Abstract
Recent discoveries have revealed the key role of mTOR (target of rapamycin) in aging. Furthermore, rapamycin extends lifespan in mice, especially in female mice. Here, we treated obese male mice on high-fat diet with rapamycin given intermittently: either weekly (once a week) or alternating bi-weekly (three injections every other week). While only marginally reducing obesity, intermittent administration of rapamycin significantly extended lifespan. Significance was achieved for weekly treated group and for the three rapamycin-received groups combined. In weekly treatment group, 100% mice were alive by the age of 2 years, whereas 60% of mice died in untreated group by this age. The effect of weekly treatment on survival was highly significant and cannot be fully explained by partial reduction in obesity. Alternating bi-weekly treatments seem to be less effective than weekly treatment, although effects of additional factors (see Discussion) may not be excluded. After one year of treatment, all survived mice were sacrificed 8 days after the last administration of rapamycin to avoid its direct interference with parameters examined. Fasting levels of cardiac and hepatic p-S6, a marker of mTORC1 activity, were lower in weekly treatment group compared with control mice. In contrast, levels of p-Akt (S473), glucose, triglycerides and insulin were unchanged, whereas leptin and IGF-1 tended to be lower. Thus, weekly treatment with rapamycin may slow down aging in obese male mice on high-fat diet.
Keywords: aging, anti-aging agent, longevity, mammalian or mechanistic target of rapamycin, mortality, obesity, rapamycin
Introduction
mTOR (mammalian or mechanistic target of rapamycin) is involved in organismal aging (Blagosklonny & Hall, 2009; Stanfel et al., 2009; Kapahi et al., 2010; Sengupta et al., 2010b; Bjedov & Partridge, 2011; Zoncu et al., 2011; Cornu et al., 2012; Flynn et al., 2013; Johnson et al., 2013). Inhibition of mTOR suppresses geroconversion from reversible arrest to senescence (Demidenko et al., 2010; Leontieva et al., 2012c, 2013a). An increasing number of studies have demonstrated that rapamycin extends lifespan in mice and prevents age-related pathologies including cancer (Harrison et al., 2009; Miller et al., 2011; Anisimov et al., 2011b, 2010; Ramos et al., 2014; Wilkinson et al., 2012; Spong & Bartke, 2012; Selman & Partridge, 2012; Neff et al., 2013; Ye et al., 2013; Flynn et al., 2013; Zhang et al., 2014). Given that rapamycin is a clinically approved drug, it potentially can be used to slow aging in humans. The effect of rapamycin on longevity has been studied in mice on regular diet. Yet, most humans abuse high-calorie diet and suffer from obesity. Will obesity and high-fat (HF) diet counteract beneficial effects of rapamycin? Also, rapamycin extends lifespan in male mice to a lesser degree compared with female mice (Harrison et al., 2009; Miller et al., 2011), probably in part due to different sensitivity to rapamycin (Leontieva et al., 2012a). Most previous studies used chronic (everyday) administration of rapamycin. Here, we investigated the effect of intermittent treatment with rapamycin followed by treatment-free breaks. Albeit based on theoretic considerations (Blagosklonny, 2012b), the choice of treatment schedules was relatively arbitrary. Two groups (R1 and R3) of mice on high-fat diet received 3 i.p. injections of 1.5 mg kg−1 or 0.5 mg kg−1 of rapamycin during one week followed by a treatment-free week (alternating bi-weekly schedule). Also we included a simple, every-week treatment with one injection of 1.5 mg kg−1 of rapamycin (weekly or R2 group). This weekly schedule (group R2) resulted in superior effects.
Results
Rapamycin tended to decrease body weight on HF diet
Three groups of male mice on high-fat diet were treated by i.p. injections with the following intermittent schedules: R1 – 1.5 mg kg−1 three times/week every other week; R2 (weekly schedule) – 1.5 mg kg−1 week−1; and R3 – 0.5 mg kg−1 three times per week every other week (Fig. 1). These groups of mice were fed with HF food for 3 months before the start of the current study (Experimental procedures, prehistory). So at the start of the treatment, all three treatment groups were obese (Fig. 1) and were similar to control HF diet group (compare weights at week 0 in Fig. 1). When this treatment started (Fig. 1), there was a transient decrease in absolute body weight in three rapamycin-treated groups (Fig. 1). In contrast, control mice on high-fat diet continued to gain weight until reaching a plateau after 8 months (Fig. 1 and Fig. S1, Supporting information). By the end of the experiment, mice in group R2 showed tendency to weigh less than control HF mice (Fig. 1). There was a statistically significant, albeit transient reduction in weight gain in R2 group compared with HF control group (Fig. S1, Supporting information indicated by asterisks).
