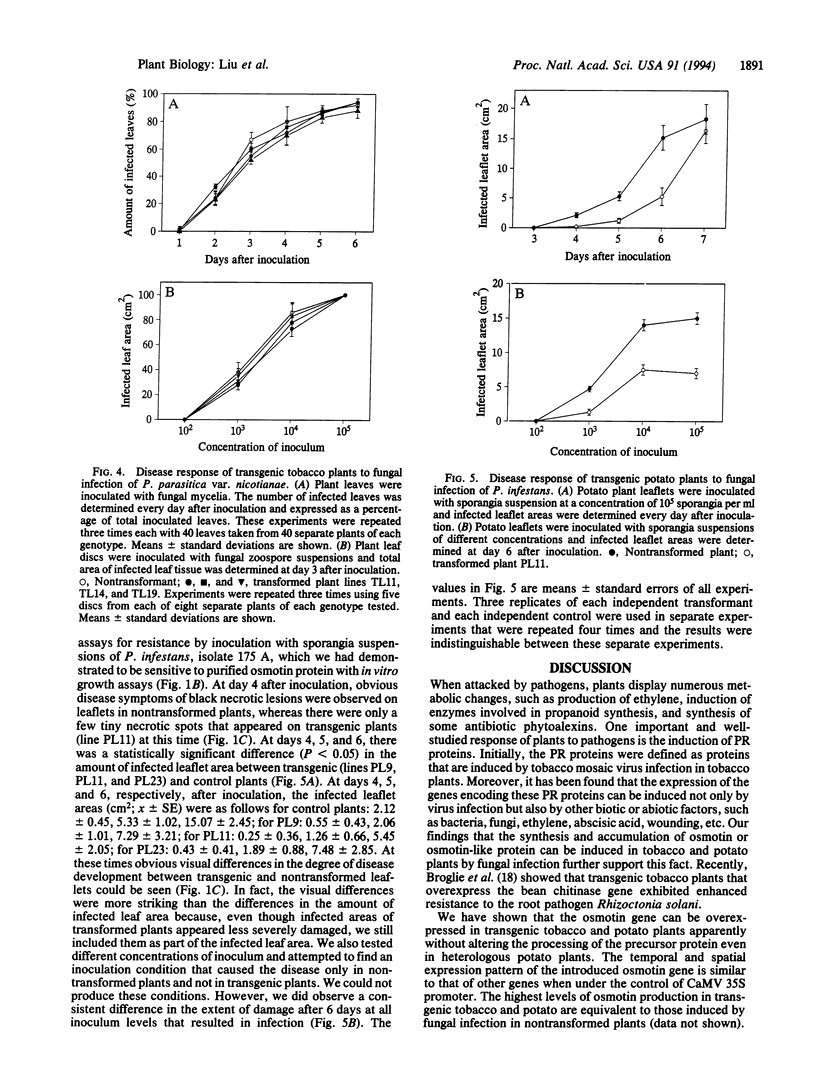
Abstract
Transgenic potato and tobacco plants carrying the osmotin gene under the control of the cauliflower mosaic virus 35S promoter constitutively overexpressed osmotin to a level of approximately 2% of total cellular protein. Leaves of transgenic potato plants exhibited delayed development of disease symptoms after inoculation with spore suspensions of Phytophthora infestans, which is the cause of late blight disease of potato. In contrast, transgenic tobacco plants did not display any change in the development of disease symptoms when challenged with either spore suspensions or fungal mycelia of Phytophthora parasitica var. nicotianae. Using in vitro assays, purified osmotin was found to be more effective against P. infestans. Some inhibition of P. parasitica also was observed in vitro even though no in vivo effect could be established.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Brogue K., Chet I., Holliday M., Cressman R., Biddle P., Knowlton S., Mauvais C. J., Broglie R. Transgenic Plants with Enhanced Resistance to the Fungal Pathogen Rhizoctonia solani. Science. 1991 Nov 22;254(5035):1194–1197. doi: 10.1126/science.254.5035.1194. [DOI] [PubMed] [Google Scholar]
- Casas A. M., Nelson D. E., Raghothama K. G., D'Urzo M. P., Singh N. K., Bressan R. A., Hasegawa P. M. Expression of Osmotin-Like Genes in the Halophyte Atriplex nummularia L. Plant Physiol. 1992 May;99(1):329–337. doi: 10.1104/pp.99.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutt J. R., Harpster M. H., Dixon D. C., Carr J. P., Dunsmuir P., Klessig D. F. Disease response to tobacco mosaic virus in transgenic tobacco plants that constitutively express the pathogenesis-related PR1b gene. Virology. 1989 Nov;173(1):89–97. doi: 10.1016/0042-6822(89)90224-9. [DOI] [PubMed] [Google Scholar]
- Hejgaard J., Jacobsen S., Svendsen I. Two antifungal thaumatin-like proteins from barley grain. FEBS Lett. 1991 Oct 7;291(1):127–131. doi: 10.1016/0014-5793(91)81119-s. [DOI] [PubMed] [Google Scholar]
- Kononowicz A. K., Nelson D. E., Singh N. K., Hasegawa P. M., Bressan R. A. Regulation of the Osmotin Gene Promoter. Plant Cell. 1992 May;4(5):513–524. doi: 10.1105/tpc.4.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Ryals J. A., Ward E. R., Dixon R. A. Emerging strategies for enhancing crop resistance to microbial pathogens. Biotechnology (N Y) 1992 Nov;10(11):1436–1445. doi: 10.1038/nbt1192-1436. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Chen Z., Nelson D. E., Singh N. K., Hasegawa P. M., Bressan R. A. Osmotin gene expression is posttranscriptionally regulated. Plant Physiol. 1992 Sep;100(1):409–415. doi: 10.1104/pp.100.