Abstract
Previously, we reported that the kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca showed potent anti-HSV activity. In the present study the anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside are investigated at different concentrations (100, 50, 25 and 10 μg/ml) using HIV-1 p24 Antigen kit. Real-time Polymerase chain reaction (RT-PCR) assay was also used for quantification of full range of virus load observed in treated and untreated cells. According to the results of RT- PCR, tested compounds at a concentration of 100 μg/ml exerted potent inhibitory effect. Time of drug addition experiments demonstrated that these compounds exerted their inhibitory effects on the early stage of HIV infection. The results also showed potent anti-HIV-1 reverse transcriptase activity. Antiviral activity of kaempferol-7-O-glucoside was more pronounced than that of kaempferol. These findings demonstrate that kaempferol-7-O-glucoside could be considered as a new potential drug candidate for the treatment of HIV infection which requires further assessments.
Keywords: HIV-1, Antiviral activity, Flavonol, Real Time PCR assay
INTRODUCTION
Human immunodeficiency virus I (HIV-1) is one of the most important pathogens which causes the majority of HIV infections worldwide (1). Therapeutic success of present anti HIV drugs has been restricted by the development of drug resistant viruses (2). Some studies have accomplished to isolate the active novel compounds from plants useful for preventing transmission of HIV and treatment of patients (3). A variety of secondary metabolites obtained from these plants are alkaloids, polyphenolics, flavonoids, sulphated polysaccharides, coumarines, phenolics and triterpenes (4,5). Flavonoid compounds have been reported as an important natural source of anti-HIV agents due to their significant anti-HIV-1 activity and low toxicity. These compounds inhibit reverse transcription process, virus entry, the integrase and protease (4,6). Therefore, we were willing to evaluate Iranian plant species with respect to their anti-HIV-1 activity and screen their active constituents. Securigera securidaca (Fabaceae), also called goat pea, is an annual herb used against a variety of diseases such as malaria, gastric influx, epilepsy, hypertension and hyperlipidemia in folk eastern medicine (7,8). Some compounds such as cardenolides, steroidal and pentacyclic have been detected in extract of S. securidaca seeds (9). Kaempferol and kaempferol-7-O-glucoside have also been isolated from S. securidaca with potent anti-HSV1 activity (10). Therefore, in the present study, anti-HIV-1 activity of these two compounds from Securigera securidaca was investigated for the first time.
MATERIALS AND METHODS
Extraction and isolation of compounds
The seeds of S. securidaca were collected from University of Isfahan plant herbarium, Iran (No. 13656). The plant seeds were carefully dried in a well-ventilated dark room and powdered. The S. securidaca seeds powder was extracted trice with methanol (98%) at 40 °C. Seeds (1000 g) was done thrice. Then, the resulting liquid was collected, filtered and its volume reduced through evaporation under vacuum by a rotary evaporator (Stroglass, Italy) at 45 °C and dried using a freeze dryer (Zirbus, Germany). Silica-gel column fractionation and high-performance liquid chromatography (HPLC) was carried out according to our previous work (10). Briefly, methanol extract of S. securidaca, eluted with n-hexane: acetone (8:2 to 2:8, v/v) and then with 100% methanol. Kaempferol and kaempferol-7-O-glucoside were isolated and identified using nuclear magnetic resonance (NMR) analysis.
Spectroscopic analysis
High resolution nuclear magnetic resonance (1H NMR) screening was used to detect trace compounds in each fraction. 1H NMR spectra were recorded on Bruker 400 MHz spectrometer using dimethyl sulfoxide (DMSO) as residual solvent with chemical shifts expressed in parts per million (ppm). Fourier Transform Infrared Spectroscopy (FTIR) analysis was recorded on Jasco FT/IR- 6300 (400-4000 cm-1), Japan, (νmax, KBr).