Figure 1.
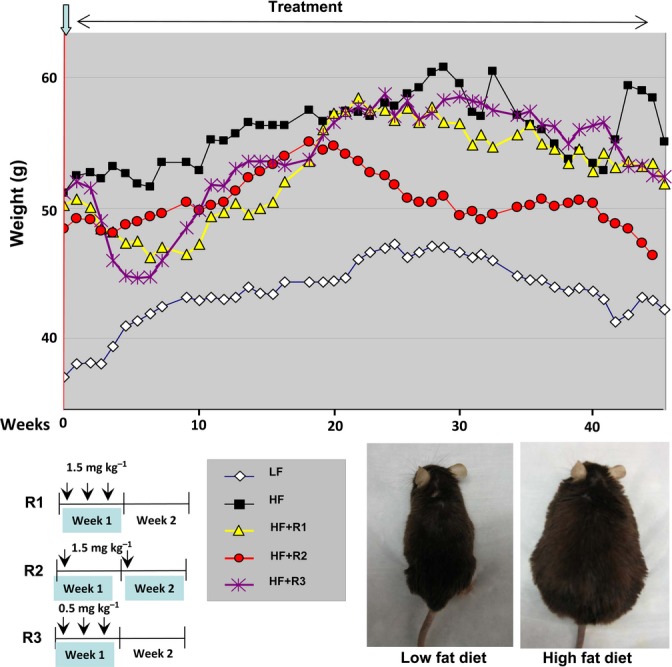
Weekly body weight during the experiment. Absolute body weights. Nine-month-old male mice were divided into five groups: low-fat (LF) diet control mice and four high-fat (HF) diet groups: untreated control (HF), R1 – 1.5 mg kg−1 rapamycin three times/week every other week; R2 group – 1.5 mg kg−1 per a week every week and R3 group – 0.5 mg kg−1 three times/week every other week. Treatment was continued for 11 months, and weight was measured every week. Upper panel – average weights for each group are presented. Bottom panel – schemas of rapamycin treatment schedules and photos of representative mice from low-fat and high-fat control groups are shown.
Rapamycin extends lifespan in obese male mice
Analysis of overall survival of individual rapamycin-treated groups versus HF control mice revealed that weekly treatment with rapamycin (schedule R2) significantly prevented morbidity and death in obese male mice on HF diet (Fig. 2A). Whereas 60% of control mice on high-fat diet died or were sacrificed due to morbidity, all mice in R2 group survived. (The cause of death was not always determined so we do not provide these data). There was a high statistically significant difference (P = 0.0063) in overall survival of mice in group R2 compared with control HF group (Fig. 2A). Survival rate of all three rapamycin-treated groups taken together for analysis also was significantly higher when compared to control HF group (P = 0.028, Fig. 2B). Thus, significance was achieved for group R2 and for the three rapamycin-received groups combined, relative to the HF group. Alternating bi-weekly treatments (groups R1 and R3) showed tendency to increase survival, but it was not statistically significant (Fig. S2, Supporting information).
Figure 2.
