Abstract
Silver nanoparticles (NPs) have been the subjects of researchers because of their unique properties (e.g., size and shape depending optical, antimicrobial, and electrical properties). A variety of preparation techniques have been reported for the synthesis of silver NPs; notable examples include, laser ablation, gamma irradiation, electron irradiation, chemical reduction, photochemical methods, microwave processing, and biological synthetic methods. This review presents an overview of silver nanoparticle preparation by physical, chemical, and biological synthesis. The aim of this review article is, therefore, to reflect on the current state and future prospects, especially the potentials and limitations of the above mentioned techniques for industries.
Keywords: Nanoparticle synthesis, Silver nanoparticles, Physical synthesis, Chemical synthesis, Biological synthesis
INTRODUCTION
Nanotechnology is an important field of modern research dealing with design, synthesis, and manipulation of particle structures ranging from approximately 1-100 nm. Nanoparticles (NPs) have wide range of applications in areas such as health care, cosmetics, food and feed, environmental health, mechanics, optics, biomedical sciences, chemical industries, electronics, space industries, drug-gene delivery, energy science, optoelectronics, catalysis, single electron transistors, light emitters, nonlinear optical devices, and photo-electrochemical applications (1,2,3,4,5,6).
Nanobiotechnology is a rapidly growing scientific field of producing and constructing devices. An important area of research in nanobiotechnology is the synthesis of NPs with different chemical compositions, sizes and morphologies, and controlled dispersities. Nanobiotechnology has turned up as an elementary division of contemporary nanotechnology and untied novel epoch in the fields of material science receiving global attention due to its ample applications. It is a multidisciplinary approach resulting from the investigational use of NPs in biological systems including the disciplines of biology, biochemistry, chemistry, engineering, physics and medicine. Moreover, the nanobio-technology also serves as an imperative technique in the development of clean, nontoxic, and eco-friendly procedures for the synthesis and congregation of metal NPs having the intrinsic ability to reduce metals by specific metabolic pathways (1,2,3,4,5,6).
Nowadays, there is a growing need to develop eco-friendly processes, which do not use toxic chemicals in the synthesis protocols. Green synthesis approaches include mixed-valence polyoxometalates, polysaccharides, Tollens, biological, and irradiation method which have advantages over conventional methods involving chemical agents associated with environmental toxicity. Selection of solvent medium and selection of eco-friendly nontoxic reducing and stabilizing agents are the most important issues which must be considered in green synthesis of NPs.
Silver NPs are of interest because of the unique properties which can be incorporated into antimicrobial applications, biosensor materials, composite fibers, cryogenic super-conducting materials, cosmetic products, and electronic components. Some important applications of silver NPs in pharmaceutics, medicine, and dentistry are shown in Table 1. Several physical and chemical methods have been used for synthesizing and stabilizing silver NPs (Table 2) (7,8).
Table 1.
Important applications of silver nanoparticles.
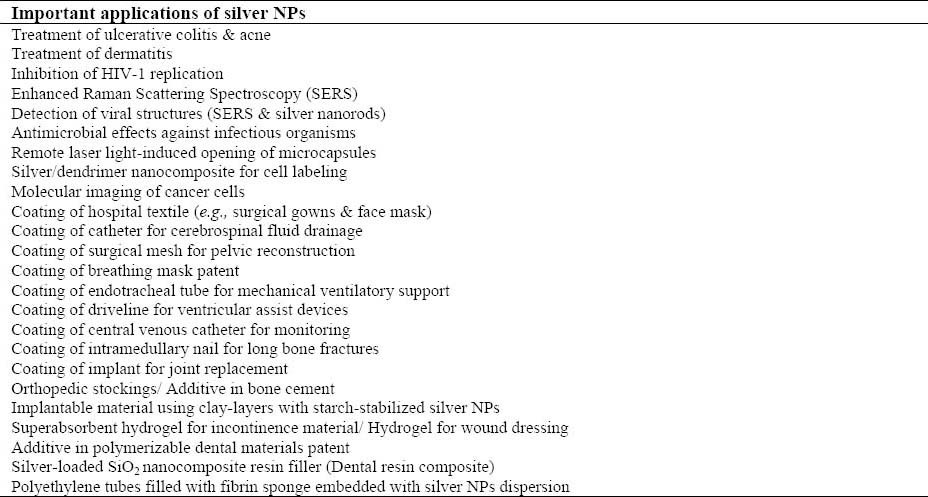
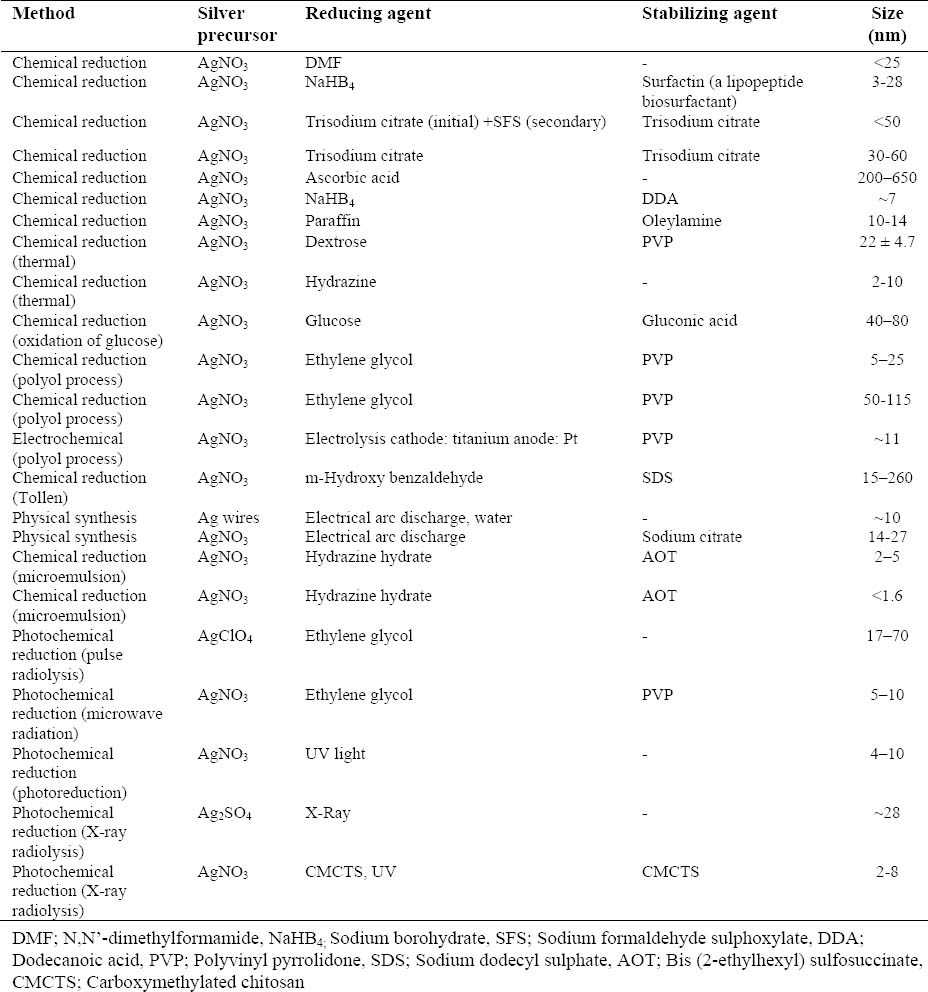
Table 2.
Some important physical, chemical and photochemical methods for synthesizing and stabilizing silver NPs.
The most popular chemical approaches, including chemical reduction using a variety of organic and inorganic reducing agents, electrochemical techniques, physicochemical reduction, and radiolysis are widely used for the synthesis of silver NPs. Most of these methods are still in development stage and the experienced problems are the stability and aggregation of NPs, control of crystal growth, morphology, size and size distribution. Furthermore, extraction and purification of produced NPs for further applications are still important issues (9,10,11).
This review article presents an overview of silver nanoparticle preparation by physical, chemical, and green synthesis approaches.
Synthesis of silver NPs
Physical methods
Evaporation-condensation and laser ablation are the most important physical approaches. The absence of solvent contamination in the prepared thin films and the uniformity of NPs distribution are the advantages of physical synthesis methods in comparison with chemical processes. Physical synthesis of silver NPs using a tube furnace at atmospheric pressure has some disadvantages, for example, tube furnace occupies a large space, consumes a great amount of energy while raising the environmental temperature around the source material, and requires a lot of time to achieve thermal stability. Moreover, a typical tube furnace requires power consumption of more than several kilowatts and a preheating time of several tens of minutes to reach a stable operating temperature (12,13). It was demonstrated that silver NPs could be synthesized via a small ceramic heater with a local heating area (14). The small ceramic heater was used to evaporate source materials. The evaporated vapor can cool at a suitable rapid rate, because the temperature gradient in the vicinity of the heater surface is very steep in comparison with that of a tube furnace.
This makes possible the formation of small NPs in high concentration. The particle generation is very stable, because the temperature of the heater surface does not fluctuate with time. This physical method can be useful as a nanoparticle generator for long-term experiments for inhalation toxicity studies, and as a calibration device for nanoparticle measurement equipment (14). The results showed that the geometric mean diameter, the geometric standard deviation and the total number concentration of NPs increase with heater surface temperature. Spherical NPs without agglomeration were observed, even at high concentration with high heater surface temperature. The geometric mean diameter and the geometric standard deviation of silver NPs were in the range of 6.2-21.5 nm and 1.23-1.88 nm, respectively.
Silver NPs could be synthesized by laser ablation of metallic bulk materials in solution (15,16,17,18,19). The ablation efficiency and the characteristics of produced nano-silver particles depend upon many parameters, including the wavelength of the laser impinging the metallic target, the duration of the laser pulses (in the femto-, pico- and nanosecond regime), the laser fluence, the ablation time duration and the effective liquid medium, with or without the presence of surfactants (20,21,22,23).
One important advantage of laser ablation technique compared to other methods for production of metal colloids is the absence of chemical reagents in solutions. Therefore, pure and uncontaminated metal colloids for further applications can be prepared by this technique (24). Silver nanospheroids (20-50 nm) were prepared by laser ablation in water with femtosecond laser pulses at 800 nm (25). The formation efficiency and the size of colloidal particles were compared with those of colloidal particles prepared by nanosecond laser pulses. As a result, the formation efficiency for femtosecond pulses was significantly lower than that for nanosecond pulses. The size of colloids prepared by femtosecond pulses were less dispersed than that of colloids prepared by nanosecond pulses. Furthermore, it was found that the ablation efficiency for femtosecond ablation in water was lower than that in air, while in the case of nanosecond pulses, the ablation efficiency was similar in both water and air.
Tien and coworkers (26) used the arc discharge method to fabricate silver NPs suspension in deionized water with no added surfactants. In this synthesis, silver wires (Gredmann, 99.99%, 1 mm in diameter) were submerged in deionized water and used as electrodes. With a silver rod consumption rate of 100 mg/min, yielding metallic silver NPs of 10 nm in size, and ionic silver obtained at concentrations of approximately 11 ppm and 19 ppm, respectively. Siegel and colleagues (27) demonstrated the synthesis of silver NPs by direct metal sputtering into the liquid medium. The method, combining physical deposition of metal into propane-1,2,3-triol (glycerol), provides an interesting alternative to time-consuming, wet-based chemical synthesis techniques. Silver NPs possess round shape with average diameter of about 3.5 nm with standard deviation 2.4 nm. It was observed that the NPs size distribution and uniform particle dispersion remains unchanged for diluted aqueous solutions up to glycerol-to-water ratio 1:20.
