table S2) were maintained and handled as described previously (Brenner, 1974).
Analysis of LifeSpan
Lifespan analyses were conducted at 15 or 20 °C as previously described (Kenyon et al., 1993). At least 100 worms were used for each experiment. Age refers to days following adulthood, and P-values were calculated using the log-rank (Mantel-Cox) method (Lawless, 1982) in Prism 6 software.
RNA-interference (RNAi) experiments
HT115 bacteria transformed with RNAi vectors expressing dsRNA of the genes of interest were grown at 37 °C in LB with 100 μg mL−1 ampicillin and then seeded onto NG-ampicillin plates supplemented with 2 mm IPTG and 30 μm of FUDR. One-day-old young adult worms were added to the plates and transferred to new plates every 4 days.
RNA extraction and qRT-PCR Analysis
Wild-type worms and transgenic lines carrying POPTOP and POPFOP reporters were synchronized by hypochlorite treatment and hatching on unseeded NGM agar plates, and then grown to the three different stages (2, 5, and 10 days) of adulthood. Approximately 400 worms were harvested for each strain per time point. Total RNA was extracted using RNAqueous Kit (Ambion, Austin, TX, USA Cat #AM1931) according to the manufacturer’s instructions. 200 ng of total RNA was reverse-transcribed to cDNA using oligo (dT) primers (Fermentas, ThermoFisher Scientific, Netherlands, Cat #SO131) and Maxima Reverse Transcriptase (Fermentas, Cat #EP0741). Quantitative real-time PCR (qRT-PCR) analysis was performed in triplicate with Power SYBR Green RNA-to-Ct 1-Step Kit (Applied Biosystems, Foster City, CA, USA, Part #4389986), according to the manufacturer’s instructions. Vector pD4H1 containing mCherry sequence was used to build a standard curve. The primers mCherry-Forward AGGGTTTTAAGTGGGAACGC and mCherry-Reverse GCATAACAGGTCCATCCGAG were used to determine the expression levels of mCherry. Mom-2 Fw (qRT) CACATCAACACGCCAGTTCT and mom-2 Rev (qRT) CACATTCGCTTCTGTTGAGG were used to detect mom-2 expression level; cwn-2 Fw (qRT) GCATTGCCAACGGATTTAGT and cwn-2 Rev (qRT) TATCCTCGTCCACAGCACAA were used to detect cwn-2 expression level.
Imaging and quantification of GFP and Cherry expression
To examine changes in expression of GFP and mCherry reporters with respect to age and RNAi treatment, we picked 20 worms at 2 different ages (2 and 5 days of adulthood), grown on control or anti-Wnts RNAi plates, and then measured for levels of GFP and mCherry expression using quantitative fluorescence microscopy. Specifically, we used pixel intensity to quantify the level of GFP or mCherry expression for both wild-type and RNAi treatment for each gene. Twenty hermaphrodites from each strain were analyzed for GFP and mCherry expression using a Zeiss Axioplan microscope. Comparison of all images was carried out on the same day with the same microscope settings. Images were analyzed using ImageJ, a public domain Java image-processing program (Rasband, 2004).
Oxidative stress and heat-shock survival assays
Oxidative stress resistance assays were performed in 24-well plates as previously described (Budovskaya et al., 2008). Briefly, 1-day-old adult hermaphrodites were immersed in S-basal media containing 200 mm of paraquat. Worms were scored every hour until all worms were scored as dead by touch-provoked movement. Three independent trials were pooled for analysis. Heat-shock survival assay were performed by placing worms at 35 °C and recording their rate of death, as previously described (Budovskaya et al., 2008). Three-independent trials were pooled for analysis.
Acknowledgments
We thank all members of Stanley Brul lab for discussions and comments on the manuscript; the whole C. elegans community and Caenorhabditis Genetic Center (CGC) funded by NIH office of Research Infrastructure Programs (P40 OD010440) for most of the worm strains used in this study. This work was supported by MacGillavry fellowship at the University of Amsterdam.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Fig. S1 Expression patterns of lin-44pro::4xNLSGFP, elt-20pro::4xNLS GFP, and mom-2pro::4xNLS GFP Wnt ligands during aging.
Fig. S2 Expression patterns cwn-1pro::4xNLSGFP and cwn-2pro::4xNLSGFP Wnt ligands in young adult worms.
Fig. S3 Post-translational stability of ELT-3 GATA is not affected in Wnts RNAi treatment.
Fig. S4 mom-2(RNAi) treatment does not slow the rate of reproduction of wild type worms.
Table S1 The median life span of the wild type and Wnt ligand mutant worms does not change after exposure to the cellular or the oxidative stress.
Table S2 Strains used in this study.