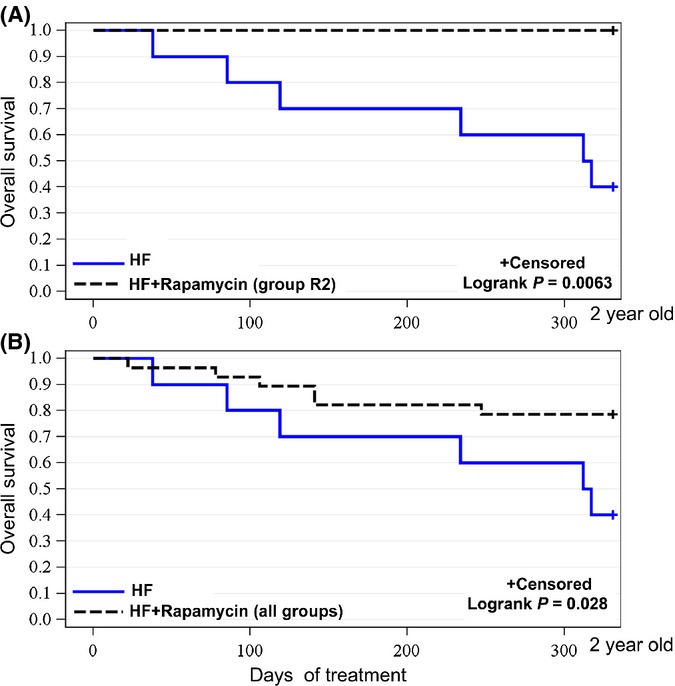
Rapamycin extended lifespan of mice on high-fat (HF) diet. (A). Kaplan–Meier survival rate curve for control mice on HF diet (HF; n = 10) and mice treated with rapamycin following schedule R2 (HF+rapamycin (group R2); n = 9). (B). Kaplan–Meier survival rate curve for control mice on HF diet (HF; n = 10) and all groups of mice treated with rapamycin (all groups) taken together (n = 28).
Levels of p-S6 in surviving mice
After eleven months of treatment, when 60% of mice in HF control group died, the experiment was stopped to examine the surviving mice. In all three rapamycin-treated groups, mice were sacrificed 8 days after the last treatment, to eliminate direct effects of rapamycin on examined parameters. We focused the study on the heart and the liver. Previous reports point to the heart (the cardiac muscle) as to also being very important tissue to measure phospho-S6 (Hua et al., 2011; Leontieva et al., 2012a, 2013b; Ramos et al., 2012; Flynn et al., 2013; Wu et al., 2013; Zhou et al., 2013). The heart is one of the most commonly studied organs in aging, and rapamycin treatment improves the heart condition. Furthermore, cardiovascular diseases are the most common cause of death in humans. Importantly, our previous studies implicate the cardiac phospho-S6 as a potential marker of longevity and animal hypertrophy (Leontieva et al., 2013b) (Leontieva et al., 2012a; Flynn et al., 2013).
In R2 group, levels of cardiac p-S6 were statistically lower than in control HF group (Fig. 3). P-S6 reflects the activity of mTORC1, which is known to be involved in aging. We also measured phosphorylation of AKT at S473, which is in part a marker of mTORC2 activity. In contrast to p-S6, p-AKT(S473) was not decreased in R2 group (Fig. 3).
Figure 3.
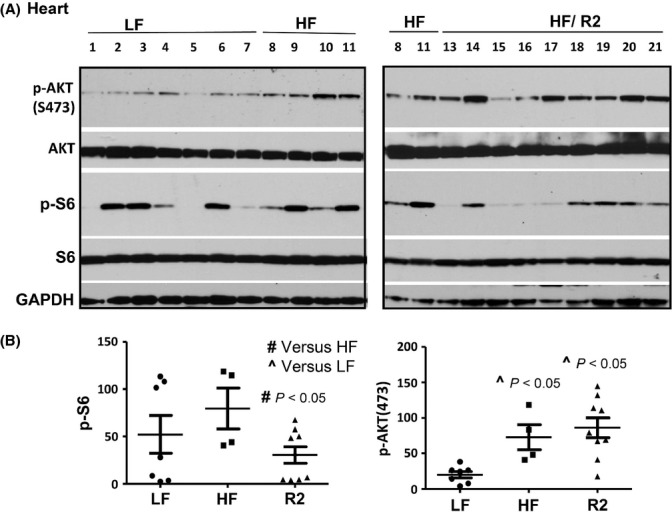
p-S6 in the heart of the survived mice. (A). Immunoblot analysis of protein lysates from the heart of mice on low-fat (LF) or high-fat (HF) diet: control (HF – untreated) or rapamycin treated (group R2 presented – HF/R2). Immunoblotting was performed with the indicated antibodies. Numbers indicate individual mice. (B). Quantitative analysis of data shown in Fig. 3A. Quantified intensities of p-S6 signal (left panel) and signal of p-AKT(Ser473) (right panel) presented as mean ± SE.