Chemical methods
Chemical reduction
The most common approach for synthesis of silver NPs is chemical reduction by organic and inorganic reducing agents. In general, different reducing agents such as sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, polyol process, Tollens reagent, N, N-dimethylformamide (DMF), and poly (ethylene glycol)-block copolymers are used for reduction of silver ions (Ag+) in aqueous or non-aqueous solutions. These reducing agents reduce Ag+ and lead to the formation of metallic silver (Ag0), which is followed by agglomeration into oligomeric clusters. These clusters eventually lead to the formation of metallic colloidal silver particles (28,29,30). It is important to use protective agents to stabilize dispersive NPs during the course of metal nanoparticle preparation, and protect the NPs that can be absorbed on or bind onto nanoparticle surfaces, avoiding their agglomeration (31). The presence of surfactants comprising functionalities (e.g., thiols, amines, acids, and alcohols) for interactions with particle surfaces can stabilize particle growth, and protect particles from sedimentation, agglomeration, or losing their surface properties.
Polymeric compounds such as poly (vinyl alcohol), poly (vinylpyrrolidone), poly (ethylene glycol), poly (methacrylic acid), and polymethylmethacrylate have been reported to be the effective protective agents to stabilize NPs. In one study, Oliveira and coworkers (31) prepared dodecanethiol-capped silver NPs, according to Brust procedure (32) based on a phase transfer of an Au3+ complex from aqueous to organic phase in a two-phase liquid-liquid system, which was followed by a reduction with sodium borohydride in the presence of dodecanethiol as stabilizing agent, binding onto the NPs surfaces, avoiding their aggregation and making them soluble in certain solvents. They reported that small changes in synthetic factors lead to dramatic modifications in nanoparticle structure, average size, size distribution width, stability and self-assembly patterns. Kim and colleagues (33) reported synthesis of spherical silver NPs with a controllable size and high monodispersity using the polyol process and a modified precursor injection technique. In the precursor injection method, the injection rate and reaction temperature were important factors for producing uniform-sized silver NPs with a reduced size.
Silver NPs with a size of 17 ± 2 nm were obtained at an injection rate of 2.5 ml/s and a reaction temperature of 100 °C. The injection of the precursor solution into a hot solution is an effective means to induce rapid nucleation in a short period of time, ensuring the fabrication of silver NPs with a smaller size and a narrower size distribution. Zhang and coworkers (34) used a hyper branched poly (methylene bisacrylamide aminoethyl piperazine) with terminal dimethylamine groups (HPAMAM-N(CH3)2) to produce colloids of silver. The amide moieties, piperazine rings, tertiary amine groups and the hyper-branched structure in HPAMAM-N(CH3)2 are important to its effective stabilizing and reducing abilities. Chen and colleagues (35) have shown the formation of monodispersed silver NPs using simple oleylamine-liquid paraffin system. It was reported that the formation process of these NPs could be divided into three stages: growth, incubation and Oatwald ripening stages. The higher boiling point of 300 °C of paraffin affords a broader range of reaction temperature and makes it possible to effectively control the size of silver NPs by varying the heating temperature alone without changing the solvent. Moreover, the size of the colloidal silver NPs could be regulated not only by changing the heating temperature, or the ripening time, but also by adjusting the ratio of oleylamine to the silver precursor.
Silver NPs can be prepared at room temperature, by simple mixing of the corresponding metal ions with reduced polyoxometalates which serves as reducing and stabilizing agents. Polyoxometalates are soluble in water and have the capability of undergoing stepwise, multielectron redox reactions without disturbing their structure. It was demonstrated that silver NPs were produced by illuminating a deaerated solution of polyoxometalate/S/Ag+ (36). Furthermore, green chemistry-type one-step synthesis and stabilization of silver nanostructures with MoV–MoVI mixed-valence polyoxometalates in water at room temperature has been reported (37).
Microemulsion techniques
Uniform and size controllable silver NPs can be synthesized using microemulsion techniques. The NPs preparation in two-phase aqueous organic systems is based on the initial spatial separation of reactants (metal precursor and reducing agent) in two immiscible phases. The interface between the two liquids and the intensity of inter-phase transport between two phases, which is mediated by a quaternary alkyl-ammonium salt, affect the rate of interactions between metal precursors and reducing agents. Metal clusters formed at the interface are stabilized, due to their surface being coated with stabilizer molecules occurring in the non-polar aqueous medium, and transferred to the organic medium by the inter-phase transporter (38). One of the major disadvantages is the use of highly deleterious organic solvents.
Thus large amounts of surfactant and organic solvent must be separated and removed from the final product. For instance, Zhang and coworkers (39) used dodecane as oily phase (a low deleterious and even nontoxic solvent), but there was no need to separate the prepared silver solution from the reaction mixture. On the other hand, colloidal NPs prepared in nonaqueous media for conductive inks are well-dispersed in a low vapor pressure organic solvent, to readily wet the surface of polymeric substrate without any aggregation. The advantages can also be found in the applications of metal NPs as catalysts to catalyze most organic reactions, which have been conducted in non-polar solvents. It is very important to transfer metal NPs to different physicochemical environments in practical applications (40).
UV-initiated photoreduction
A simple and effective method, UV-initiated photoreduction, has been reported for synthesis of silver NPs in the presence of citrate, polyvinylpyrrolidone, poly (acrylic acid), and collagen. For instance, Huang and Yang produced silver NPs via photoreduction of silver nitrate in layered inorganic laponite clay suspensions which served as stabilizing agent for prevention of NPs aggregation. The properties of produced NPs were studied as a function of UV irradiation time. Bimodal size distribution and relatively large silver NPs were obtained when irradiated under UV for 3 h. Further irradiation disintegrated the silver NPs into smaller sizes with a single distribution mode until a relatively stable size and size distribution was obtained (41). Silver NPs (nanosphere, nanowire, and dendrite) have been prepared by UV irradiation photoreduction technique at room temperature using poly (vinylalcohol) (as protecting and stabilizing agent). Concentration of both poly (vinylalcohol) and silver nitrate played significant role in the growth of the nanorods and dendrites (42).
Sonoelectrochemistry technique utilizes the ultrasonic power primarily to manipulate the material mechanically. Pulsed sonoelectro-chemical synthetic method involves alternating sonic and electric pulses, and electrolyte composition plays a crucial role in shape formation (43). It was reported that silver nanospheres could be prepared by sono-electrochemical reduction using a complexing agent, nitrilotriacetate to avoid aggregation (43).
Photoinduced reduction
Silver NPs can be synthesized by using a variety of photoinduced or photocatalytic reduction methods. Photochemical synthesis is a clean process which has high spatial resolution, convenience of use, and great versatility. Moreover, photochemical synthesis enables one to fabricate the NPs in various mediums including cells, emulsion, polymer films, surfactant micelles, glasses, etc. Nano-sized silver particles with an average size of 8 nm were prepared by photoinduced reduction using poly (styrene sulfonate)/poly (allylamine hydrochloride) polyelectrolyte capsules as microreactors (44). Moreover, it was demonstrated that photoinduced method could be used for converting silver nanospheres into triangular silver nanocrystals (nanoprisms) with desired edge lengths in 30-120 nm range (45). Particle growth process was controlled using dual-beam illumination of NPs. Citrate and poly (styrene sulfonate) were used as stabilizing agents. In another study, silver NPs were prepared through a very fast reduction of Ag+ by α-aminoalkyl radicals generated from hydrogen abstraction toward an aliphatic amine by the excited triplet state of 2-substituted thioxanthone series (TX-O-CH2-COO− and TX-S-CH2-COO−). Quantum yield of this prior reaction was tuned by substituent effect on thioxanthones, and led to a kinetic control of conversion of silver ion (Ag+) to silver metal (Ag0) (46).
The direct photo-reduction process of AgNO3 in the presence of sodium citrate (NaCit) was carried out with different light sources (UV, white, blue, cyan, green and orange) at room temperature. Sato-Berrú and coworkers (47) have shown that this light-modification process results in a colloid with distinctive optical properties which can be related to the size and shape of the particles. Moreover, Ghosh and colleagues (48) reported a simple and reproducible UV photo-activation method for the preparation of stable silver NPs in aqueous Triton X-100 (TX-100). The TX-100 molecules act as reducing agent and also as NPs stabilizer through template/capping action.
Furthermore, surfactant solution helps to carry out the process of NPs growth in the diffusion controlled way by decreasing the diffusion or mass transfer co-efficient of the system. It also helps to improve the NPs size distributions by increasing the surface tension at the solvent-NPs interface. Huang and coworkers (49) reported the synthesis of silver NPs in an alkaline aqueous solution of AgNO3/carboxymethylated chitosan (CMCTS) using UV light irradiation. CMCTS, a water-soluble and biocompatible chitosan derivative, served simultaneously as a reducing agent for silver cation and a stabilizing agent for the silver NPs. The diameter range of produced silver NPs was 2–8 nm, and they can be dispersed stably in the alkaline CMCTS solution for more than 6 months.
Electrochemical synthetic method
Electrochemical synthetic method can be used to synthesize silver NPs. It is possible to control particle size by adjusting electrolysis parameters and to improve homogeneity of silver NPs by changing the composition of electrolytic solutions. Polyphenylpyrrole coated silver nanospheroids (3-20 nm) were synthesized by electrochemical reduction at the liquid/liquid interface. This nano-compound was prepared by transferring the silver metal ion from aqueous phase to organic phase, where it reacted with pyrrole monomer (50). In another study, monodisperse silver nanospheroids (1-18 nm) were synthesized by electrochemical reduction inside or outside zeolite crystals according to silver exchange degree of compact zeolite film modified electrodes (51). Furthermore, spherical silver NPs (10-20 nm) with narrow size distributions were conveniently synthesized in aqueous solution by an electrochemical method (52). Poly N-vinylpyrrolidone was chosen as the stabilizer for the silver clusters in this study. Poly N-vinylpyrrolidone protects NPs from agglomeration, significantly reduces silver deposition rate, and promotes silver nucleation and silver particle formation rate. Application of rotating platinum cathode effectively solves the technological difficulty of rapidly transferring metallic NPs from cathode vicinity to bulk solution, avoiding the occurrence of flocculates in vicinity of cathode, and ensures monodispersity of particles. Addition of sodium dodecyl benzene sulfonate to the electrolyte improved particle size and particle size distribution of silver NPs (52).
Irradiation methods
Silver NPs can be synthesized by using a variety of irradiation methods. Laser irradiation of an aqueous solution of silver salt and surfactant can produce silver NPs with a well defined shape and size distribution (53). Furthermore, laser was used in a photo-sensitization synthetic method of making silver NPs using benzophenone. At short irradiation times, low laser powers produced silver NPs of about 20 nm, while an increased irradiation power produced NPs of about 5 nm. Laser and mercury lamp can be used as light sources for production of silver NPs (54). In visible light irradiation studies, photo-sensitized growth of silver NPs using thiophene (sensitizing dye) and silver nanoparticle formation by illumination of Ag(NH3)+ in ethanol has been done (55,56).
Microwave-assisted synthesis
Microwave-assisted synthesis is a promising method for synthesis of silver NPs. Microwave heating is better than a conventional oil bath when it comes to consistently yielding nanostructures with smaller sizes, narrower size distributions, and a higher degree of crystallization (57). Microwave heating has shorter reaction times, reduced energy consumption, and better product yields which prevents the agglomeration of the particles formed (57). Moreover, other than the elimination of the oil bath, microwave-assisted synthesis, in conjunction with benign reaction media, can also drastically reduce chemical wastes and reaction times in several organic syntheses and chemical transformations (58).
