Abstract
Objective:
Currently, the method of early nasal continuous positive airway pressure (nCPAP) and selective administration of surfactant via an endotracheal tube is widely used in the treatment of respiratory distress syndrome (RDS) in premature infants. To prevent complications related to endotracheal intubation and even a brief period of mechanical ventilation, in this study, we compared the effectiveness of surfactant administration via a thin intratracheal catheter versus the current method using an endotracheal tube.
Methods:
Thirty eight preterm infants ≤34 weeks' gestation with birth weight of 1000–1800 g who were putted on nCPAP for RDS within the first hour of life, were randomly assigned to receive surfactant either via endotracheal tube (ET group) or via thin intratracheal catheter (CATH group). The primary outcomes were the need for mechanical ventilation and duration of oxygen therapy. Data were analyzed by independent t-test, Mann-Whitney U-test, and Chi-square test, using SPSS v. 21.
Findings:
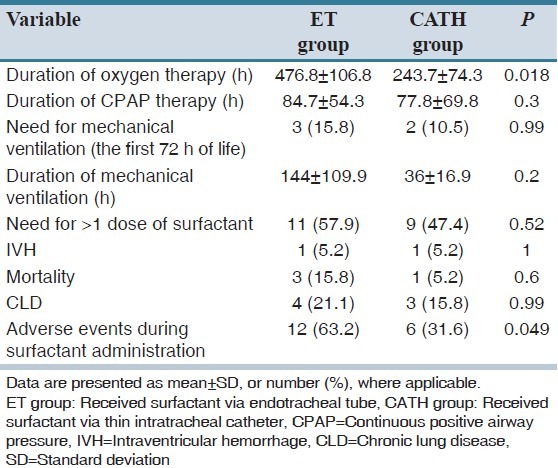
There was no significant difference between groups regarding to need for mechanical ventilation during the first 72 h of birth (3 [15.8%] in ET group vs. 2 [10.5%] in CATH group; P = 0.99). Duration of oxygen therapy in CATH group was significantly lower than ET group (243.7 ± 74.3 h vs. 476.8 ± 106.8 h, respectively; P = 0.018). The incidence of adverse events during all times of surfactant administration was not statistically significant between groups (P = 0.14), but the number of infants who experienced adverse events during surfactant administration was significantly lower in CATH group than ET group (6 [31.6%] vs. 12 [63.2%], respectively; P = 0.049). All other outcomes, including duration of treatment with CPAP and mechanical ventilation, times of surfactant administration and the need for more than one dose of the drug, the rate of intraventricular hemorrhage, mortality and combined outcome of chronic lung disease or mortality were statistically similar between the groups
Conclusion:
Surfactant administration via thin intratracheal catheter in preterm infants receiving nCPAP for treatment of RDS has similar efficacy, feasibility and safety to its administration via endotracheal tube.