We also evaluated these parameters in the liver. In agreement, levels of hepatic p-S6 were significantly lower in R2 group compared with HF control. Similar to the hearts, the hepatic levels of p-AKT(S473) had tendency to be higher in R2 group although it did not reach statistical difference in this particular test (Fig. 4). Noteworthy, the levels of p-S6 and p-Akt in the kidneys were not different in control and rapamycin-treated groups and p-S6 and p-Akt highly correlated (Fig. S4, Supporting information).
Figure 4.
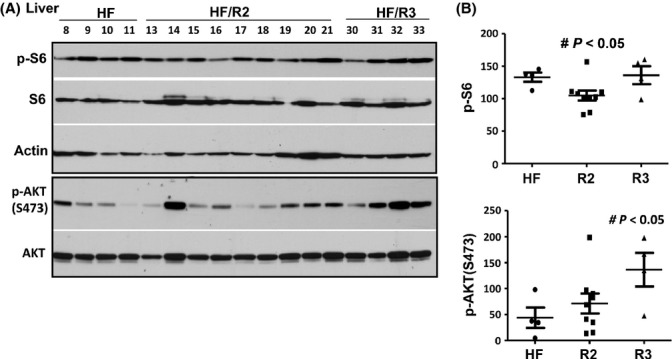
p-S6 and p-Akt in the liver of survived mice. (A). Immunoblot analysis of protein lysates from the liver of ~ 2-year-old male mice on high-fat (HF) diet: control (HF – untreated) or rapamycin treated (groups R2 and R3 presented – HF/R2, HF/R3). Numbers indicate individual mice. Equal loading was also confirmed by staining the membrane with Commassie Blue (Fig. S3, Supporting information). (B). Quantitative analysis of data shown in Fig. 4A. Quantified intensities of p-S6 signal and signal of p-AKT(Ser473) presented as mean ± SE.
Weekly treated mice did not have metabolic abnormalities
As we discussed, there was a tendency to a lesser weight gain in rapamycin-treated mice, albeit only transiently statistically significant (Fig. 1 and Fig. S1, Supporting information). Levels of leptin mirror body weight, consistent with leptin production by fat cells. Importantly, rapamycin treatment did not impair metabolic parameters: It did not increase triglyceride, insulin and IGF1 levels (Fig. 5). Glucose levels were similar in all groups including mice on LF diet. There was no indication on either insulin resistance or overt diabetes in rapamycin-treated groups. This conclusion is supported by comparison of fasted and nonfasted (fed) levels of glucose and insulin (Fig. S5, Supporting information). Glucose levels were tightly controlled and were not increased in nonfasted mice compared with fasted mice (Fig. S5, Supporting information). In agreement, insulin levels were induced in fed mice, controlling glucose levels. In some mice in rapamycin-treated R2 group, there was a strong induction of insulin in response to food, suggesting preserved beta-cell function. As a small number of surviving mice in control HF group precluded statistically significant differences, we used correlations between parameters to estimate potential changes. It was shown that rapamycin decreased body weight gains on HF diet (Chang et al., 2009). We found a strong correlation between weight and leptin levels (Fig. S6, Supporting information). Leptin levels also correlated with insulin, glucose and IGF-1 levels (Fig. S6, Supporting information). Except for one mouse in control HF group, other HF control mice tended to have higher leptin, glucose, IGF-1 and insulin levels compared with mice in R2 group (Fig. S6, Supporting information).
Figure 5.