It was reported that silver NPs could be synthesized by microwave-assisted synthesis method employing carboxymethyl cellulose sodium as reducing and stabilizing agent. The size was depended on concentration of sodium carboxymethyl cellulose and silver nitrate. The produced NPs were uniform and stable, and were stable at room temperature for 2 months without any visible changes (59). Production of silver NPs in the presence of Pt seeds, polyvinyl pyrrolidine and ethylene glycol was also reported (60).
Furthermore, starch has been employed as a template and reducing agent for synthesis of silver NPs with an average size of 12 nm, using microwave-assisted synthetic method. Starch functions as a template, preventing the aggregation of produced silver NPs (61). Microwaves in combination with polyol process were applied for synthesis of silver nanospheroids using ethylene glycol and poly N-vinylpyrrolidone as reducing and stabilizing agents, respectively (62). In a typical polyol process inorganic salt is reduced by the polyol (e.g., ethylene glycol which serves as both solvent and reducing agent) at a high temperature. Yin and coworkers (63) reported that large-scale and size-controlled silver NPs could be rapidly synthesized under microwave irradiation from an aqueous solution of silver nitrate and trisodium citrate in the presence of formaldehyde as a reducing agent. Size and size distribution of produced silver NPs are strongly dependent on the states of silver cations in the initial reaction solution. Silver NPs with different shapes can be synthesized by microwave irradiation of a silver nitrate-ethylene-glycol-H2[PtCl6]-poly(vinylpyrrolidone) solution within 3 min (64). Moreover, the use of microwave irradiation to produce monodispersed silver NPs using basic amino acids (as reducing agents) and soluble starch (protecting agent) has been reported (65). Radiolysis of silver ions in ethylene glycol, in order to synthesize silver NPs, was also reported (66). Moreover, silver NPs supported on silica aero-gel were produced using gamma radiolysis. The produced silver clusters were stable in 2-9 pH range and started agglomeration at pH>9 (67). Oligochitosan as a stabilizer can be used in preparation of silver NPs by gamma radiation. It was reported that stable silver NPs (5-15 nm) were synthesized in a 1.8-9.0 pH range by this method (68). Silver NPs (4-5 nm) were also synthesized by γ-ray irradiation of acetic water solutions containing silver nitrate and chitosan (69).
Silver nanospheroids (1-4 nm) have been produced by γ-ray irradiation of silver solution in optically transparent inorganic mesoporous silica. Reduction of silver ions within the matrix is brought about by hydrated electrons and hydroalkyl radicals generated during radiolysis of 2-propanol solution. The produced NPs within the silica matrix were stable in the presence of oxygen for at least several months (70). Moreover, silver NPs have been produced by irradiating a solution, prepared by mixing silver nitrate and poly-vinyl-alcohol, with 6-MeV electrons (71). Pulse radiolysis technique has been applied to study reactions of inorganic and organic species in silver nanoparticle synthesis, to understand the factors controlling the shape and size of the NPs synthesized by a common reduction method using citrate ions (as reducing and stabilizing agents) (72), and to demonstrate the role of phenol derivatives in formation of silver NPs by the reduction of silver ions with dihydroxy benzene (73). Dihydroxy benzene could be used to reduce silver ions to synthesize stable silver NPs (with an average size of 30 nm) in air-saturated aqueous solutions (73).
Silver, gold, platinum, and gold-palladium nanostructures have been prepared using microwave-assisted synthetic approach. Morphologies and sizes of NPs can be controlled by altering some experimental parameters such as the concentration of metallic precursors, surfactant polymers, solvents, and temperature. Moreover, monodisperse silver NPs can be synthesized in large quantities using microwave-assisted chemistry method in an aqueous system. In this method, amino acids act as reducing agents and soluble starch acts as a protecting agent.
Not only silver, but silver doped lanthanum chromites can also be synthesized with microwave energy (74). Microwave energy and thermal reduction can be coupled to synthesize silver NPs that can be deposited on oxidized carbon paper electrodes. The silver NPs that are synthesized through this method maintain a uniform size between particles and are well-dispersed over the carbon paper substrate. The microwave-assisted synthesis of silver NPs is made possible by depositing the silver catalysts on carbon paper electrodes. This method can potentially be used in alkaline fuel cells because the synthesis occurs quickly, there is high activity, and the process is very simple (75).
Nanosized calcium deficient hydroxy-apatites can be used to generate nanosized calcium deficient hydroxyapatite with a silver substitution in three different concentrations by microwave-assisted synthesis. This study showed that controlling the parameters of the microwave process could influence the size of the crystals produced. It was shown that the microwave power had more of an impact on the size of the particles than the length of time of the treatment. The ensuing powder product could be used in the field of medicine and biomedical engineering to make grafts and coating metal implants in addition to work against bacterial infections without the use of antibiotics. This method can reduce medicinal costs and time of hospitalization (76).
Polymer based silver composites were produced using microwave energy on the basis of interfacial polymerization. A water/chloroform interface was used under microwave irradiation with no oxidizing agent. The produced silver NPs (about 20 nm in size) were spherical and well-dispersed (77). Silver nitrate provided silver ions for the thermal polymerization of pyrrole. The ions were converted to silver/polypyyrole nano-composites. Transmission electron microscopy (TEM) images proved that the particles were about 5−10 nm in size. The silver/polypyyrole had a thick film, which could sense ammonia, hydrogen sulfide, and carbon dioxide at 100, 250, and 350°C, respectively (78).
Microwave radiation and ethylene glycol can be used to synthesize silver powders from silver nitrate at temperatures of 100-200°C. It was reported that when polyvinyl pyrrolidone was used in the mixture of silver nitrate, the NPs ranged from 62 to 78 nm in diameter (79). Moreover, Fe-Ag bimetallic NPs could be synthesized by using microwave heating and an oil-soluble silver salt (80). The produced silver NPs were characterized through freeze-etching replication TEM which revealed the nanoparticle diameter and distribution. The produced silver NPs (30 nm) were spherical in shape (81).
Hydrolysis of alkoxysilanes along with the silver salt, in the presence of microwave irradiation, can produce silver/SiO2 composite sols, which displayed antimicrobial properties (82). Meng and coworkers (83) discussed the utilization of various water-based synthesis routes toward the shape-controlled synthesis of silver NPs and microstructures. Several one-pot methods employing commercial microwave ovens, inexpensive/low power ultrasound cleaners, or two-electrode electro-chemistry were described. Synthesis of silver nanostructures with various shapes in solution and their doping on unmodified silica and on/inside carbon spheres were investigated.
Microwave-assisted synthesis was used to prepare different kinds of nanosilver colloids. Silver nitrate was mixed with sodium citrate and then split into five groups. Each group was heated for varying durations of time at different temperatures. It was determined that the nanosilver colloids had a negatively charged surface when heated for a long period of time and a positively charged surface when heated for a short period of time (84). Moreover, silica-alumina can be used to synthesize silver NPs with precursors like Ag2O or AgNO3. The particles were as small as 3 nm in diameter or as big as 50 nm. They were not oxidized, and the particles were well spread out (85). In another study, nanosilver/polyvinylpyrrolidone composite materials were synthesized using the microwave approach. The produced NPs ranged from 15-25 nm and were evenly spread out in the polyvinylpyrrolidone matrix (86).
Polymers and polysaccharides
Silver NPs were prepared using water as an environmentally friendly solvent and polysaccharides as capping/reducing agents. For instance, synthesis of starch-silver NPs was carried out with starch (capping agent) and β-D-glucose (reducing agent) in a gently heated system (87).
The binding interactions between starch and produced silver NPs were weak and could be reversible at higher temperatures, allowing separation of the synthesized NPs. In dual polysaccharide function, silver NPs were synthesized by reduction of silver ions inside of nanoscopic starch templates (87,88). The extensive network of hydrogen bands in templates provided surface passivation or protection against nanoparticle aggregation. Green synthesis of silver NPs using negatively charged heparin (reducing/stabilizing agent and nucleation controller) was also reported by heating a solution of silver nitrate and heparin to 70 °C for about 8 h (89). TEM micrographs demonstrated an increase in particle size of silver NPs with increased concentrations of silver nitrate (substrate) and heparin. Moreover, changes in heparin concentration varied the morphology and size of silver NPs. The synthesized silver NPs were highly stable, and showed no signs of aggregation after two months (89). In another study, stable silver NPs (10-34 nm) were synthesized by autoclaving a solution of silver nitrate (substrate) and starch (capping/reducing agent) at 15 psi and 121 °C for 5 min (90). These NPs were stable in solution for three months at about 25 °C. Smaller silver NPs (≤ 10 nm) were synthesized by mixing two solutions of silver nitrate containing starch (capping agent), and NaOH solutions containing glucose (reducing agent) in a spinning disk reactor with a reaction time of less than 10 min (91).
Silver nitrate, glucose, sodium hydroxide, and starch can be used, respectively, to serve as precursor, reducing agent, accelerator, and stabilizer for the reduction synthesis of silver nitrate. Polyethylene glycol (reducing agent and stabilizing agent) was used to prepare stable monodisperse silver colloids (~10 nm) (92). Biodegradable starch worked as a stabilizing agent to synthesize silver NPs (5-20 nm). The analyses showed that the NPs were coated with a layer of starch (93).
Silver NPs (~13 ± 3 nm) can be synthesized using sulfated polysaccharide which can be obtained from marine red algae; Porphyra vietnamensis. It was reported that sulfate moiety from the polysaccharides was involved in silver nitrate reduction. Zeta potential measurements of −35.05 mV showed that the anionic polysaccharide had indeed capped the nanoparticles’ surfaces and contributed to the electrostatic stability. The NPs were stable at a very broad pH range, from 2 to 10, and electrolyte concentration of 10−2 M (94).
Polymers that have ion-exchangeable capacity can be used in many fields of science. The polymer often used contained phosphonic acid groups and had a low molecular weight. For instance, silver NPs were stabilized in the presence of an ion-exchange polymer. The surface morphology indicated that cubes and rectangular prism structures were formed (95). Co-polymers like cyclodextrin, grafted with poly acrylic acid, can be used to produce silver NPs where potassium per sulfate was used as the initiator. The co-polymer reduces and stabilizes the silver ions that yielded silver NPs. The concentration of the alkali, silver nitrate, the co-polymer, and the method of heating all played an important role in determining the size of the produced NPs (96).
Poly (methyl vinyl etherco- maleic anhydride) could be used as a reducing and stabilizing agent as well. The produced NPs were stable at room temperature for up to a month and had a 5-8 nm coat of poly (methyl vinyl etherco-maleic anhydride) surrounding them (97). It was reported that the NPs (10.2-13.7 nm) were face-centered cubic (FCC) structures, not aggregating, and very spherical in shape (98). Sarkar and colleagues (99) examined the synthesis of silver nanowires and NPs. Through a polypol process, with the help of a polymer, silver nanowires and NPs were formed. It was reported that the NPs were 60-200 nm in size and held prismatic and hexagonal shapes while the nanowires had diameters from 50 to 190 nm and lengths between 40 and 1000 μm. The reaction occurred at 210°C when ethylene glycol was used as the solvent. The different photoluminescence emission from the nano-clusters spread out through the methanol and the ethylene glycol at room temperature. The excitation wavelengths were measured between 300 and 414 nm (99). By changing the reducing and capping agents that are used to synthesize silver NPs, one can change the morphologies of the NPs, as well. The synthesis yielded NPs which were spherical in shape and around 15-43 nm in size after being heated at 70°C for 30 min; while at room temperature, the particles were only 8-24 nm. Sodium hydroxide reduced salt in ethylene glycol and cubes were formed upon some aggregation. By adding 5 wt % poly-vinylpyrrolidone to 1 wt % of starch solution (aq), mixtures of spherical and anisotropic structures were produced. The reaction took place at 70°C for 1 h (100).