Keywords: Endotracheal tube, preterm infants, respiratory distress syndrome, surfactant, thin catheter
INTRODUCTION
Respiratory distress syndrome (RDS) is considered one of the important causes of morbidity and mortality in preterm infants especially among extremely low birth weight ones. Mechanical ventilation through endotracheal tube even for a short time could cause lung tissue damage and surfactant inactivation.[1,2]
It has been shown that early use of continuous positive airway pressure (CPAP) after birth could decrease the need for mechanical ventilation without any significant increase in the complications of the disease.[3,4] Considering the beneficial effects of early surfactant administration and possibility of treatment failure by the application of CPAP alone, combined use of both surfactant and CPAP in the treatment of RDS has been developed in some clinical trials. Results of these studies have indicated that use of the method called intubation, surfactant, extubation (INSURE) significantly reduces the need for mechanical ventilation.[2] However, this method also requires positive pressure ventilation for at least a short time after intratracheal administration of surfactant. So the researchers have been looking for other methods of the drug administration without the need for endotracheal intubation.[5] These methods include nebulizing surfactant,[6] administration through laryngeal mask airway[7] or intrapartum pharyngeal instillation of the drug before infant begins to breathe.[1,8]
A number of researchers modified the INSURE method and developed a new one. In this method, surfactant was administered via a thin intratracheal catheter during spontaneous breathing with nasal continuous positive airway pressure (nCPAP). The method was experienced for the first time by Kribs et al. in Germany.[9] A number of studies on the effectiveness and advantages of this method over the standard method of endotracheal intubation and surfactant administration is limited.[5,6] Most of these studies have retrospective or prospective observational design.[2,9,10,11] There are only three clinical trials conducted in this field to date; results of two studies have been published.[12,13,14] In mentioned studies the new method have been compared either with method in which neonates was intubated, received surfactant and then mechanically ventilated[2,9,10,14] or with method of continuing nCPAP and administering surfactant via endotracheal tube, only when respiratory distress was progressed and infants needed to receive mechanical ventilation.[11,12,13] In some of these studies, the primary outcomes were only feasibility and safety of the new method.[9,10,12] In this study, we compared two methods of surfactant administration in the early phases of RDS: Via either an endotracheal tube by INSURE method or a thin intratracheal catheter while the infants spontaneously breathe on nCPAP.
METHODS
From December 2012 to May 2013, we conducted a randomized controlled trial in two Neonatal Intensive Care Units (NICUs) in Shahid Beheshti Maternity Hospital and Al-Zahra General Hospital, two teaching tertiary hospitals affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. The study protocol was approved by the Ethics Committee of the University; the registration number was 392176. Inborn infants with gestational age ≤34 weeks and birthweight 1000–1800 g in whom signs of RDS were appeared within the 1st h of life and surfactant was required after 30 min treatment with nCPAP (based on the predefined criteria as indicated below) were included. Infants with a history of chorioamnionitis in mother, Apgar score of 4 or less at 5 min after birth, life-threatening congenital anomalies and those who need endotracheal intubation and mechanical ventilation for stabilization at birth or need mechanical ventilation for more than a few minutes after surfactant administration were excluded from the study. Based on data extracted from the mentioned previous studies, if d was considered equal to 0.4, 34 infants were needed for enrollment to achieve 80% power and an α of 0.05. We enrolled a total of 38 infants in this study. Informed consent was obtained from the parents as soon as the first sign of respiratory distress was observed.
Interventions
Infants were treated with nCPAP on a pressure of 6 cmH2O as early symptoms of RDS were appeared. CPAP was administered by Neopuff infant resuscitator (Fisher and Paykel, Auckland, New Zealand) via an oral mask in the delivery room and continued using bubble CPAP (Fisher and Paykel, Auckland, New Zealand) via binasal prongs in the NICU. A 6F orogastric tube was inserted into the stomach in all infants. SpO2(arterial oxyhemoglobin saturation measured by pulse oximetry) and heart rate were continuously monitored. For infants with birth weight < 1250 g intravenous aminophylline was administered with a loading dose of 6 mg/kg and continued with a maintenance dose of 1.5 mg/kg every 8 h.