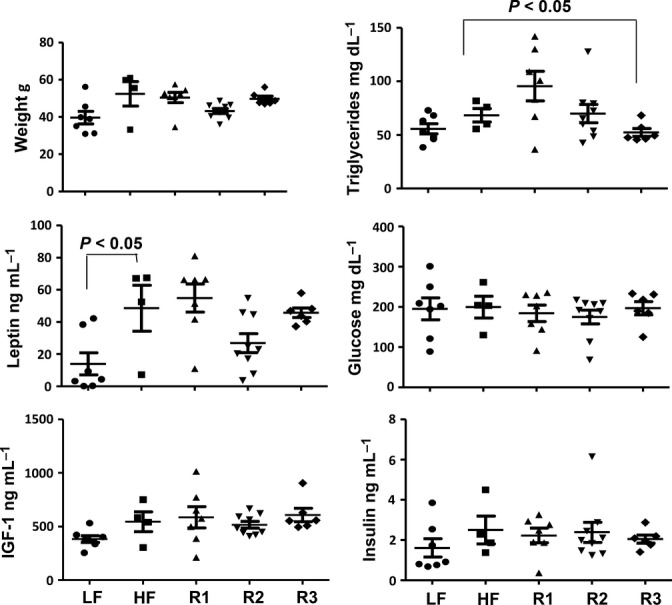
Metabolic parameters in survived mice. Weights and metabolic parameters (leptin, IGF1, triglycerides, glucose and insulin) were determined in fasted plasma and presented as mean ± SE. Fasted blood was collected in the morning after overnight fasting.
Discussion
Here, we demonstrated for the first time that rapamycin extended lifespan in obese mice on high-fat (HF) diet. A significant increase in survival was achieved by intermittent administration of rapamycin. The most prominent effect was observed in mice treated once a week. All mice in this (R2) group survived until the end of experiment. Two other schedules of rapamycin administration (every other week: R1 and R3) showed tendency to decrease mortality, albeit statistical significance was not achieved. Nevertheless, survival in all three rapamycin-treated groups combined (80%) was significantly higher (P < 0.05) than in control HF mice (40%). Noteworthy, this effect was achieved in male mice, which according to previous studies are less responsive to rapamycin compared with female mice (Harrison et al., 2009) (Miller et al., 2011; Leontieva et al., 2012a). It is remarkable that rapamycin was administrated only once a week. Weekly administration of rapamycin partially reduced obesity, but this cannot completely explain the improvement in overall survival. In fact, mortality in lean mice group (control mice on low-fat diet – LF group) was higher than in R2 mice, even though difference in survival was not statistically significant at the end of experiment (Fig. S7, Supporting information). Small group size is one of limitations of this study. Another limitation is slightly different prehistory between R1, R2 and R3 groups. Prior beginning of current study, mice in R3 and R1 groups received low dose of resveratrol for 3 months. However, we believe that this difference is not consequential for several reasons: (i) at the end of the pretreatments, three groups were similar in metabolic parameters; (ii) the group (R2) that showed the best result in the current study did not receive resveratrol, so we cannot attribute the survival effect of rapamycin in this group to prehistory of resveratrol administration.
We also evaluated metabolic parameters in survived mice. Rapamycin treatment was discontinued 8 days before mice were sacrificed, to eliminate direct inhibitory effects of rapamycin on mTOR/S6 pathway. Rapamycin-treated mice did not develop metabolic abnormalities by the end of the study. Fasting levels of glucose, triglyceride and insulin were normal. Leptin and IGF-1 levels showed the tendency to be lower in R2 group than in control HF group. Levels of p-Akt (S473) tended to be higher in R2 mice compared with control mice, indicating that intermittent rapamycin did not obviously affect mTOR complex two, which phosphorylates p-Akt at S473. Levels of phosphorylated S6 (p-S6), a marker of mTOR/S6K activity, were statistically significantly lower in both the cardiac muscle and the liver in R2 group, received rapamycin every week, than in control HF group. Intriguingly, as shown by Sengupta et al. in figure 4C, fasting levels of p-S6 are higher in old mice than in young mice (Sengupta et al., 2010a). Given that p-S6 levels distinguish quiescence from senescence (Blagosklonny, 2012a; Leontieva et al., 2012b), fasting levels of p-S6 may be a potential marker of aging. Levels of p-S6 in the heart muscle are correlated with body size and tend to be higher in male than in female mice (Leontieva et al., 2012a). This is in agreement with observations that big males tend to live shorter than females and small strains of mice. Taken together, these data support the notion that the mTOR/S6K pathway drives both growth and aging (Blagosklonny & Hall, 2009).