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst H. J., Meuwissen R. L., Kauffmann S., Bol J. F. Constitutive expression of pathogenesis-related proteins PR-1, GRP, and PR-S in tobacco has no effect on virus infection. Plant Cell. 1989 Mar;1(3):285–291. doi: 10.1105/tpc.1.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. E., Raghothama K. G., Singh N. K., Hasegawa P. M., Bressan R. A. Analysis of structure and transcriptional activation of an osmotin gene. Plant Mol Biol. 1992 Jul;19(4):577–588. doi: 10.1007/BF00026784. [DOI] [PubMed] [Google Scholar]
- Neuhaus J. M., Ahl-Goy P., Hinz U., Flores S., Meins F., Jr High-level expression of a tobacco chitinase gene in Nicotiana sylvestris. Susceptibility of transgenic plants to Cercospora nicotianae infection. Plant Mol Biol. 1991 Jan;16(1):141–151. doi: 10.1007/BF00017924. [DOI] [PubMed] [Google Scholar]
- Neuhaus J. M., Flores S., Keefe D., Ahl-Goy P., Meins F., Jr The function of vacuolar beta-1,3-glucanase investigated by antisense transformation. Susceptibility of transgenic Nicotiana sylvestris plants to Cercospora nicotianae infection. Plant Mol Biol. 1992 Aug;19(5):803–813. doi: 10.1007/BF00027076. [DOI] [PubMed] [Google Scholar]
- Reimmann C., Dudler R. cDNA cloning and sequence analysis of a pathogen-induced thaumatin-like protein from rice (Oryza sativa). Plant Physiol. 1993 Mar;101(3):1113–1114. doi: 10.1104/pp.101.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo I., Vera P., Frank R., Conejero V. Identification of the viroid-induced tomato pathogenesis-related (PR) protein P23 as the thaumatin-like tomato protein NP24 associated with osmotic stress. Plant Mol Biol. 1991 May;16(5):931–934. doi: 10.1007/BF00015088. [DOI] [PubMed] [Google Scholar]
- Schardl C. L., Byrd A. D., Benzion G., Altschuler M. A., Hildebrand D. F., Hunt A. G. Design and construction of a versatile system for the expression of foreign genes in plants. Gene. 1987;61(1):1–11. doi: 10.1016/0378-1119(87)90359-3. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes S., Mauch-Mani B., Moyer M., Potter S., Williams S., Dincher S., Chandler D., Slusarenko A., Ward E., Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992 Jun;4(6):645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers A. J., Roberts W. K., Selitrennikoff C. P. A new family of plant antifungal proteins. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):315–323. doi: 10.1094/mpmi-4-315. [DOI] [PubMed] [Google Scholar]
- Woloshuk C. P., Meulenhoff J. S., Sela-Buurlage M., van den Elzen P. J., Cornelissen B. J. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991 Jun;3(6):619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Hatada M., van der Wel H., Krabbendam H., Peerdeman A. F., Kim S. H. Three-dimensional structure of thaumatin I, an intensely sweet protein. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1406–1409. doi: 10.1073/pnas.82.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]