[15] Thirty minutes after application of nCPAP in the NICU, if the required fraction of inspired oxygen (FIO2) for maintaining SpO2 in the range of 87-92% was more than 30% or the infant had moderate to severe respiratory distress (Silverman-Andersen score > 4), curosurf (Chiesi Farmaceutisi SPA, Parma, Italy) was administrated with a dose of 200 mg/kg. Using cards provided in consecutively numbered, opaque and sealed envelopes that were opened just after making the decision of surfactant administration, infants were randomly allocated to one of two groups to receive surfactant via an endotracheal tube (ET group) or via a thin catheter inserted in trachea (CATH group). A level 2 resident in perinatal-neonatal subspecialty program who did not perform randomization and didn't involve in outcome measurements was administered all doses of the drug. Before administration of surfactant, infants took intravenous atropine in a dose of 0.025 mg/kg.[9] In ET group surfactant was administered via a 2.5F or 3F endotracheal tube inserted into the trachea and fixed after vocal cord guide was aligned with vocal cords. The infant was temporarily separated from the CPAP. After bolus injection of the drug into the trachea, positive pressure ventilation was applied using neopuff and continued for at least 1 min or until SpO2 was reached to 87% or more. The endotracheal tube was then removed, and the infant was switched back to nCPAP on the previous pressure. The FIO2 was decreased at a rate of 5% every 1-2 min as long as SpO2 was maintained at the above desired level. In CATH group, surfactant was administrated via a 4F end hole feeding tube (thin catheter) with one orifice at the tip. The catheter was marked 1.5 cm above the tip with a permanent marker. The catheter was connected to the syringe contained surfactant and filled with the drug. It was then clamped with a Magill forceps in an angle of 120°. The head of the infant was positioned as for tracheal intubation. Using laryngoscope in one hand and the forceps in another hand, the catheter was inserted into the trachea so far as the marker was aligned at the level of vocal cords. It was fixed with two fingers, and the laryngoscope was removed. Then, the surfactant was injected into the trachea over 1–3 min. At the end of the procedure, to insure that the drug was not accidentally injected into the stomach, the orogastric tube was aspirated. During the procedure, the nCPAP was continuously applied. After surfactant administration, FIO2 was decreased gradually as described in ET group.[10] In both studied groups, the procedure was discontinued in following conditions: Heart rate drop below 100 beats/min, SpO2 decrease to < 80% and severe cough or chocking. It was restarted after stabilization with positive pressure ventilation (via endotracheal tube and oral mask in ET group and 2, respectively).[9] Arterial blood gas analysis was performed as indicated by the clinical condition of the studied preterm infants.
Twelve hours after the first dose of surfactant, if FIO2 ≥ 30% was still required to maintain SpO2 at 87% and more, the second dose of the drug would be administered in an amount of 100 mg/kg using the same method of administration. Infants in whom the above criteria were still met 12 h after the second dose took the drug in a similar amount for the third time. The infants were intubated for mechanical ventilation, if the above treatment was failed as defined as follows:
Required FIO2 ≥ 70% for more than 2 h or above 40% for more than 12 h (despite surfactant administration) to maintain SpO2 ≥ 87%
pH < 7.2 or PaCO2 more than 65 mmHg
Occurrence of one major apnea (requires bag and mask ventilation) or six minor apnea (requires only a brief stimulation) within 6 h.
The infants were followed until 36 weeks postmenstrual age or 28th day of birth (whichever came later). To detect intraventricular hemorrhage (IVH), cranial ultrasound was performed on days 3, 7 and 14 of birth.
Assessments
Demographic data included infants' gestational age, birth body weight and gender, multiple gestation, route of delivery, rupture of membranes > 18 h and administration of antenatal glucocorticoids to the mother. Primary outcome was the need for mechanical ventilation within 72 h of birth.
Secondary outcomes included duration of mechanical ventilation, duration of treatment with nCPAP, duration of oxygen requirement, the number of attempts for successful insertion of endotracheal tube or thin catheter into the trachea, times of surfactant administration and the need for more than one dose of the drug, rate of mortality, chronic lung disease (CLD), defined as oxygen requirement by the later of 36 weeks postmenstrual age or 28 day after birth), IVH and adverse events during the procedure (including heart rate < 100/min or more than 200/min and SpO2 ≤ 80%, coughing, choking).
Data were analyzed with the use of SPSS software for windows version 21 (SPSS Inc., Chicago, IL, USA) software. Independent t-test and Mann-Whitney test was used to compare parametric and nonparametric continuous variables. Chi-square test was applied for qualitative variables. P < 0.05 was considered to be statistically significant.