Cells and viruses
Peripheral blood mononuclear cells (PBMCs) were obtained from HIV-1 seronegative donors by Lymphodex density centrifugation. The cells were grown in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% (v/v) Fetal Calf Serum (FCS; Gibco-BRL, Grand Island, NY, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine and 1 mM Na-pyruvate. All materials were purchased from (Gibco, Germany). A virus stock of drug resistant mutation of HIV-1 subtype A was obtained from Azahra Hospital, Isfahan, Iran. Aliquots of 6 × 105 cells were infected with a low multiplicity of virus and incubated for 4 days. Then, supernatant of viral stock was harvested every day over a period of 4 days post-infection. Virus titers were determined by the HIV-1 p24 antigen kit (BioMerieux, France). The viruses were stored at -70 °C until use.
Peripheral blood mononuclear cells cytotoxicity assay
The cytotoxicity of PBMC stimulated by phytohemagglutinin(PHA). Phytohem-agglutinin was determined in the presence of methanol extract, kaempferol and kaempferol-7-O-glucoside isolated from S. securidaca. The extracts were initially dissolved in DMSO and then diluted with RPMI medium to prepare working concentrations of 2500, 2000, 1500, 1000, 750, 500, 250, 120, 60, 30, 15 μg/ml. Cell viability was examined according to previous report with some modification (11). 6 × 105 PBMCs were cultured in a 96-well plate and incubated for 72 h at 37 °C. 20 μL of 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution (5 mg/ml) was added to each well, and the plate was incubated for 4 h. Finally, 50 μl of PrOH/HCl/TX (0.04M HCl in 2-propanol plus 10% Triton X-100) was added to solubilize the formed formazan crystals. The amount of formazan crystal was determined by measuring the absorbance at A540 nm using a microplate spectrophotometer (Awareness Technology Inc., stat fax 2100, USA).
Antiviral activity
Anti-HIV activity of kaempferol and kaempferol-7-O-glycoside was investigated via the HIV-1 p24 Antigen kit. The PBMCs were grown in microtitre tissue culture plates. The cells were infected with 0.3 Multiplicity of Infection (MOI) HIV-1 and supplemented with different concentrations (100, 50, 20 and 10 μg/ml) of extracts and incubated at 37 °C for 12 h. Infected cells were washed with phosphate buffered saline (PBS) and overlaid with medium. 0.1% DMSO and different concentrations of zidovudine (AZT) (10, 5, 2.5 and 1 μg/ml) were also used as negative and positive controls, respectively. After 3-4 days of incubation, the overlay medium was used to detect and quantify HIV-1 Gag p24 antigen by kit. The supernatant was transferred to a coated 96-well plate for the p24 assay. The protocol was followed as described by the manufacturer, with absorbance measured at 450 nm.
Time of addition study
The time-of-addition effect of kaempferol and kaempferol-7-O-glucoside was examined as described by Behbahani and coworkers (10). PBMCs, 2 × 105 per well, were seeded into 24-well culture plates (Nunc; Nalge Nunc International, Rochester, NY, USA) and incubated for 24 h. Then, the cultures were treated with 50 μg/ml of kaempherol and 32 μg/ml kaempherol-7-O-glucoside both concurrent with HIV-1 (0 h) or at intervals of 2, 4 and 6 h pre-infection and also post-infection. After incubation at 37 °C for 72 h, the reduction in the virus titer was obtained by real-time polymerase chain reaction (RT-PCR) assay and infected cultures containing the extracts were compared with control cultures for each treatment.
