India exports medicines to more than 200 countries worldwide. Pharmaceutical market in India consists of more than 20,000 manufacturers and is termed as the 3rd largest market in the world, by volume. In spite of that, more than half of its population has no access to essential medications in government hospitals due to heavy dependence of a majority of patients on private sector. A significant proportion of the Indian population pays for their health care expenditures by shelling money out of their own pockets. About 60-90% of this money is spent on medications and for procuring health services. Pharmaceutical policy in India is perceived to be favouring the industry rather than the public. The main focus of pharmaceutical health policies in India is to focus on the progression of the industrial sector while the issues of availability, pricing, and affordability of drugs remain ignored. Although, it is a common notion that drug prices in India are relatively low, studies have reported that medications in India are overpriced and unaffordable. The margin in medication sales across the same generic class of medications is extremely high, often ranging from 1000% to 4000%.[1] Since independence, the objectives of the pharmaceutical industry in India have remained the same: To promote the domestic industry and manufacture good quality drugs. However, the promulgation of law and policy from time to time has severely affected the progress toward these goals.
In 1997, the National Pharmaceutical Pricing Authority (NPPA) was established under the ministry of chemicals and fertilizers, Government of India with the aim of controlling the prices of medicines and ensure its availability.[2] In 1979, 347 drugs were included in the price control list and later the drug list was shortened to 166 in 1987 and further to 142. This drug list has been revised and cut down considerably over the years and finally in 1995, the Drug Price Control Order (DPCO) included only 76 drugs that were subjected to price control.[3] Recently, on May 15, 2013, the Ministry of Chemical and Fertilizers (Department of Pharmaceuticals) authorized NPPA to regulate the availability and pricing of all the drugs mentioned in National Essential List of India (National List of Essential Medicines [NLEM], 2011).[2] As a result, the prices of the 348 essential medicines originally included in 1979 were reduced dramatically, and they were made available to the public at low cost.
Almost a year later on May 29, 2014, another amendment was made in DPCO, which authorized the NPPA to control prices of other 108 life-saving drugs which were not originally included in the NLEM.[2] This patient friendly policy significantly reduced the prices of some important life-saving drugs for disease conditions such as cancer, HIV/AIDS, tuberculosis, cardiovascular diseases, diabetes, etc. The efforts of NPPA were widely acknowledged nationwide by the public as NPAA was viewed as an organization making a paradigm shift its industry-friendly policy to health policy. On September 20, 2014, the NPPA announced to cap the prices of 36 more drugs to increase their affordability to the public. However, just 2 days later, on 22 September, 2014, the Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Government of India issued a notification in which the internal guidelines given by NPPA on May 29, 2014 regarding the inclusion of 108 drugs under price control policy were withdrawn with immediate effect.[2]
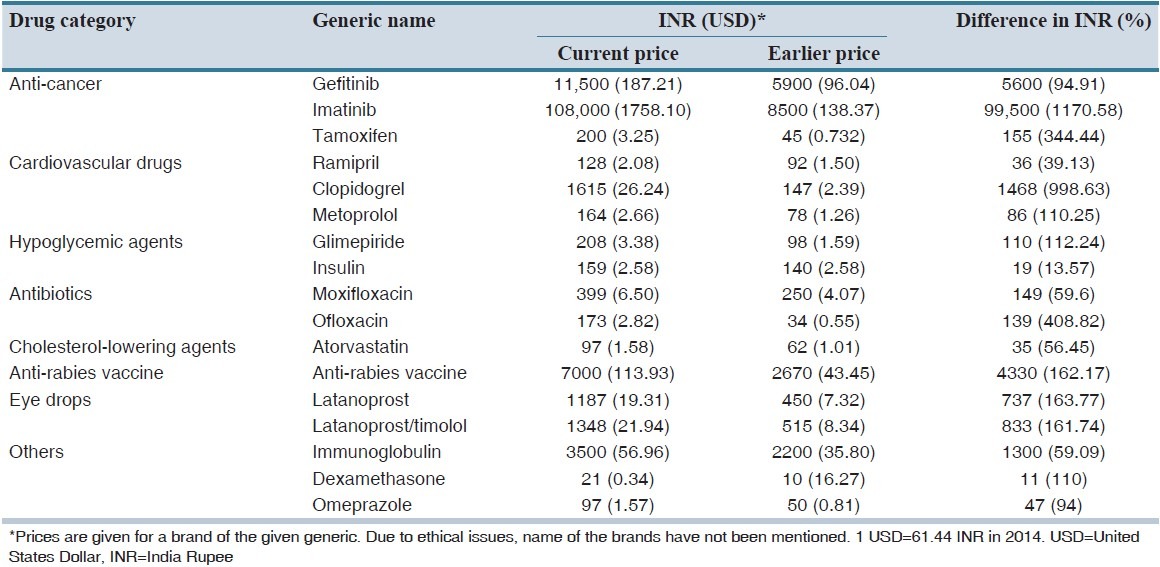