RESULTS
Sixty-one infants were assessed for eligibility. Parents of seven infants refused participation of their child in the study. Sixteen infants responded to nCPAP alone and hence they did not meet all inclusion criteria [Figure 1]. A total of 38 newborn infants, consisted of 21 male and 17 female, were enrolled in the study with characteristics described in Table 1. The groups were similar regarding baseline characteristics. As shown in Table 2, there was no significant difference between the two groups in terms of the need for mechanical ventilation within 72 h of birth, duration of treatment with CPAP, duration of mechanical ventilation, times of surfactant administration and the need for more than one dose of the drug, the rate of IVH, mortality and CLD. The duration of oxygen requirement in CATH group (surfactant via thin intratracheal catheter) was significantly lower than ET group (surfactant via endotracheal tube) (243.7 ± 74.3 h vs. 476.8 ± 106.8 h, respectively; P = 0.018). A total of 71 doses of surfactant were administered in the two groups: 36 doses in ET group and 35 doses in CATH group (P = 0.77). The median number of times of surfactant administration in CATH group was less than ET group, but the difference was not statistically significant (1 vs. 2 times, respectively; P = 0.62). The first attempt for tracheal intubation was successful in 26 of 36 cases (72.2%) of surfactant administration in ET group. The success rate of first attempt for catheter insertion into the trachea was 88.5% (31 of 35 cases) in CATH group (P = 0.08).
Figure 1.
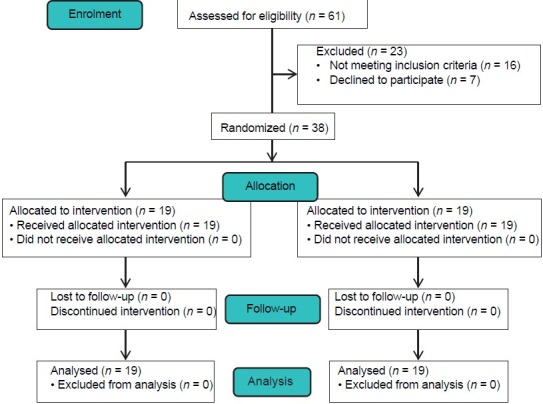
Patients' flow diagram (CONSORT)
Table 1.
Demographic and baseline characteristics of the preterm infants (N=19, for each study group)
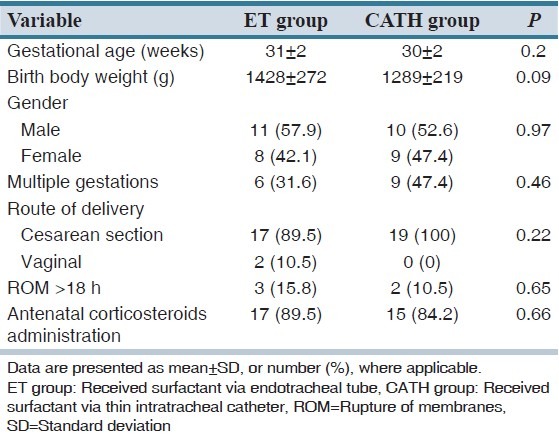
Table 2.
Comparison of clinical course and complications in the two study groups (N=19)
The overall incidence of adverse events and each of them individually during administration of all doses of surfactant in each group are presented in Figure 2.
Figure 2.