Quantitative real-time polymerase chain reaction assay for HIV-1
For RT-PCR, RNA was purified from each specimen by high pure viral nucleic acid kit (Roche Diagnostics, Meylan, France) according to the standard protocol. HIV-1 RNA was detected and quantified by RT-PCR. As previously described, forward primer NEC152 (5′-CCTCAATAAAGCTTGCCTTGA-3′), the reverse primer NEC131 (5′-GGCGCCACTGCTAGAGATTTT-3′), and the dually labeled NEC-LTR probe (5′-6-carboxyfluorescein-AGTAGTGTGTGCCCGTCTGTTRTKTGACT-6-carboxytetramethylrhodamine-3′) in the long terminal repeat gene were used (12). Primers and probes were synthesized by Metabion Co. (Germany). The master mix contained × RNA master hybridization buffer, including the Taq polymerase and deoxynucleoside triphosphate mix (containing dUTP instead of dTTP), 2.5 mM Mn(OAc)2 solution and 0.3 μM concentrations of each primer and probe. Cycling conditions were as follows: initial reverse transcription at 61 °C for 30 min, denaturation at 95 °C for 30 s, and 45 cycles of denaturation at 95 °C for 1 s, annealing at 55 °C for 15 s and elongation at 65 °C for 1 min with a ramp of 5 °C/s (with fluorescence acquisition at the end of each elongation stage). The positive control was consisted of culture supernatant of the HIV-1. As a control for cross-contamination, a sample of distilled water was subjected to the RNA extraction procedure, and the resulting extract was amplified in duplicate (13).
Reverse transcriptase assay
The special effects of kaempherol and kaempherol-7-O-glucoside at IC50 concentration on reverse transcriptase (RT) activity were estimated with recombinant HIV-enzyme, using a non-radioactive HIV-RT colorimetric ELISA kit from Roche, Germany. The protocol summarized in the kit was pursued using 2 ng of enzyme in each well and the reaction was incubated for 2 h at 37 °C. Bovine serum albumin was also added to assay buffers to avoid the tannins interference in a concentration of 0.2% (w/v) to adsorb probable tannins from crude extract (14).
Statistical analysis
Data from three independent experiments were presented as mean ± SD. The IC50 and CC50 values were calculated by Microsoft Excel 2000. A selectivity index (SI) was calculated for each viral strain by the ratio of CC50 to IC50 value. Student's unpaired t-test was used to assess significance of the test sample and solvent control. p<0.05 was considered statistically significant.
RESULTS
Spectroscopic analysis
The most active fraction obtained from the first column was fraction 6, which were further analyzed by 1H-NMR. Then, it was fractionated by use of normal phase chromatography to get two active compounds 6a and 6b and analyzed using 1H NMR and FT-IR.
Compound 6a: 1HNMR (DMSO, 400 MHz): δ 11.89 (s, 1H, 5-OH), 9.82 (s, 1H, 3-OH), 7.85 (d, J = 8.8Hz, 2H), 7.20 (d, J = 8.8Hz, 2H), 6.80 (d, J = 1.8Hz, 1H), 6.72 (d, J = 1.8Hz, 1H), 5.92 (s, 2H, 7, 4’-OH).
FT-IR (νmax, KBr): 3335, 2928, 1668, 1584, 1495, 1392, 1075.
Compound 6b : 1HNMR (DMSO, 400 MHz): δ 11.78 (s, 1H, 5-OH), 9.80 (s, 1H, 3-OH), 7.80 (d, J = 8.8Hz, 2H), 7.20 (d, J = 8.8Hz, 2H), 6.78 (d, J = 1.8Hz, 1H), 6.70 (d, J = 1.8Hz, 1H), 5.88 (s, 1H, 4’-OH); Glucose moiety: 4.87 (d, J = 7.3Hz, 1H), 4.79 (dd, J1 = 9.2, J2 =8.3Hz, 1H), 4.38 (t, J = 7.5Hz, 1H), 3.90 (m, 1H), 3.76 (dd J1 = 9.7Hz, J2 =2.1Hz, 1H,), 3.72 (d, J = 11.8Hz, 2H), 3.60 (s, 4H). FT-IR (νmax, KBr): 3393, 3298, 2926, 2895, 1704, 1660, 1605, 1513, 1460, 1420, 1245, 1139, 1072, 998.
Spectroscopic analyses suggested that compounds 6a and 6b which showed anti-HIV-1 activity were identified as kaempferol and kaempferol-7-O-glucoside respectively.