References
- Baugh LR, Hill AA, Claggett JM, Hill-Harfe K, Wen JC, Slonim DK, Brown EL, Hunter CP. The homeodomain protein PAL-1 specifies a lineage-specific regulatory network in the C. elegans embryo. Development. 2005;132:1843–1854. doi: 10.1242/dev.01782. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya YV, Wu K, Southworth LK, Jiang M, Tedesco P, Johnson TE, Kim SK. An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell. 2008;134:291–303. doi: 10.1016/j.cell.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassata G, Shemer G, Morandi P, Donhauser R, Podbilewicz B, Baumeister R. ceh-16/engrailed patterns the embryonic epidermis of Caenorhabditis elegans. Development. 2005;132:739–749. doi: 10.1242/dev.01638. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Ceron J, Rual J-F, Chandra A, Dupuy D, Vidal M, Van den Heuvel S. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Dev. Biol. 2007;7:30. doi: 10.1186/1471-213X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-Catenin Signaling and Disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336:638–646. doi: 10.1038/336638a0. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM. 2005. pp. 1–17. Wnt signaling. WormBook: the online review of C. elegans biology. [DOI] [PMC free article] [PubMed]
- Ewbank JJ, Barnes TM, Lakowski B, Lussier M, Bussey H, Hekimi S. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science. 1997;275:980–983. doi: 10.1126/science.275.5302.980. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Falk MJ, Zhang Z, Rosenjack JR, Nissim I, Daikhin E, Sedensky MM, Yudkoff M, Morgan PG. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans. Mol. Genet. Metab. 2008;93:388–397. doi: 10.1016/j.ymgme.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstbrein B, Stamatas G, Kollias N, Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005;4:127–137. doi: 10.1111/j.1474-9726.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- Gleason JE, Szyleyko EA, Eisenmann DM. Multiple redundant Wnt signaling components function in two processes during C. elegans vulval development. Dev. Biol. 2006;298:442–457. doi: 10.1016/j.ydbio.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008;134:646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Honigberg L, Robinson N, Kenyon C. Neuronal cell migration in C. elegans: regulation of Hox gene expression and cell position. Development. 1996;122:3117–3131. doi: 10.1242/dev.122.10.3117. [DOI] [PubMed] [Google Scholar]
- Harterink M, Kim DH, Middelkoop TC, Doan TD, Van Oudenaarden A, Korswagen HC. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development. 2011;138:2915–2924. doi: 10.1242/dev.064733. (Cambridge, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Vassilieva LL, Horvitz HR, Shaw JE, Herman RK. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83:101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Ishii N, Goto S, Hartman PS. Protein oxidation during aging of the nematode Caenorhabditis elegans. Free Radical Biol. Med. 2002;33:1021–1025. doi: 10.1016/s0891-5849(02)00857-2. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Koh K, Peyrot S, Wood C. Cell fates and fusion in the C. elegans vulval primordium are regulated by the EGL-18 and ELT-6 GATA factors—Apparent direct targets of the LIN-39 Hox protein. Development. 2002;129:5171–5180. doi: 10.1242/dev.129.22.5171. [DOI] [PubMed] [Google Scholar]
- Lawless JF. Statistical Models and Methods for Lifetime Data. 2nd edn. New York: John Wiley & Sons; 1982. 2011. [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro MF, Hill RJ, Heid PJ, Newman-Smith ED, Zhu J, Priess JR, Rothman JH. Genetic redundancy in endoderm specification within the genus Caenorhabditis. Dev. Biol. 2005a;284:509–522. doi: 10.1016/j.ydbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Kasmir JJ, Zhu J, Rothman JH. The Wnt effector POP-1 and the PAL-1/Caudal homeoprotein collaborate with SKN-1 to activate C. elegans endoderm development. Dev. Biol. 2005b;285:510–523. doi: 10.1016/j.ydbio.2005.06.022. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage? FASEB J. 2012;26:4821–4826. doi: 10.1096/fj.12-210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloof JN, Whangbo J, Harris JM, Jongeward GD, Kenyon C. A Wnt signaling pathway controls hox gene expression and neuroblast migration in C. elegans. Development. 1999;126:37–49. doi: 10.1242/dev.126.1.37. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- McCarroll SSA, Murphy CCT, Zou S, Pletcher SD, Chin C-S, Jan YN, Kenyon C, Bargmann CI, Li H. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nature. 2004;36:197–204. doi: 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated surviving signaling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Sumida T, Nomura S, Liu M-L, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee J-K, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C-L, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev. Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. 2004.
- Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 Activates the LIT-1 Protein Kinase to Transduce Anterior/Posterior Polarity Signals in C. elegans. Cell. 1999;97:717–726. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- Sawa H, Kouike H, Okano H. Components of the SWI/SNF complex are required for asymmetric cell division in C. elegans. Mol. Cell. 2000;6:617–624. doi: 10.1016/s1097-2765(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Shapira M, Hamlin BJ, Rong J, Chen K, Ronen M, Tan M-W. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc. Natl Acad. Sci. USA. 2006;103:14086–14091. doi: 10.1073/pnas.0603424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 2005;280:20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- Shetty P, Lo M, Robertson S, Lin R. C. elegans TCF protein, POP-1, converts from repressor to activator as a result of Wnt-induced lowering of nuclear levels. Dev. Biol. 2005;285:584–592. doi: 10.1016/j.ydbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Song S, Zhang B, Sun H, Li X, Xiang Y, Liu Z, Huang X, Ding M. A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Genet. 2010;6:pii–e1001056. doi: 10.1371/journal.pgen.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, Van Beest M, Van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Expression patterns of lin-44pro::4xNLSGFP, elt-20pro::4xNLS GFP, and mom-2pro::4xNLS GFP Wnt ligands during aging.
Fig. S2 Expression patterns cwn-1pro::4xNLSGFP and cwn-2pro::4xNLSGFP Wnt ligands in young adult worms.
Fig. S3 Post-translational stability of ELT-3 GATA is not affected in Wnts RNAi treatment.
Fig. S4 mom-2(RNAi) treatment does not slow the rate of reproduction of wild type worms.
Table S1 The median life span of the wild type and Wnt ligand mutant worms does not change after exposure to the cellular or the oxidative stress.
Table S2 Strains used in this study.