Tollens method
A simple one-step process, Tollens method, has been used for synthesis of silver NPs with a controlled size. This green synthesis technique involves reduction of Ag(NH3)2+ (as Tollens reagent) by an aldehyde (101). In the modified Tollens procedure, silver ions are reduced by saccharides in the presence of ammonia, yielding silver nanoparticle films (50-200 nm), silver hydrosols (20-50 nm) and silver NPs of different shapes. In this method, ammonia concentration and nature of the reducing agent play an important role in controlling size and morphology of silver NPs. It was revealed that the smallest particles were formed at the lowest ammonia concentration. Glucose and the lowest ammonia concentration (5 mM) resulted in the smallest average particle size of 57 nm with an intense maximum of surface plasmon absorbance at 420 nm. Moreover, increase in NH3 from 0.005 M to 0.2 M resulted in a simultaneous increase in particle size and polydispersity (102). Silver NPs with controllable sizes were synthesized by reduction of [Ag(NH3)2]+ with glucose, galactose, maltose, and lactose (103).
The nanoparticle synthesis was carried out at various ammonia concentrations (0.005-0.20 M) and pH conditions of 11.5-13.0 resulting in average particle sizes of 25-450 nm. The particle size was increased by increasing (NH3), and the difference in structure of reducing agent (monosaccharides and disaccharides) and pH (particles obtained at pH 11.5 were smaller than those at pH 12.5) influenced the particle size. Polydispersity also decreased by lowering the pH. Produced silver NPs were stabilized and protected by sodium dodecyl sulfate (SDS), polyoxyethylene sorbitane monooleate (Tween 80), and polyvinylpyrrolidone (PVP 360) (104,105).
Bio-based methods
A number of reports prevailed in the literatures indicate that synthesis of nanoparticles by chemical approaches are eco-unfriendly and expensive. Thus, there is a growing need to develop environmentally and economically friendly processes, which do not use toxic chemicals in the synthesis protocols. This has conducted researchers to look at the organisms. The potential of organisms in nanoparticle synthesis ranges from simple prokaryotic bacterial cells to eukaryotic fungi and plants (106). Some examples of nanoparticle production include using bacteria for gold, silver, cadmium, zinc, magnetite, and iron NPs; yeasts for silver, lead and cadmium NPs; fungi for gold, silver and cadmium NPs; algae for silver and gold NPs; plants for silver, gold, palladium, zinc oxide, platinum, and magnetite NPs (7,107).
Bio-based protocols could be used for synthesis of highly stable and well-characterized NPs when critical aspects, such as types of organisms, inheritable and genetical properties of organisms, optimal conditions for cell growth and enzyme activity, optimal reaction conditions, and selection of the biocatalyst state have been considered. Sizes and morphologies of the NPs can be controlled by altering some critical conditions, including substrate concentration, pH, light, temperature, buffer strength, electron donor (e.g., glucose or fructose), biomass and substrate concentration, mixing speed, and exposure time. In the following section, we discussed the synthesis of NPs using microorganisms and biological systems (Fig. 1 and Table 3).
Fig. 1.
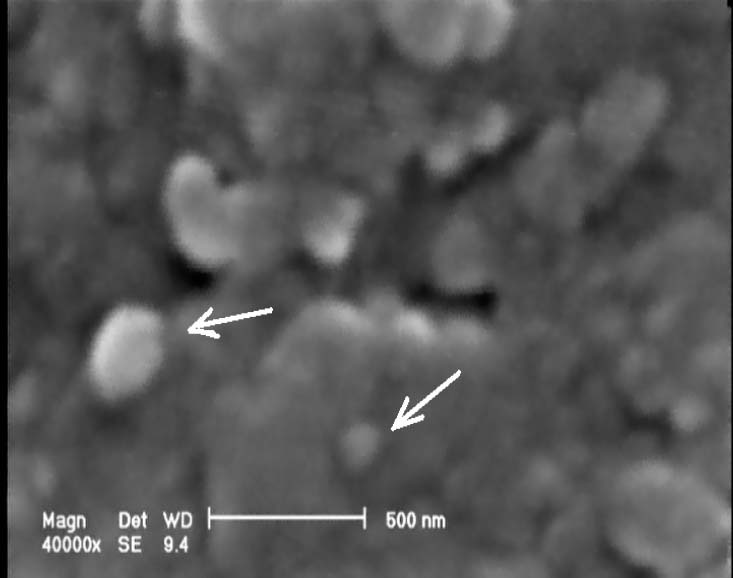
Biosynthesis of silver NPs; We demonstrated the bioreductive synthesis of silver nanoparticles using F. oxysporum (an unpublished scanning electron microscopy micrograph recorded from silver NPs produced by reaction of silver nitrate solution (1 mM) with F. oxysporum).
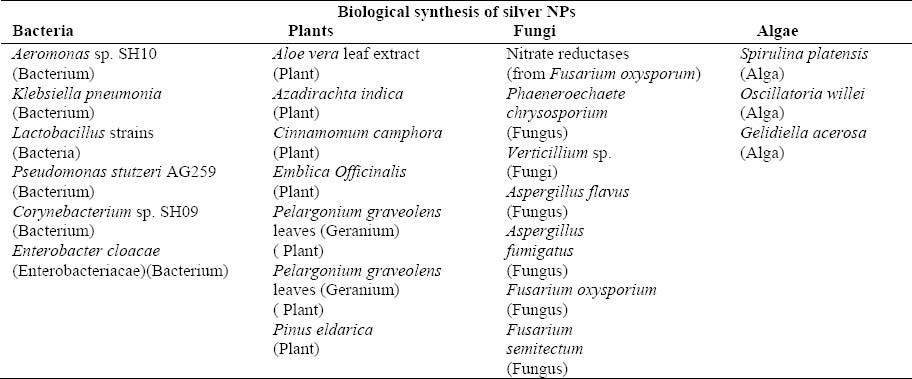
Table 3.
Some important examples of organisms used for synthesizing silver nanoparticles.
Bacteria
Bacteria have been explored in the synthesis of silver NPs. It was reported that highly stable silver NPs (40 nm) could be synthesized by bioreduction of aqueous silver ions with a culture supernatant of nonpathogenic bacterium, Bacillus licheniformis (108). Moreover, well-dispersed silver nanocrystals (50 nm) were synthesized using the bacterium B. licheniformis (109). Saifuddin and coworkers (110) have described a novel combinational synthesis approach for the formation of silver NPs by using a combination of culture supernatant of B. subtilis and microwave irradiation in water. They reported the extracellular biosynthesis of monodispersed Ag NPs (5-50 nm) using supernatants of B. subtilis, but in order to increase the rate of reaction and reduce the aggregation of the produced NPs, they used microwave radiation which might provide uniform heating around the NPs and could assist the digestive ripening of particles with no aggregation.
Silver nanocrystals of different compositions were successfully synthesized by Pseudomonas stutzeri AG259 (111). The silver-resistant bacterial strain, P. stutzeri AG259, isolated from a silver mine, accumulated silver NPs intracellularly, along with some silver sulfide, ranging in size from 35 to 46 nm (112). Larger particles were formed when P. stutzeri AG259 challenged with high concentrations of silver ions during culturing, resulted intracellular formation of silver NPs, ranging in size from a few nm to 200 nm (8,111). P. stutzeri AG259 detoxificated silver through its precipitation in the periplasmic space and its reduction to elemental silver with a variety of crystal typologies, such as hexagons and equilateral triangles, as well as three different types of particles: elemental crystalline silver, monoclinic silver sulfide acanthite (Ag2S), and a further undetermined structure (111). The periplasmic space limited the thickness of the crystals, but not their width, which could be rather large (100-200 nm) (8).
In another study, rapid biosynthesis of metallic NPs of silver using the reduction of aqueous Ag+ ions by culture supernatants of Klebsiella pneumonia, Escherichia coli, and Enterobacter cloacae was reported (113). The synthetic process was quite fast and silver NPs were formed within 5 min of silver ions coming in contact with the cell filtrate. It seems that nitroreductase enzymes might be responsible for bioreduction of silver ions.
It was also reported that visible-light emission could significantly increase synthesis of silver NPs (1-6 nm) by culture supernatants of K. pneumoniae (114). Monodispersed and stable silver NPs were also successfully synthesized with bioreduction of [Ag (NH3)2]+ using Aeromonas sp. SH10 and Corynebacterium sp. SH09 (115). It was speculated that [Ag (NH3)2]+ first reacted with OH− to form Ag2O, which was then metabolized independently and reduced to silver NPs by the biomass.
Lactobacillus strains, when exposed to silver ions, resulted in biosynthesis of NPs within the bacterial cells (116,117). It has been reported that exposure of lactic acid bacteria present in the whey of buttermilk to mixtures of silver ions could be used to grow NPs of silver. The nucleation of silver NPs occurred on the cell surface through sugars and enzymes in the cell wall, and then the metal nuclei were transported into the cell where they aggregated and grew to larger-sized particles. Korbekandi and colleagues (117) demonstrated the bioreductive synthesis of silver NPs using L. casei subsp. casei at room temperature. Researchers have reported qualitative production of silver NPs by Lactobacillus sp., but they did not optimize the reaction mixture. Biosynthesized silver NPs were almost spherical, single (25-50 nm) or in aggregates (100 nm), attached to the surface of biomass or were inside and outside of the cells. The reduction of metal ions and stabilization of the silver NPs was confirmed to occur by an enzymatic process. Electron microscopy analysis indicated that the silver NPs were formed on the surface of the cytoplasmic cell membrane, inside the cytoplasm and outside of the cells, possibly due to the bioreduction of the metal ions by enzymes present on the cytoplasmic membrane and within the cytoplasm (117).
Fungi
Silver NPs (5-50 nm) could be synthesized extracellularly using Fusarium oxysporum, with no evidence of flocculation of the particles even a month after the reaction (118). The long-term stability of the nanoparticle solution might be due to the stabilization of the silver particles by proteins. The morphology of NPs was highly variable, with generally spherical and occasionally triangular shapes observed in the micrographs. Silver NPs have been reported to interact strongly with proteins including cytochrome c (Cc). This protein could be self-assembled on citrate-reduced silver colloid surface (119). Interestingly, adsorption of (Cc)-coated colloidal gold NPs onto aggregated colloidal Ag resulted Ag: Cc: Au nanoparticle conjugate (120). In UV-vis spectra from the reaction mixture after 72 h, the presence of an absorption band at ca. 270 nm might be due to electronic excitations in tryptophan and tyrosine residues in the proteins. In F. oxysporum, the bioreduction of silver ions was attributed to an enzymatic process involving NADH-dependent reductase (121). The exposure of silver ions to F. oxysporum, resulted in the release of nitrate reductase and subsequent formation of highly stable silver NPs in solution (122). The secreted enzyme was found to be dependent on NADH cofactor. They mentioned high stability of NPs in solution was due to capping of particles by release of capping proteins by F. oxysporum. Stability of the capping protein was found to be pH dependent. At higher pH values (>12), the NPs in solution remained stable, while they aggregated at lower pH values (<2) as the protein was denatured. Kumar and coworkers (122) have demonstrated enzymatic synthesis of silver NPs with different chemical compositions, sizes and morphologies, using α-NADPH-dependent nitrate reductase purified from F. oxysporum and phytochelatin, in vitro. Silver ions were reduced in the presence of nitrate reductase, leading to formation of a stable silver hydrosol 10-25 nm in diameter and stabilized by the capping peptide. Use of a specific enzyme in vitro synthesis of NPs showed interesting advantages. This would eliminate the downstream processing required for the use of these NPs in homogeneous catalysis and other applications such as non-linear optics. The biggest advantage of this protocol based on purified enzyme was the development of a new approach for green synthesis of nanomaterials over a range of chemical compositions and shapes without possible aggregation. Korbekandi and colleagues (123) demonstrated the bioreductive synthesis of silver NPs using F. oxysporum. Previous researchers reported qualitative production of silver NPs by F. oxysporum, but they did not optimize the reaction mixture. In SEM micrographs, silver NPs were almost spherical, single (25-50 nm) or in aggregates (100 nm), attached to the surface of biomass. The reduction of metal ions and stabilization of the silver NPs was confirmed to occur by an enzymatic process. It seems that the first step involves trapping of the Ag+ ions by F. oxysporum cells. More details of the location of NPs production by this fungus were revealed, and the previous theories were corrected. In contrast with the previous studies, it is claimed that the nanoparticle production in F. oxysporum is intracellular by engulfing the NPs in vesicles, transporting, and excreting of them through exocytosis outside of the cells (123). Ingle and coworkers (124) demonstrated the potential ability of Fusarium acuminatum Ell. and Ev. (USM-3793) cell extracts in biosynthesis of silver NPs. The NPs produced within 15-20 min and were spherical with a broad size distribution in the range of 5-40 nm with the average diameter of 13 nm. A nitrate-dependent reductase enzyme might act as the reducing agent. The white rot fungus, Phanerochaete chrysosporium, also reduced silver ions to form nano-silver particles (125). The most dominant morphology was pyramidal shape, in different sizes, but hexagonal structures were also observed.