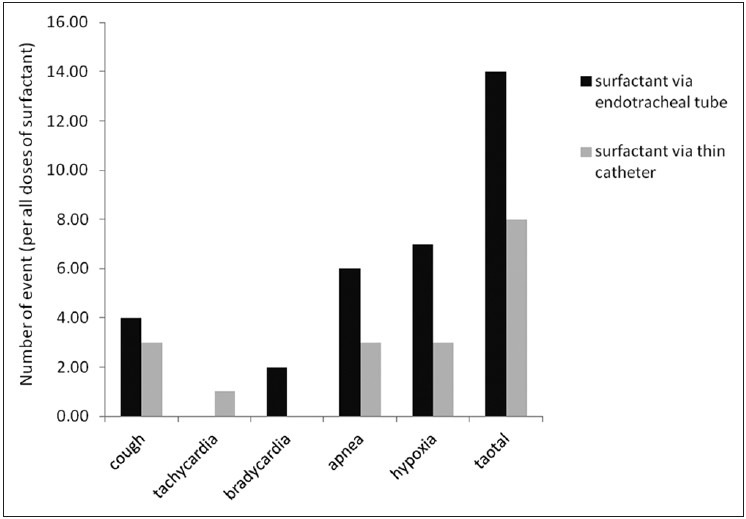
Rate of adverse events during all times of surfactant administration in the two study groups
Accordingly the incidence of each of adverse events individually was not different between the two groups (P ≥ 0.3). The overall incidence of adverse events was higher in ET group than CATH group, but the difference was not statistically significant (P = 0.14). Number of preterm infants who experienced adverse events during surfactant therapy was significantly lower in CATH group than ET group (6 [31.6%] vs. 12 [63.2%], respectively; P = 0.049).
DISCUSSION
The findings of our study showed that in preterm infants with RDS who need surfactant while treating on nCPAP, instillation of surfactant into the trachea either via an endotracheal tube or a thin catheter did not significantly change duration of treatment with nCPAP, need for mechanical ventilation within 72 h of birth, duration of mechanical ventilation, rate of the drug administration, rate of mortality, CLD and IVH. The duration of oxygen requirement, however was significantly lower with the drug instillation via a thin intratracheal catheter. The results of studies evaluated the feasibility, efficacy and safety of surfactant administration via thin intratracheal catheter have shown decrease in: Need for mechanical ventilation within 1–3 days of birth[9,10,11,12,14] and its duration,[10,14] duration of oxygen therapy,[12] rate of oxygen requirement at discharge or 28 days of birth[10,14] and rate of CLD and mortality.[9,11] Compared with enrolled infants in our study, all infants in some of these studies[2,9,11] and a number of them in others have lower gestational age and/or birth weight.[10,12,14] In most of these studies, one group of infants has received surfactant via thin intratracheal catheter based on defined criteria while treating with nCPAP and compared with another group, which has been intubated and mechanically ventilated with the same criteria.[2,9,10,11,14] Factors such as early application of mechanical ventilation in control group and the lower infants' gestational age and birth weight which increases the risk of complications of this intervention could explain the significant differences in above mentioned short- and long-term outcomes between the two groups. In the group of infants who took surfactant via a thin intratracheal catheter, the drug was administered earlier and thus more frequent than the other group. As the surfactant has an important role in the improvement of infants' pulmonary outcome, it may be a strong reason for decreased rate of complications in this group. In one of their studies, Kribs et al. reported that although infants who received surfactant via a thin intratracheal catheter had lower gestational age and birth weight than those treated with standard care, the rate of mechanical ventilation, CLD and the combined outcome of mortality or need for oxygen at discharge was lower among them. They suggested that the improved outcomes may be due to the surfactant itself not a new intervention.[10] Based on the results of their similar study, Kribs et al. explained that outcome improvement in the group took surfactant via thin intratracheal catheter may be due to early and prophylactic use of the drug rather than the new method of its administration.[2] In some of these studies use of caffeine or theophylline was significantly higher in group in whom surfactant was administered by a new method (via a thin intratracheal catheter). It could have an important role in the improvement of pulmonary function and decrease in need for mechanical ventilation and rate of CLD.[2] Notably, the majority of studies in this field had observational design[2,9,10,11,12] with some limitations including that they were not randomized and in some of them the indications and conditions of surfactant administration were not definitively determined.[10] In our study, the rate of effort for insertion of a thin catheter into the trachea was lower than that for endotracheal intubation, although the difference was not statistically significant. It means that the feasibility of this new method is at least the same as of the standard method. In addition, the number of infants who developed adverse events during surfactant administration via thin intratracheal catheter was significantly lower. In our center, it is for the first time that insertion of a thin catheter into the trachea for surfactant administration is examined, whereas there have been enough experience for the drug administration by endotracheal intubation for many years. With increasing experience, it seems that the number of infants who develop adverse events during surfactant administration using the new method and the rate of effort for insertion of a thin catheter into the trachea would be decreased significantly. In conclusion, the results of our study showed that during management of preterm infants with RDS who need surfactant while treating on nCPAP, surfactant administration via a thin intratrachal catheter have similar feasibility, efficacy and safety as its administration via an endotracheal tube. This study did not include infants with birth weight <1000 g. To compare the feasibility and efficacy of a new method in this group of neonates as the most immature infants at the highest risk for adverse outcomes, similar randomized controlled trials with larger sample size is recommended.