mTOR is activated by insulin, IGF-1 and inflammatory cytokines (Zoncu et al., 2011; Cornu et al., 2012), all of which are markers of fast aging and poor health. Furthermore, activated mTOR/S6K pathway can cause insulin resistance (Khamzina et al., 2005; Krebs et al., 2007). Therefore, lower fasting levels of p-S6 may be a marker of slower aging and metabolic health. Our study probably underestimated positive effects of rapamycin, because metabolic parameters were evaluated only in mice that survived until the age of 2 years. Whereas all mice in R2 group survived until the end of experiment, only 40% mice survived in control group. In control HF group, mice with shorter lifespan (less healthy and faster aging by definition) did not survive and thus were not included in the investigation of p-S6 and metabolic parameters.
This study is a starting point to further evaluate intermittent schedules of rapamycin and to increase their life-extending potential by modulating doses and frequency. The life extension by intermittent treatment with rapamycin may be further potentiated with diet, the antidiabetic drug metformin (Anisimov et al., 2011a; Martin-Montalvo et al., 2013) and, if possible, physical exercise. Taken together, these modalities may improve health and increase lifespan in aging humans.
Experimental procedures
Mice
All animal studies were conducted in accordance with the regulations of the Committee of Animal Care and Use at Roswell Park Cancer Institute.
Prehistory [previous study (Leontieva et al., 2013c)]: 9-month-old male mice (C57BL/6NCr strain) were divided into 5 groups: one group received standard laboratory chow (5% fat, low fat) (LF) diet. Four other groups received high-fat 60% diet (Research Diets, Inc, Cat # D12492 Rodent Diet 60% kCal% fat; New Brunswick, NJ, USA) (HF) for 3 months. These four groups on HF diet were as follows: HF group – control (HF), R1 group received orally low dose of resveratrol; R2 group received orally low dose of rapamune; and R3 group received a combination of resveratrol and rapamune, as described previously (Leontieva et al., 2013c).
Current study
One week after the first study ended, experimental groups (R1–R3) were treated with rapamycin (LC Laboratories) via i.p injections, according to the following schedules: R1 group received 1.5 mg kg−1 three times/week/every other week; R2 group was injected with 1.5 mg kg−1 week−1 per every week; and R3 group was administered 0.5 mg kg−1 three times/week/every other week. Treatment was continued for 11 months, and weight was measured every week. On the eighth day after the last treatment, mice were fasted overnight and sacrificed. Blood was collected at the end of the day before food was removed for overnight fasting. Next morning, fasted blood was collected and mice were sacrificed. Nonfasted and fasted plasma were prepared, accordingly, for biochemical analysis.
Rapamycin (LC Laboratories, Woburn, MA, USA) was dissolved in ethanol at 15 mg mL−1 (stock) and then diluted to 0.15 mg mL−1 in PBS containing 5% Tween-80, 5% PEG 400 and 4% ethanol.
Immunoblot analysis
Tissues were homogenized, and immunoblotting was performed as previously described (Leontieva et al., 2012a). Rabbit antiphospho S6(Ser 240/244), antiphospho-AKT(Ser473), total AKT, and anti-S6 were used by us as previously described (Leontieva et al., 2012c) and purchased from Cell Signaling Biotechnology (Danvers, MA, USA); monoclonal anti-β-actin –peroxidase (AC-15) and mouse anti-GAPDH antibodies were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Invitrogen (Grand Island, NY, USA), respectively.
Glucose levels in blood plasma were measured using Accu-Chek Aviva strips (McKesson, Atlanta, GA, USA).
Insulin, IGF1, leptin, and triglyceride concentration in blood plasma, were measured using Insulin (Mouse) Ultrasensitive ELISA kit (ALPCO Diagnostics, Salem, NH, USA), IGF1 (Mouse/Rat) ELISA kit (ALPCO), Mouse Leptin ELISA kit (Crystal Chem Inc, Downers Grove, IL, USA), and Triglyceride Colorimetric Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA), respectively. Data were analyzed using range of standards and four parameter logistic fit or linear regression.
Statistical analysis
T test and correlation analyses [Pearson r coefficient and P value (two tailed)] were performed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San-Diego California, USA. www.graphpad.com.