Stable silver NPs could be achieved by using Aspergillus flavus (126). These NPs were found to be stable in water for more than 3 months with no significant aggregation because of surface binding of stabilizing materials secreted by the fungus (126). Extracellular biosynthesis of silver NPs (5-25 nm) using Aspergillus fumigatus has also been investigated (127). Most of the NPs were spherical in nature with some others having occasionally triangular shapes (127).
The extracellular filtrate of Cladosporium cladosporioides biomass was used to synthesize silver NPs (128). It was suggested that proteins, organic acids and polysaccharides released by C. cladosporioides were responsible for formation of spherical crystalline silver NPs. Kathiresan and coworkers (129) have shown that when the culture filtrate of Penicillium fellutanum was incubated with silver ions and maintained under dark conditions, spherical silver NPs could be produced. Penicillium genus were used for green synthesis of silver NPs (130). Penicillium sp. J3 isolated from soil was able to produce silver NPs (131). The bioreduction of the silver ions occurred on the surface of the cells and proteins might have critical role in the formation and stabilization of the synthesized NPs.
Sanghi and colleagues (132) have investigated the ability of Coriolus versicolor in formation of monodisperse spherical silver NPs. the time taken for production of silver NPs was reduced from 72 h to 1 h under alkaline conditions (pH 10). It was indicated that alkaline conditions might be involved in bioreduction of silver ions, water hydrolysis and interaction with protein functionalities. Findings of this study have shown that glucose was necessary for the reduction of silver NPs, and S-H of the protein played an important role in the bioreduction.
Algae
A few reports are available regarding gold accumulation using algal genera including cyanobacteria as biological reagent. Cyanobacteria and eukaryotic alga genera such as Lyngbya majuscule, Spirulina subsalsa, Rhizoclonium heiroglyphicum, Chlorella vulgaris, Cladophora prolifera, Padina pavonica, Spirulina platensis, and Sargassum fluitans can be used as cost effective means for biorecovery of gold out of the aqueous solutions, as well as the formation of gold NPs (133,134,135,136). Marine algae like Chaetoceros calcitrans, Chlorella salina, Isochrysis galbana and Tetraselmis gracilis can also be used for reduction of silver ions and thereby synthesis of Ag NPs (113). Marine cyanobacterium, Oscillatoria willei NTDM01 has been used for synthesis of silver NPs (100-200 nm). Silver nitrate solution incubated with washed marine cyanobacteria changed to a yellow color from 72 h onwards, indicating the formation of silver NPs. When Spirulina platensis biomass was exposed to 10−3 M aqueous AgNO3, extracellular formation of spherical silver NPs (7-16 nm) has been resulted in 120 h at 37 °C at pH 5.6 (137). Proteins might be responsible for reduction and stabilization of the NPs. In the case of C. vulgaris, the proteins in the extract have dual function of Ag+ ion reduction, and shape controlled synthesis of NPs. The Ag nano plates are obtained at room temperature. Reduction of Ag+ ions is done by the hydroxyl groups in Tyr residues and carboxyl groups in Asp/Glu residues. This is responsible for the anisotropic growth of Ag nano plates which yields rod-like particles with a mean length of 44 nm and width of 16-24 nm.
Plants
Synthesis of NPs using plants is very cost effective, and thus can be used as an economic and valuable alternative for the large-scale production of NPs (10). Camellia sinensis (green tea) extract has been used as a reducing and stabilizing agent for the biosynthesis of silver NPs (138). Phenolic acid-type biomolecules (e.g., caffeine and theophylline) present in the C. sinensis extract seemed to be responsible for the formation and stabilization of silver NPs. Black tea leaf extracts were also used in the production of silver NPs (139). The NPs were stable and had different shapes, such as spheres, trapezoids, prisms, and rods. Polyphenols and flavonoids seemed to be responsible for the biosynthesis of these NPs.
Plant extracts from alfalfa (Medicago sativa), lemongrass (Cymbopogon flexuosus), and geranium (Pelargonium graveolens) have served as green reactants in silver nanoparticle synthesis. Moreover, a high density of extremely stable silver NPs (16-40 nm) was rapidly synthesized by challenging silver ions with Datura metel leaf extract (140). The leaf extracts of this plant contains biomolecules, including alkaloids, proteins/enzymes, amino acids, alcoholic compounds, and poly-saccharides which could be used as reductant to react with silver ions, and therefore used as scaffolds to direct the formation of silver NPs in the solution.
Song and colleagues elucidated the fact that Pinus desiflora, Diospyros kaki, Ginko biloba, Magnolia kobus and Platanus orientalis leaf broths synthesized stable silver NPs with average particle size ranging from 15 to 500 nm, extracellularly. In the case of M. kobus and D. kaki leaf broths, the synthesis rate and final conversion to silver NPs was faster, when the reaction temperature was increased. But the average particle sizes produced by D. kaki leaf broth decreased from 50 nm to 16 nm, when temperature was increased from 25°C to 95°C (141). The researchers also illustrated that only 11 min was required for more than 90% conversion at the reaction temperature of 95°C using M. kobus leaf broth (141). It was further demonstrated that leaf extracts from the aquatic medicinal plant, Nelumbo nucifera, was able to reduce silver ions and produce silver NPs (45 nm) in different shapes (142).
Capsicum annuum can be used for green synthesizing silver NPs (143). Proteins with amine groups reduced the silver ions and acted as a control during synthesis. After interacting with the silver ions, the protein's secondary structure was found to be altered. The crystalline phase of the NPs changed from polycrystalline to single crystalline and increased in size with increasing reaction time. A recognition-reduction-limited nucleation and growth model was suggested to explain the possible formation mechanism of silver NPs in Capsicum annuum L. extract (143).
Spherical silver NPs (40-50 nm) were produced using leaf extract of Euphorbia hirta (144). These NPs had potential and effective antibacterial property against Bacillus cereus and Staphylococcus aureus.
Acalypha indica (Euphorbiaceae) leaf extracts have produced silver NPs (20-30 nm) within 30 min (145). These NPs had excellent antimicrobial activity against water borne pathogens, E. coli and V. cholera (minimum inhibitory concentration (MIC)=10 μg/ml).
Moreover, silver NPs (57 nm) were produced when 10 ml of Moringa oleifera leaf extract was mixed to 90 ml of 1 mM aqueous of AgNO3 and was heated at 60-80°C for 20 min. These NPs had considerable antimicrobial activity against pathogenic microorganisms, including Staphylococcus aureus, Candida tropicalis, Klebsiella pneumoniae, and Candida krusei (146).
It has been reported that cotton fibers loaded with biosynthesized silver NPs (~20 nm) using natural extracts of Eucalyptus citriodora and Ficus bengalensis had excellent antibacterial activity against E. coli. These fibers had potential for utilization in burn/wound dressings as well as in the fabrication of antibacterial textiles and finishings (147). Garcinia mangostana leaf extract could be used as reducing agent in order to biosynthesize silver NPs (35 nm).
These NPs had high effective antimicrobial activity against E. coli and S. aureus (148). It was reported that Ocimum sanctum leaf extract could reduce silver ions into crystalline silver NPs (4-30 nm) within 8 min of reaction time. These NPs were stable due to the presence of proteins which may act as capping agents. O. sanctum leaves contain ascorbic acid which may play an important role in reduction of silver ions into metallic silver NPs. These NPs have shown strong antimicrobial activity against E. coli and S. aureus (149).
Green synthesis of silver NPs using Cacumen platycladi extract was investigated. Reducing sugars and flavonoids in the extract seemed to be mainly responsible for reduction of silver ions, and their reductive capability promoted at 90°C, leading to formation of silver NPs (18.4 ± 4.6 nm) with narrow size distribution. The produced NPs had significant antibacterial activity against both gram negative and gram positive bacteria (E. coli and S. aureus) (150). In case of Cinnamon zeylanicum, the bark extract could be used in biosynthesis of cubic and hexagonal silver nanocrystals (31-40 nm) (151).
The particle size distribution varied with variation in the amount of C. zeylanicum bark extract. The number of particles increased with increasing dosage due to the variation in the amount of reductive biomolecules. Small NPs were formed at high pH. The shape of silver NPs at high pH was more spherical in nature rather than ellipsoidal. Bactericidal effect of produced nano-crystalline silver particles was tested against E. coli strain. As a result, the various tested concentrations of 2, 5, 10, 25, and 50 mg/L produced inhibition of 10.9, 32.4, 55.8, 82, and 98.8 %, respectively. The MIC was found to be 50 mg L-1. In another study, silver NPs were successfully produced using P. eldarica bark extract. Characterization by UV-visible and TEM techniques confirmed the reduction of silver ions to silver NPs. The preparation of nanostructured silver particles using P. eldarica bark extract provides an environmentally friendly option, as compared to currently available chemical and/or physical methods (152).
Studying synthesis of silver NPs with isolated/purified bio-organics may give better insight into the system mechanism. Glutathione (γ-Glu-Cys-Gly) as a reducing/capping agent can synthesize water-soluble and size tunable silver NPs which easily bind to a model protein (bovine serum albumin) (153). Tryptophan residues of synthetic oligo-peptides at the C-terminus were identified as reducing agents producing silver NPs (154). Moreover, silver NPs were synthesized by vitamin E in Langmuir-Blodgett technique, by bio-surfactants (sophorolipids) and by L-valine-based oligopeptides with chemical structures, Z-(L-Val)3-OMe and Z-(L-Val)2-L-Cys (S-Bzl)-OMe (155,156). The sulfur content in the Z-(L-Val)2-L-Cys(S-Bzl)-OMe controls the shape and size of silver NPs, which suggests interaction between silver ions and thioether moiety of the peptide (156).