The exclusion of these 108 drugs from the price control list can negatively influence public health. The implication of this policy has already seen an unbelievable rise in pricing of some of the commonly used drugs as shown in Table 1.[4,5] It is noteworthy to mention that out of the 108 drugs that were recently excluded from price control list, more than 50 drugs were cardiovascular drugs, anti-diabetic drugs and cancer drugs. These diseases account for 53% of deaths and 44% of disability-adjusted life-years in India.[6] World Health Organization recently stated that cardiovascular disorders, diabetes mellitus, and cancer were among the major causes of mortality in India.[7] A study on the global burden of diseases showed that the number of deaths is expected to rise from 3.78 billion in 1990 to 7.63 million in 2020.[6] Currently in India, patients with diabetes amount to 4.1 crores, with coronary heart disease amount to 4.7 crores, with tuberculosis amount to 22 lakhs, with cancer amount to 11 lakhs and with HIV/AIDS amount to 25 lakhs, respectively.[4] In India, more than 80% of healthcare expenditure is borne by patients.[8] The daily income of 70% of Indian population is <2$.[2] Keeping in view the above statistics, it would not be wrong to say that the repercussion of this new policy would leave the middle and lower class Indians struggling to get basic access to healthcare.
Table 1.
Differences in medication pricing upon recent exclusion from drug price control list: A few examples
As health care professionals, we urge the stakeholders to review the necessity of their policy of excluding 108 drugs from price control list. The extremely essential medications that are needed by the majority of the Indian population should be reconsidered for inclusion in the drug price control list. Another possible solution could be to negotiate drug pricing with the manufacturers to keep prices under control and in return, the manufacturers could be provided with tax benefits.
Pharmaceutical companies should be brought on board to partner with the government to achieve the vision of healthcare access for all. There is a wide variation in the prices of different branded drugs and generics that are available in the market. There is a wide variation even among the branded or generic drugs manufactured by different companies, even though they might represent the same drug molecule. Medical professionals should be urged to prescribe cost-effective medications in the interest of the patients without being influenced by pharmaceutical companies. Consumer knowledge about generics could be enhanced through effective educational interventions. The issue of pricing needs a holistic solution generated by the synchronized efforts of stakeholders, pharmaceutical companies, and healthcare professionals in order to catalyze equity in access to healthcare.
REFERENCES
- 1.Ahmad A, Patel I, Sanyal S, Balkrishnan R, Mohanta GP. Availability, cost and affordability of antimalarial medicines in India. Int J Pharm Clin Res. 2014;6:7–12. [Google Scholar]
- 2.National Pharmaceutical Pricing Authority, Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Government of India. [Last accessed on 2014 Oct 05]. Available from: http://www.nppaindia.nic.in/index1.html .
- 3.Selvaraj S. How Effective Is India's Drug Price Control Regime? Boston, MA, USA: Harvard School of Public Health; 2007. [Last accessed on 2014 Oct 06]. Available from: https://www.hsph.harvard.edu/wp-content/uploads/sites/114/2012/10/RP256.pdf . [Google Scholar]
- 4.Iftikhar G. New Delhi, Cancer Drug Price Goes Up from Rs. 8,000 to Rs. 1.08 lakh. [Last accessed on 2014 Oct 06]. Available from: http://www.dnaindia.com/india/report-cancer-drug-price-goes-up-fromrs-8000-to-rs-108-lakh-2022667 .
- 5.Maken A. How Indian Cancer/TB/AIDS and Heart Patients to Bear the Cost of Modi's American Jamboree. [Last accessed on 2014 Oct 05]. Available from: http://www.ajaymakenblog.wordpress.com/
- 6.Srinath Reddy K, Shah B, Varghese C, Ramadoss A. Responding to the threat of chronic diseases in India. Lancet. 2005;366:1744–9. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- 7.Sharma K. Burden of non-communicable diseases in India: Setting priority for action. Int J Med Sci Public Health. 2013;2:6–11. [Google Scholar]
- 8.Kotwani A, Ewen M, Dey D, Iyer S, Lakshmi PK, Patel A, et al. Prices and availability of common medicines at six sites in India using a standard methodology. Indian J Med Res. 2007;125:645–54. [PubMed] [Google Scholar]