Acknowledgments
This work was supported by RPCI. Authors thank Wei Tan for help with statistical analysis of mice survival.
Conflict of interest
Authors declare no conflict of interests.
Funding
This work was funded by Roswell Park Cancer Institute (Buffalo, NY, USA).
Author contributions
OVL executed experiments, analyzed data, and wrote the paper. GMP executed experiments and analyzed data. MVB designed the study, analyzed data, and wrote the paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Fig. S1 Percent of initial body weight.
Fig. S2 Kaplan–Meier survival curves for control mice on HF diet (HF, n = 10) and mice treated with schedule R1 (n = 10) and R3 (n = 9).
Fig. S3 Commassie Blue-stained membrane of liver blots shown in Figure 4A to confirm equal loading.
Fig. S4 Levels of p-S6 and p-AKT in kidneys of surviving mice.
Fig. S5 Levels of fasted and nonfasted glucose and insulin in blood plasma of male mice.
Fig. S6 Correlation between metabolic parameters in fasted plasma.
Fig. S7 Kaplan–Meier survival curves.
Table S1 Survival curve high-fat control (HF) and high fat + rapamycin-treated group 2 (HF+rapamycin (group 2)).
Table S2 Survival curve high-fat control (HF) and high fat + rapamycin-treated groups (HF+rapamycin all groups).
References
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am. J. Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011a;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011b;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem. Soc. Trans. 2011;39:460–465. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012a;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012b;4:350–358. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging. 2009;1:357–362. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, Mao FC. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J. Pharmacol. Sci. 2009;109:496–503. doi: 10.1254/jphs.08215fp. [DOI] [PubMed] [Google Scholar]
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2012;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc. Natl. Acad. Sci. USA. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;5:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature. 2009;460:392–396. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res. Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, FŸrnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- Leontieva OV, Geraldine Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012a;4:899–916. doi: 10.18632/aging.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012b;11:4642–4649. doi: 10.4161/cc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc. Natl. Acad. Sci. USA. 2012c;109:13314–13318. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Dis. 2013a;20:1241–1249. doi: 10.1038/cdd.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Novototskaya LR, Paszkiewicz GM, Komarova EA, Gudkov AV, Blagosklonny MV. Dysregulation of the mTOR pathway in p53-deficient mice. Cancer Biol. Ther. 2013b;14:1182–1188. doi: 10.4161/cbt.26947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Paszkiewicz G, Demidenko ZN, Blagosklonny MV. Resveratrol potentiates rapamycin to prevent hyperinsulinemia and obesity in male mice on high fat diet. Cell Death Dis. 2013c;4:e472. doi: 10.1038/cddis.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Hölter SM, Moreth K, Prehn C, Puk O, Rácz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Höfler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Partridge L. A double whammy for aging? Rapamycin extends lifespan and inhibits cancer in inbred female mice. Cell Cycle. 2012;11:17–18. doi: 10.4161/cc.11.1.18736. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010a;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell. 2010b;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle. 2012;11:845. doi: 10.4161/cc.11.5.19607. [DOI] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim. Biophys. Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013;5:539–550. doi: 10.18632/aging.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Réndon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. GSK-3alpha is a central regulator of age-related pathologies in mice. J. Clin. Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Percent of initial body weight.
Fig. S2 Kaplan–Meier survival curves for control mice on HF diet (HF, n = 10) and mice treated with schedule R1 (n = 10) and R3 (n = 9).
Fig. S3 Commassie Blue-stained membrane of liver blots shown in Figure 4A to confirm equal loading.
Fig. S4 Levels of p-S6 and p-AKT in kidneys of surviving mice.
Fig. S5 Levels of fasted and nonfasted glucose and insulin in blood plasma of male mice.
Fig. S6 Correlation between metabolic parameters in fasted plasma.
Fig. S7 Kaplan–Meier survival curves.
Table S1 Survival curve high-fat control (HF) and high fat + rapamycin-treated group 2 (HF+rapamycin (group 2)).
Table S2 Survival curve high-fat control (HF) and high fat + rapamycin-treated groups (HF+rapamycin all groups).