Pure natural constituents could be used to bio-reduce and stabilize silver NPs. Kasthuri and coworkers (157) have shown the ability of apiin extracted from henna leaves to produce anisotropic gold and quasi-spherical silver NPs. Secondary hydroxyl and carbonyl groups of apiin were responsible for the bioreduction of metal salts. In order to control size and shape of NPs, they used different amounts of apiin (as the reducing agent). The NPs were stable in water for 3 months, which could be attributed to surface binding of apiin to NPs. Furthermore, geraniol as a volatile compound obtained from P. graveolens was used for biosynthesis of silver NPs (1-10 nm). They also evaluated in vitro cytotoxicity of silver NPs against Fibrosarcoma Wehi 164 at different concentrations (1-5 μg/ml). The presence of 5 μg/ml of silver NPs significantly inhibited the cell line's growth (up to 60 %) (158). The various synthetic and natural polymers such as poly(ethylene glycol), poly-(N-vinyl-2-pyrrolidone), starch, heparin, poly-cationic chitosan (1-4-linked 2-amino-2-deoxy -β-D-glucose), sodium alginate, and gum acacia have been reported as reducing and stabilizing agents for biosynthesis of silver NPs. For instance, it was reported that monodisperse spherical silver NPs (~3 nm) could be synthesized using gum kondagogu (non-toxic polysaccharide derived as an exudate from the bark of Cochlospermum gossypium) (159). It was suggested that carboxylate and hydroxyl groups were involved in complexation and bioreduction of silver ions into NPs. This method was compatible with green chemistry principles as the gum serves a matrix for both bioreduction and stabilization of the synthesized NPs. Due to availability of low cost plant derived biopolymer, this method could be implemented for large-scale synthesis of highly stable monodispersed NPs.
CONCLUSION
Silver NPs have gained considerable interest because of their unique properties, and proven applicability in diverse areas such as medicine, catalysis, textile engineering, biotechnology, nanobiotechnology, bio-engineering sciences, electronics, optics, and water treatment. These NPs have significant inhibitory effects against microbial pathogens, and are widely used as antimicrobial agents in a diverse range of products.
The flexibility of silver nanoparticle synthetic methods and facile incorporation of silver NPs into different media have encouraged researchers to further investigate the mechanistic aspects of antimicrobial, antiviral and anti-inflammatory effects of these NPs. Shape, size and size distribution of silver NPs can be controlled by adjusting the reaction conditions such as reducing agent, stabilizer or employing different synthetic methods. Therefore, it is important to elucidate the effects of reaction conditions on morphology and size of NPs.
Different methods have been developed to obtain silver NPs of various shapes and sizes, including laser ablation, gamma irradiation, electron irradiation, chemical reduction, photochemical methods, microwave processing, and thermal decomposition of silver oxalate in water and in ethylene glycol, and biological synthetic methods. Biosynthetic methods of NPs provide a new possibility of conveniently synthesizing NPs using natural reducing and stabilizing agents. As possible environmentally and economically friendly alternatives to chemical and physical approaches, biosynthesis of metal and semiconductor NPs using organisms has been suggested. Monodispersity and particle size and shape are very important parameters in the evaluation of NPs synthesis. Therefore, the efficient control on the morphology and monodispersity of NPs must be explored. The reaction conditions should be optimized. By using screened organisms with high production capability and controlling the reaction conditions, well- characterized NPs can be obtained by synthesis rates faster or compatible to those of chemical and physical approaches. This eco-friendly method can potentially be used in various areas, including pharmaceuticals, cosmetics, foods, and medical applications.
REFERENCES
- 1.Colvin VL, Schlamp MC, Alivisatos A. Light emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature. 1994;370:354–357. [Google Scholar]
- 2.Wang Y, Herron N. Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photophysical properties. J Phys Chem. 1991;95:525–532. [Google Scholar]
- 3.Schmid G. Large clusters and colloids. Metals in the embryonic state. Chem Rev. 1992;92:1709–1027. [Google Scholar]
- 4.Hoffman AJ, Mills G, Yee H, Hoffmann M. Q-sized cadmium sulfide: synthesis, characterization, and efficiency of photoinitiation of polymerization of several vinylic monomers. J Phys Chem. 1992;96:5546–5552. [Google Scholar]
- 5.Hamilton JF, Baetzold R. Catalysis by Small Metal Clusters. Science. 1979;205:1213–1220. doi: 10.1126/science.205.4412.1213. [DOI] [PubMed] [Google Scholar]
- 6.Mansur HS, Grieser F, Marychurch MS, Biggs S, Urquhart RS, Furlong D. Photoelectrochemical properties of ‘q-state’ cds particles in arachidic acid langmuir-blodgett films. J Chem Soc Faraday Trans. 1995;91:665–672. [Google Scholar]
- 7.Senapati S. Ph.D. Thesis. India: University of pune; 2005. Biosynthesis and immobilization of nanoparticles and their applications; pp. 1–57. [Google Scholar]
- 8.Klaus-Joerger T, Joerger R, Olsson E, Granqvist CG. Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001;19:15–20. doi: 10.1016/s0167-7799(00)01514-6. [DOI] [PubMed] [Google Scholar]
- 9.Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–170. [Google Scholar]
- 10.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. [Google Scholar]
- 11.Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotech. 2009;29:279–306. doi: 10.3109/07388550903062462. [DOI] [PubMed] [Google Scholar]
- 12.Kruis F, Fissan H, Rellinghaus B. Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater Sci Eng B. 2000;69:329–334. [Google Scholar]
- 13.Magnusson M, Deppert K, Malm J, Bovin J, Samuelson L. Gold nanoparticles: production, reshaping, and thermal charging. J Nanoparticle Res. 1999;1:243–251. [Google Scholar]
- 14.Jung J, Oh H, Noh H, Ji J, Kim S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J Aerosol Sci. 2006;37:1662–1670. [Google Scholar]
- 15.Mafune F, Kohno J, Takeda Y, Kondow T, Sawabe H. Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation. J Phys Chem B. 2000;104:8333–8337. [Google Scholar]
- 16.Mafune F, Kohno J, Takeda Y, Kondow T, Sawabe H. Formation of gold nanoparticles by laser ablation in aqueous solution of surfactant. J Phys Chem B. 2001;105:5114–5120. [Google Scholar]
- 17.Kabashin AV, Meunier M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J Appl Phys. 2003;94:7941–7943. [Google Scholar]
- 18.Sylvestre JP, Kabashin AV, Sacher E, Meunier M, Luong JHT. Stabilization and size control of gold nanoparticles during laser ablation in aqueous cyclodextrins. J Am Chem Soc. 2004;126:7176–7177. doi: 10.1021/ja048678s. [DOI] [PubMed] [Google Scholar]
- 19.Dolgaev SI, Simakin AV, Voronov VV, Shafeev GA, Bozon-Verduraz F. Nanoparticles produced by laser ablation of solids in liquid environment. Appl Surf Sci. 2002;186:546–551. [Google Scholar]
- 20.Kim S, Yoo B, Chun K, Kang W, Choo J, Gong M, et al. Catalytic effect of laser ablated Ni nanoparticles in the oxidative addition reaction for a coupling reagent of benzylchloride and bromoacetonitrile. J Mol Catal A: Chem. 2005;226:231–234. [Google Scholar]
- 21.Link S, Burda C, Nikoobakht B, El-Sayed M. Laser-Induced shape changes of colloidal gold nanorods using femtosecond and nanosecond laser pulses. J Phys Chem B. 2000;104:6152–6163. [Google Scholar]
- 22.Tarasenko N, Butsen A, Nevar E, Savastenko N. Synthesis of nanosized particles during laser ablation of gold in water. Appl Surf Sci. 2006;252:4439–4444. [Google Scholar]
- 23.Kawasaki M, Nishimura N. 1064-nm laser fragmentation of thin Au and Ag flakes in acetone for highly productive pathway to stable metal nanoparticles. Appl Surf Sci. 2006;253:2208–2216. [Google Scholar]
- 24.Tsuji T, Iryo K, Watanabe N, Tsuji M. Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl Surf Sci. 2002;202:80–85. [Google Scholar]
- 25.Tsuji T, Kakita T, Tsuji M. Preparation of nano-size particle of silver with femtosecond laser ablation in water. Applied Surface Science. 2003;206:314–320. [Google Scholar]
- 26.Tien D-C, Tseng K-H, Liao C-Y, Huang J-C, Tsung TT. Discovery of ionic silver in silver nanoparticle suspension fabricated by arc discharge method. Journal of Alloys and Compounds. 2008;463:408–411. [Google Scholar]
- 27.Siegel J, Kvítek Ondřej, Ulbrich Pavel, Kolská Z, Slepička P, Švorčík V. Progressive approach for metal nanoparticle synthesis. Materials Letters. 2012;89:47–50. [Google Scholar]
- 28.Wiley B, Sun Y, Mayers B, Xi Y. Shape-controlled synthesis of metal nanostructures: the case of silver. Chem Eur J. 2005;11:454–463. doi: 10.1002/chem.200400927. [DOI] [PubMed] [Google Scholar]
- 29.Evanoff, Chumanov G. Size-controlled synthesis of nanoparticles. 2. measurement of extinction, scattering, and absorption cross sections. J Phys Chem B. 2004;108:13957–13962. doi: 10.1021/jp0475640. [DOI] [PubMed] [Google Scholar]
- 30.Merga G, Wilson R, Lynn G, Milosavljevic B, Meisel D. Redox Catalysis on “naked” silver nanoparticles. J Phys Chem C. 2007;111:12220–12206. [Google Scholar]
- 31.Oliveira M, Ugarte D, Zanchet D, Zarbin A. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J Colloid Interface Sci. 2005;292:429–435. doi: 10.1016/j.jcis.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 32.Brust M, Kiely C. Some recent advances in nanostructure preparation from gold and silver particles: a short topical review. Colloids Surf A: Phys Eng Aspects. 2002;202:175–186. [Google Scholar]
- 33.Kim D, Jeong S, Moon J. Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology. 2006;17:4019. doi: 10.1088/0957-4484/17/16/004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Peng H, Huang W, Zhou Y, Yan D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J Colloid Interface Sci. 2008;325:371–376. doi: 10.1016/j.jcis.2008.05.063. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Feng Y-G, Wang X, Li T-C, Zhang J-Y, Qian DJ. Silver nanoparticles capped by oleylamine: formation, growth, and self-organization. Langmuir. 2007;23:5296–5304. doi: 10.1021/la700553d. [DOI] [PubMed] [Google Scholar]
- 36.Troupis A, Hiskia A, Papaconstantinou E. Synthesis of metal nanoparticles by using polyoxometalates as photocatalysts and stabilizers. Angew Chem Int Ed. 2002;41:1911–1914. [PubMed] [Google Scholar]
- 37.Zhang G, Keita B, Dolbecq A, Mialane P, Secheresse F, Miserque F, et al. Green chemistry-type one-step synthesis of silver nanostructures based on MoV–MoVI mixed-valence polyoxometalates. Chem Mater. 2007;19:5821–5823. [Google Scholar]
- 38.Krutyakov Y, Olenin A, Kudrinskii A, Dzhurik P, Lisichkin G. Aggregative stability and polydispersity of silver nanoparticles prepared using two-phase aqueous organic systems. Nanotechnol Russia. 2008;3:303–310. [Google Scholar]
- 39.Zhang W, Qiao X, Chen J. Synthesis of nanosilver colloidal particles in water/oil microemulsion. Colloids Surf A: Physicochem Eng Aspects. 2007;299:22–28. [Google Scholar]