AUTHORS' CONTRIBUTION
All authors contributed the idea of research, design of study, data analysis and manuscript preparation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE. Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. J Perinatol. 2004;24:360–5. doi: 10.1038/sj.jp.7211103. [DOI] [PubMed] [Google Scholar]
- 2.Kribs A, Vierzig A, Hünseler C, Eifinger F, Welzing L, Stützer H, et al. Early surfactant in spontaneously breathing with nCPAP in ELBW infants - a single centre four year experience. Acta Paediatr. 2008;97:293–8. doi: 10.1111/j.1651-2227.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 3.Support Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 5.Stevens TP, Sinkin RA. Surfactant replacement therapy. Chest. 2007;131:1577–82. doi: 10.1378/chest.06-2371. [DOI] [PubMed] [Google Scholar]
- 6.Shah S. Exogenous surfactant: Intubated present, nebulized future? World J Pediatr. 2011;7:11–5. doi: 10.1007/s12519-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 7.Attridge JT, Stewart C, Stukenborg GJ, Kattwinkel J. Administration of rescue surfactant by laryngeal mask airway: Lessons from a pilot trial. Am J Perinatol. 2013;30:201–6. doi: 10.1055/s-0032-1323592. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Latif ME, Osborn DA. Pharyngeal instillation of surfactant before the first breath for prevention of morbidity and mortality in preterm infants at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2011;3:CD008311. doi: 10.1002/14651858.CD008311.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Kribs A, Pillekamp F, Hünseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks) Paediatr Anaesth. 2007;17:364–9. doi: 10.1111/j.1460-9592.2006.02126.x. [DOI] [PubMed] [Google Scholar]
- 10.Kribs A, Härtel C, Kattner E, Vochem M, Küster H, Möller J, et al. Surfactant without intubation in preterm infants with respiratory distress: First multi-center data. Klin Padiatr. 2010;222:13–7. doi: 10.1055/s-0029-1241867. [DOI] [PubMed] [Google Scholar]
- 11.Mehler K, Grimme J, Abele J, Huenseler C, Roth B, Kribs A. Outcome of extremely low gestational age newborns after introduction of a revised protocol to assist preterm infants in their transition to extrauterine life. Acta Paediatr. 2012;101:1232–9. doi: 10.1111/apa.12015. [DOI] [PubMed] [Google Scholar]
- 12.Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013;98:F122–6. doi: 10.1136/archdischild-2011-301314. [DOI] [PubMed] [Google Scholar]
- 13.Dargaville PA. Innovation in surfactant therapy I: Surfactant lavage and surfactant administration by fluid bolus using minimally invasive techniques. Neonatology. 2012;101:326–36. doi: 10.1159/000337346. [DOI] [PubMed] [Google Scholar]
- 14.Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): An open-label, randomised, controlled trial. Lancet. 2011;378:1627–34. doi: 10.1016/S0140-6736(11)60986-0. [DOI] [PubMed] [Google Scholar]
- 15.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2010 update. Neonatology. 2010;97:402–17. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]