- 40.Cozzoli P, Comparelli R, Fanizza E, Curri M, Agostiano A, Laub D. Photocatalytic synthesis of silver nanoparticles stabilized by TiO2 nanorods: A semiconductor/metal nanocomposite in homogeneous nonpolar solution. J Am Chem Soc. 2004;126:3868–3879. doi: 10.1021/ja0395846. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Yang Y. Preparation of silver nanoparticles in inorganic clay suspensions. Compos Sci Technol. 2008;68:2948–2953. [Google Scholar]
- 42.Zhou Y, Yu SH, Wang CY, Li XG, Zhu YR, Chen ZY. A Novel ultraviolet irradiation photoreduction technique for the preparation of single- crystal Ag nanorods and Ag dendrites. Advanced Materials. 1999;11:850–852. [Google Scholar]
- 43.Socol Y, Abramson O, Gedanken A, Meshorer Y, Berenstein L, Zaban A. Suspensive electrode for-mation in pulsed sonoelectrochemical synthesis of silver nanoparticles. langmuir. 2002;18:4736–4740. [Google Scholar]
- 44.Shchukin DG, Radtchenko IL, Sukhorukov G. Photoinduced reduction of silver inside microscale polyelectrolyte capsules. Chem Phys Chem. 2003;4:1101–1103. doi: 10.1002/cphc.200300740. [DOI] [PubMed] [Google Scholar]
- 45.Jin R, Cao YC, Hao E, Metraux GS, Schatz GC, Mirkin C. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature. 2003;425:487–490. doi: 10.1038/nature02020. [DOI] [PubMed] [Google Scholar]
- 46.Malval J-P, Jin M, Balan L, Schneider R, Versace D-L, Chaumeil H, et al. Photoinduced size-controlled generation of silver nanoparticles coated with carboxylate-derivatized thioxanthones. J Phys Chem C. 2010;114:10396–10402. [Google Scholar]
- 47.Sato-Berrú R, Redón R, Vázquez-Olmos A, Saniger J. Silver nanoparticles synthesized by direct photoreduction of metal salts. Application in surface-enhanced Raman spectroscopy. J Raman Spectrosc. 2009;40:376–380. [Google Scholar]
- 48.Ghosh SK, Kundu S, Mandal M, Nath S, Pal T. Studies on the evolution of silver nanoparticles in micelle by uv-photoactivation. J Nanopart Res. 2003;5:577–587. [Google Scholar]
- 49.Huang L, Zhai ML, Long DW, Peng J, Xu L, Wu GZ, et al. UV-induced synthesis, characterization and formation mechanism of silver nanoparticles in alkalic carboxymethylated chitosan solution. J Nanopart Res. 2008;10:1193–1202. [Google Scholar]
- 50.Johans C, Clohessy J, Fantini S, Kontturi K, Cunnane VJ. Electrosynthesis of polyphenylpyrrole coated silver particles at a liquid-liquid interface. Electrochem Commun. 2002;4:227–230. [Google Scholar]
- 51.Zhang Y, Chen F, Zhuang J, Tang Y, Wang D, Wang Y, et al. Synthesis of silver nanoparticles via electrochemical reduction on compact zeolite film modified electrodes. Chem Commun. 2002;24:2814–2815. doi: 10.1039/b208222e. [DOI] [PubMed] [Google Scholar]
- 52.Ma H, Yin B, Wang S, Jiao Y, Pan W, Huang S, et al. Synthesis of silver and gold nanoparticles by a novel electrochemical method. Chem Phys Chem. 2004;24:68–75. doi: 10.1002/cphc.200300900. [DOI] [PubMed] [Google Scholar]
- 53.Abid JP, Wark AW, Brevet PF, Girault HH. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem Commun. 2002:792–793. doi: 10.1039/b200272h. [DOI] [PubMed] [Google Scholar]
- 54.Eutis S, Krylova G, Eremenko A, Smirnova N, Schill AW, El-Sayed M. Growth and fragmentation of silver nanoparticles in their synthesis with a fs laser and CW light by photo-sensitization with benzophenone. Photochem Photobiol Sci. 2005;4:154–159. doi: 10.1039/b411488d. [DOI] [PubMed] [Google Scholar]
- 55.Sudeep PK, Kamat PV. Photosensitized growth of silver nanoparticles under visible light irradiation: a mechanistic investigation. Chem Mater. 2005;17:5404–5410. [Google Scholar]
- 56.Zhang L, Yu JC, Yip HY, Li Q, Kwong KW, Xu A-W, et al. Ambient light reduction strategy to synthesize silver nanoparticles and silver-coated TiO2 with enhanced photocatalytic and bactericidal activities. Langmuir. 2003;19:10372–10380. [Google Scholar]
- 57.Nadagouda MN, Speth TF, Varma R. Microwave-assisted green synthesis of silver nanostructures. Acc Chem Res. 2011;44:469–478. doi: 10.1021/ar1001457. [DOI] [PubMed] [Google Scholar]
- 58.Polshettiwar V, Nadagouda MN, Varma R. Microwave assisted chemistry: A rapid and sustainable route to synthesis of organics and nanomaterials. Aust J Chem. 2009;62:16–26. [Google Scholar]
- 59.Chen J, Wang K, Xin J, Jin Y. Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater Chem Phys. 2008;108:421–424. [Google Scholar]
- 60.Navaladian S, Viswanathan B, Varadarajan TK, Viswanath RP. Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation. Nanotechnology. 2008;19:045603. doi: 10.1088/0957-4484/19/04/045603. [DOI] [PubMed] [Google Scholar]
- 61.Sreeram KJ, M N, Nair BU. Microwave assisted template synthesis of silver nanoparticles. Bull Mater Sci. 2008;31:937–942. [Google Scholar]
- 62.Komarneni S, Li D, Newalkar B, Katsuki H, Bhalla AS. Microwave-polyol process for pt and ag nanoparticles. Langmuir. 2002;18:5959–5962. [Google Scholar]
- 63.Yin H, Yamamoto T, Wada Y, Yanagida S. Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater Chem Phys. 2004;83:66–70. [Google Scholar]
- 64.Tsuji M, Matsumoto K, Jiang P, Matsuo R, Hikino S, Tang X-L, et al. The role of adsorption species in the formation of Ag nanostructures by a microwave-polyol route. Bull Chem Soc Jpn. 2008;81:393–400. [Google Scholar]
- 65.Hu B, Wang S-B, Wang K, Zhang M, Yu SH. Microwave-assisted rapid facile “green” synthesis of uniform silver nanoparticles: Self-assembly into multilayered films and their optical properties. J Phys Chem C. 2008;112:11169–11174. [Google Scholar]
- 66.Soroushian B, Lampre I, Belloni J, Mostafavi M. Radiolysis of silver ion solutions in ethylene glycol: solvated electron and radical scavenging yields. Radiat Phys Chem. 2005;72:111–118. [Google Scholar]
- 67.Ramnami SP, Biswal J, Sabharwal S. Synthesis of silver nanoparticles supported on silica aerogel using gamma radiolysis. Radiat Phys Chem. 2007;76:1290–1294. [Google Scholar]
- 68.Long D, Wu G, Chen S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat Phys Chem. 2007;76:1126–1131. [Google Scholar]
- 69.Cheng P, Song L, Liu Y, Fang YE. Synthesis of silver nanoparticles by γ-ray irradiation in acetic water solution containing chitosan. Radiat Phys Chem. 2007;76:1165–1168. [Google Scholar]
- 70.Hornebecq V, Antonietti M, Cardinal T, Treguer-Delapierre M. Stable silver nanoparticles immobilized in mesoporous silica. Chemistry of Materials. 2003;15:1993–1999. [Google Scholar]
- 71.Bogle KA, Dhole SD, Bhoraskar VN. Silver nanoparticles: synthesis and size control by electron irradiation. Nanotechnology. 2006;17:3204. doi: 10.1088/0957-4484/18/13/135602. [DOI] [PubMed] [Google Scholar]
- 72.Pillai ZS, Kamat PV. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J Phys Chem B. 2004;108:945–951. [Google Scholar]
- 73.Jacob JA, Mahal HS, Biswas N, Mukerjee T, Kappor S. Role of phenol derivatives in the formation of silver nanoparticles. Langmuir. 2008;24:528–533. doi: 10.1021/la702073r. [DOI] [PubMed] [Google Scholar]
- 74.Athawale AA, Desai PA. Silver doped lanthanum chromites by microwave combustion method. Ceram Int. 2011;37:3037–3043. [Google Scholar]
- 75.Hsieh CT, Pan C, Chen WY. Synthesis of silver nanoparticles on carbon papers for electrochemical catalysts. J Power Sources. 2011;196:6055–6061. [Google Scholar]
- 76.Ipekoglu M, Altintas S. Silver substituted nanosized calcium deficient hydroxyapatite. Mater Technol. 2010;25:295–301. [Google Scholar]
- 77.Kate K, Singh K, Khanna PK. Microwave formation of Polypyrrole/Ag nano-composite based on interfacial polymerization by use of AgNO3. Synth React Inorg Met–Org Chem. 2011;41:199–202. [Google Scholar]
- 78.Kate K, Damkale SR, Khanna PK, Jain G. Nano-silver mediated polymerization of pyrrole: Synthesis and gas sensing properties of polypyrrole (PPy)/Ag nano-composite. J Nanosci Nanotechnol. 2011;11:7863–7869. doi: 10.1166/jnn.2011.4708. [DOI] [PubMed] [Google Scholar]
- 79.Katouki H, Komarneni S. Nano- and micro-meter sized silver metal powders by microwave-polyol process. J Jpn Soc Powder Powder Metall. 2003;50:745–750. [Google Scholar]
- 80.Luo S, Yang S, Sun C, Gu JD. Improved debro-mination of polybrominated diphenyl ethers by bimetallic iron-silver nanoparticles coupled with microwave energy. Sci Total Environ. 2012;429:300–308. doi: 10.1016/j.scitotenv.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 81.Ma SN, Ou ZW, Sun XF, Bai M, Liu ZH. In-situ synthesis of ultra-dispersion-stability nano-Ag in epoxy resin and toughening modification to resin. J Funct Mater. 2009;40:1029–1032. [Google Scholar]
- 82.Mahltig B, Gutmann E, Reibold M, Meyer DC, Bottcher H. Synthesis of Ag and Ag/SiO2 sols by solvothermal method and their bactericidal activity. J Sol-Gel Sci Technol. 2009;51:204–214. [Google Scholar]
- 83.Meng XK, Tang SC, Vongehr S. A review on diverse silver nanostructures. J Mater Sci Technol. 2010;26:487–522. [Google Scholar]
- 84.Si MZ, Fang Y, Dong G. Research on nano-silver colloids prepared by microwave synthesis method and its SERS activity. Acta Photon Sin. 2008;37:1034–1036. [Google Scholar]
- 85.Soto G, Tiznado H, Contreras O, Peérez-Tijerina E, Cruz-Reyes J, Del Valle M, et al. A. Preparation of a Ag/SiO2 nanocomposite using a fluidized bed microwave plasma reactor, and its hydrodesulphurization and Escherichia coli bactericidal activities. Powder Technol. 2011;213:55–62. [Google Scholar]
- 86.Yao BH, Xu GC, Zhang HY, Han X. Synthesis of nanosilver with polyvinylpyrrolidone (PVP) by microwave method. Chin J Inorg Chem. 2010;26:1629–1632. [Google Scholar]
- 87.Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- 88.Raveendran P, Fu J, Wallen SL. A simple and “green” method for the synthesis of Au, Ag, and Au-Ag alloy nanoparticles. Green Chem. 2005;8:34–38. [Google Scholar]
- 89.Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr Res. 2004;339:2627–2631. doi: 10.1016/j.carres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan P-V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res. 2006;341:2012–2018. doi: 10.1016/j.carres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 91.Tai C, Wang Y-H, Liu HS. A green process for preparing silver nanoparticles using spinning disk reactor. AIChE J. 2008;54:445–452. [Google Scholar]
- 92.Bo L, Yang W, Chen M, Gao J, Q X. A simple and ‘green’ synthesis of polymer-based silver colloids and their antibacterial properties. Chem Biodivers. 2009;6:111–116. doi: 10.1002/cbdv.200800112. [DOI] [PubMed] [Google Scholar]
- 93.Gao X, Wei L, H Y, Xu B. Green synthesis and characteristic of core-shell structure silver/starch nanoparticles. Mater Lett. 2011;65:2963–2965. [Google Scholar]
- 94.Venkatpurwar V, Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Materials Letters. 2011;65:999–1002. [Google Scholar]
- 95.García-Serrano J, Herrera AM, Ocampo-Fernaández M. Synthesis of Ag particles using an ion-exchange polymer with phosphonic acid groups. Mater Sci Forum. 2011;691:113–118. [Google Scholar]
- 96.Hebeish A, El-Shafei A, Sharaf S, Zaghloul S. Novel precursors for green synthesis and application of silver nanoparticles in the realm of cotton finishing. Carbohyd Polym. 2011;84:605–613. [Google Scholar]
- 97.Maity D, Kanti Bain M, Bhowmick B, Sarkar J, Saha S, Acharya K, et al. In situ synthesis, characterization, and antimicrobial activity of silver nanoparticles using water soluble polymer. J Appl Polym Sci. 2011;122:2189–2196. [Google Scholar]
- 98.Medina-Ramirez I, Bashir S, Luo Z, Liu JL. Green synthesis and characterization of polymer-stabilized silver nanoparticles. Colloids Surf, B. 2009;73:185–191. doi: 10.1016/j.colsurfb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 99.Sarkar R, Kumbhakar P, Mitra AK, Ganeev RA. Synthesis and photoluminescence properties of silver nanowires. Curr Appl Phys. 2010;10:853–857. [Google Scholar]
- 100.Shervani Z, Ikushima Y, Sato M, Kawanami H, Hakuta Y, Yokoyama T, et al. Morphology and size-controlled synthesis of silver nanoparticles in aqueous surfactant polymer solutions. Colloid Polym Sci. 2008;286:403–410. [Google Scholar]
- 101.Yin Y, Li Z-Y, Zhong Z, Gates B, Venkateswaran S. Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J Mater Chem. 2002;12:522–527. [Google Scholar]
- 102.Kvítek L, Prucek R, Panáček A, Novotný R, Hrbác J, Zbořil R. The influence of complexing agent concentration on particle size in the process of SERS active silver colloid synthesis. J Mater Chem. 2005;15:1099–1105. [Google Scholar]
- 103.Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 104.Kvitek L, Panacek A, Soukupova J, Kolar M, Vecerova R, Prucek R, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs) J Phys Chem C. 2008;112:5825–5834. [Google Scholar]
- 105.Soukupova J, Kvitek L, Panacek A, Nevecna T, Zboril R. Comprehensive study on surfactant role on silver nanoparticles (NPs) prepared via modified Tollens process. Mater Chem Phys. 2008;111:77–81. [Google Scholar]
- 106.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–517. [Google Scholar]
- 107.Debaditya B, Rajinder K. Nanotechnology and potential of microorganisms. Crit Rev Biotech. 2005;25:199–204. doi: 10.1080/07388550500361994. [DOI] [PubMed] [Google Scholar]
- 108.Kalishwaralal K, Deepak V, Ramkumarpandian S, Nellaiah H, Sangiliyandi G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett. 2008;62:4411–4413. [Google Scholar]
- 109.Kalishwaralal K, Deepak V, Ramkumarpandian S, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids and Surfaces B: Biointerfaces. 2008;65:150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 110.Saifuddin N, Wong CW, Nur Yasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-Journal of Chemistry. 2009;6:61–70. [Google Scholar]
- 111.Klaus T, Joerger R, Olsson E, Granqvist CGr. Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci USA. 1999;96:13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slawson RM, Van DM, Lee H, Trevor J. Germanium and silver resistance, accumulation and toxicity in microorganisms. Plasmid. 1992;27:73–79. doi: 10.1016/0147-619x(92)90008-x. [DOI] [PubMed] [Google Scholar]
- 113.Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach Process Biochemistry. 2007;42:919–923. [Google Scholar]
- 114.Mokhtari N, Daneshpajouh S, Seyedbagheri S, Atashdehghan R, Abdi K, Sarkar S, et al. Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater Res Bull. 2009;44:1415–1421. [Google Scholar]
- 115.Mouxing FU, Qingbiao LI, Daohua SUN, Yinghua LU, Ning HE, Xu DENG, et al. Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chinese J Chem Eng. 2006;14:114–117. [Google Scholar]
- 116.Nair B, Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Crystal Growth & Design. 2002;2:293–298. [Google Scholar]
- 117.Korbekandi H, Iravani S, Abbasi S. Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. J ChemTechnol Biotechnol. 2012;87:932–937. [Google Scholar]
- 118.Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum colloids and surfaces B: Biointerfaces. 2003;28:313–318. [Google Scholar]
- 119.Macdonald IDG, Smith W. Orientation of Cytochrome c adsorbed on a citrate-reduced silver colloid surface. Langmuir. 1996;12:706–713. [Google Scholar]
- 120.Keating CD, Kovaleski KK, Natan M. Heightened electromagnetic fields between metal nanoparticles: surface enhanced Raman scattering from metal-Cytochrome c-metal sandwiches. J Phys Chem B. 1998;102:9414–9425. [Google Scholar]
- 121.Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V, et al. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology. 2003;14:824–828. [Google Scholar]
- 122.Kumar SA, Majid Kazemian A, Gosavi SW, Sulabha KK, Renu P, Absar A, et al. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnology Letters. 2007;29:439–445. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- 123.Korbekandi H, Ashari Z, Iravani S, Abbasi S. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran J Pharm Res. 2013;12:289–298. [PMC free article] [PubMed] [Google Scholar]
- 124.Ingle A, Gade A, Pierrat S, Sönnichsen C, Rai M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci. 2008;4:141–144. [Google Scholar]
- 125.Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya R. Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium Colloids and Surfaces B: Biointerfaces. 2006;53:55–59. doi: 10.1016/j.colsurfb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 126.Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya R. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus Materials Letters. 2007;61:1413–1418. [Google Scholar]
- 127.Bhainsa KC, D’Souza S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids and Surfaces B: Biointerfaces. 2006;47:160–164. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 128.Balaji DS, Basavaraja S, Deshpande R, Bedre Mahesh D, Prabhakar BK, Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids and Surfaces B: Biointerfaces. 2009;68:88–92. doi: 10.1016/j.colsurfb.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 129.Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids and Surfaces B: Biointerfaces. 2009;71:133–137. doi: 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 130.Sadowski Z, Maliszewska IH, Grochowalska B, Polowczyk I, Kozlecki T. Synthesis of silver nanoparticles using microorganisms. Materials Science-Poland. 2008;26:419–424. [Google Scholar]
- 131.Maliszewska I, Szewczyk K, Waszak K. Biological synthesis of silver nanoparticles. J Phys Conf Ser. 2009;146:1–6. [Google Scholar]
- 132.Sanghi R, Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresource Technology. 2009;100:501–504. doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 133.Chakraborty N, Banerjee A, Lahiri S, Panda A, Ghosh AN, Pal R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation -a novel phenomenon. J Appl Phycol. 2009;21:145–152. [Google Scholar]
- 134.Lengke M, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)-thiosulfate and gold (III)-chloride complexes. Langmuir. 2006;22:2780–2787. doi: 10.1021/la052652c. [DOI] [PubMed] [Google Scholar]
- 135.Lengke M, Ravel B, Fleet ME, Wanger G, Gordon RA, Southam G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III)-chloride complex. Environ Sci Technol. 2006;40:6304–6309. doi: 10.1021/es061040r. [DOI] [PubMed] [Google Scholar]
- 136.Niu H, Volesky B. Gold-cyanide biosorption with L-cysteine. J Chem Technol Biotechnol. 2000;75:436–442. [Google Scholar]
- 137.Govindaraju K, Basha SK, Kumar VG, Singaravelu G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci. 2008;43:5115–5122. [Google Scholar]
- 138.Vilchis-Nestor AR, Sánchez-Mendieta V, Camacho-López MA, Gómez-Espinosa RM, Camacho-López MA, Arenas-Alatorre J. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Materials Letters. 2008;62:3103–3105. [Google Scholar]
- 139.Begum NA, Mondal S, Basu S, Laskar RA, Mandal D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids and Surfaces B: Biointerfaces. 2009;71:113–118. doi: 10.1016/j.colsurfb.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 140.Kesharwani J, Yoon KY, Hwang J, Rai M. Phytofabrication of silver nanoparticles by leaf extract of datura metel: hypothetical mechanism involvedin synthesis. J Bionanosci. 2009;3:1–6. [Google Scholar]
- 141.Song JY, Kim B. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32:79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- 142.Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C, et al. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2010 doi: 10.1007/s00436-010-2115-4. DOI: 10.1007/s00436-010-2115-2124. [DOI] [PubMed] [Google Scholar]
- 143.Li S, Shen Y, Xie A, Yu X, Qiu L, Zhang L, et al. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9:852–858. [Google Scholar]
- 144.Elumalai EK, Prasad TNVKV, Hemachandran J, Viviyan Therasa S, Thirumalai T, David E. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci & Res. 2010;2:549–554. [Google Scholar]
- 145.Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids and Surfaces B: Biointerfaces. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 146.Prasad TNVKV, Elumalai E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed. 2011:439–442. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ravindra S, Murali Mohan Y, Narayana Reddy N, Raju KM. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloids and Surfaces A: Physicochem Eng Aspects. 2010;367:31–40. [Google Scholar]
- 148.Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2011;15:113–120. [Google Scholar]
- 149.Singhal G, Bhavesh R, Kasariya K, Sharma AR, Singh RP. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13:2981–2988. [Google Scholar]
- 150.Huang J, Zhan G, Zheng B, Sun D, Lu F, Lin Y, et al. Biogenic Silver nanoparticles by cacumen platycladi extract: synthesis, formation mechanism, and antibacterial activity. Ind Eng Chem Res. 2011;50:9095–9106. [Google Scholar]
- 151.Sathishkumar M, Sneha K, Won SW, Cho C-W, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids and Surfaces B: Biointerfaces. 2009;73:332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 152.Iravani S, Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int. 2013;2013 doi: 10.1155/2013/639725. http://dx.doi.org/10.1155/2013/639725 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu Q, Cao H, Luan Q, Zhang J, Wang Z, Warner J-H, et al. Biomolecule-assisted synthesis of water-soluble silver nanoparticles and their biomedical applications. Inorg Chem. 2008;47:5882–5888. doi: 10.1021/ic8002228. [DOI] [PubMed] [Google Scholar]
- 154.Si S, Mandal TK. Tryptophan-based peptides to synthesize gold and silver nanoparticles: a mechanistic and kinetic study. Chem A Eur J. 2007;13:3160–3168. doi: 10.1002/chem.200601492. [DOI] [PubMed] [Google Scholar]
- 155.Kasture M, Singh S, Patel P, Joy PA, Prabhune AA, Ramana CV, et al. Multiutility sophorolipids as nanoparticle capping agents: synthesis of stable and water dispersible co nanoparticles. Langmuir. 2007;23:11409–11412. doi: 10.1021/la702931j. [DOI] [PubMed] [Google Scholar]
- 156.Mantion A, Guex AG, Foelske A, Mirolo L, Fromn KM, Painsi M. Silver nanoparticle engineering via oligovaline organogels. Soft Matter. 2008;4:606–617. doi: 10.1039/b712826f. [DOI] [PubMed] [Google Scholar]
- 157.Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids and Surfaces B: Biointerfaces. 2009;68:55–60. doi: 10.1016/j.colsurfb.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 158.Safaepour M, Shahverdi AR, Shahverdi HR, Khorramizadeh MR, Gohari AR. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-Wehi 164. Avicenna J Med Biotech. 2009;1:111–115. [PMC free article] [PubMed] [Google Scholar]
- 159.Kora AJ, Sashidhar RB, Arunachalam J. Gum kondagogu (Cochlospermum gossypium): A template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydrate Polymers. 2010;82:670–679. [Google Scholar]