Anti-HIV-1 activity
Antiviral activity of kaempferol and kaempferol-7-O-glucoside as isolated compounds from S. securidaca was evaluated by the HIV-1 p24 Antigen kit. Results showed that 100 μg/ml kaempferol and kaempferol-7-O-glucoside inhibited HIV-1 multiplication with an inhibition rate of 82 ± 3.1% and 95 ± 1.2%, respectively. The antiviral activity of these two compounds was further examined by different concentrations 100, 50, 25 μg/ml of kaempferol, kaempferol-7-O-glucoside, and AZT (Fig. 1). IC50 values of kaempferol, kaempferol-7-O-glucoside and AZT were 50, 32 and 1 μg/ml, respectively. The antiviral activity of kaempferol was weaker than kaempferol-7-O-glucoside. Also the calculated SI for kaempferol and kaempferol-7-O- glucoside were 6.4 and 78, respectively.
Fig. 1.
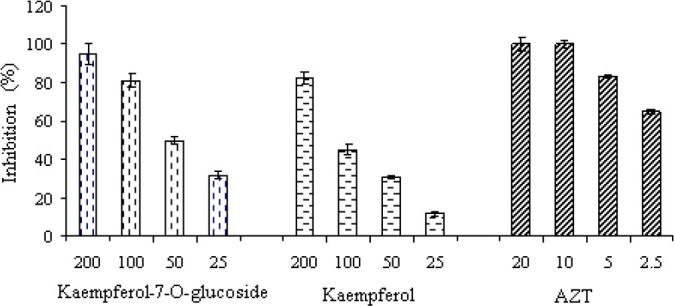
Effect of kaempferol and kaempferol-7-O-glucoside isolated from S. securidaca and zidovudine on HIV-1 replication in peripheral blood mononuclear cells. The 50% inhibitory concentration (IC50) of each extract was calculated using regression line. Each bar represents the mean ± SD of three independent experiments.
Peripheral blood mononuclear cells cytotoxicity assay
Kaempferol-7-O-glucoside significantly induced PBMCs proliferation in a dose dependent manner up to 1000 μg/ml. The result showed that 1000 μg/ml of kaempferol-7-O-glucoside can increase the number of cells more than 2.5 folds and CC50 of this compound was obtained at 2500 μg/ml (Fig. 2) whereas kaempferol exhibited strong cytotoxic effect on PBMCs with CC50 of 320 μg/ml.
Fig. 2.
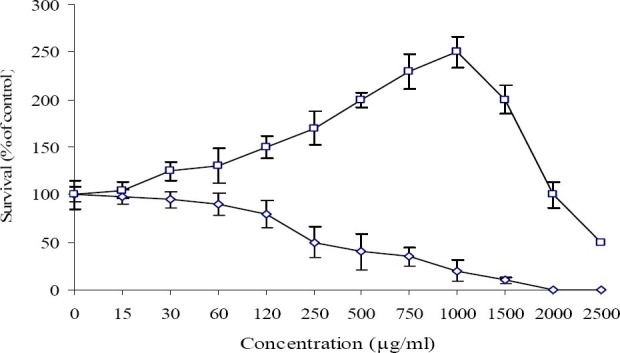
Effect of Kaempferol (◊) and Kaempferol-7-O-glucoside (□) on proliferation of lymphocyte
Mechanism of action on HIV replication
A time-of-addition experiment was performed by measuring the viral RNA yields in infected culture supernatants, by means of a RT-PCR assay. The plant extracts were added before and after virus absorption presented in the culture medium until the end of experiment. The curves generated during PCR reactions showed a prominent drop in HIV-1 DNA amounts in treated cultures compared to those of virus control (Fig. 3). The calculated viral DNA concentrations in cultures treated with 32 μg/ml of the kaempferol-7-O-glucoside before (-4 and −2 h), during (0 h), and after (2 and 4 h) period of HIV-1 infection and in untreated virus control were 214, 218, 784, 5, 765, 8, 373 and 11, 826 copies/ml, respectively.
Fig. 3.
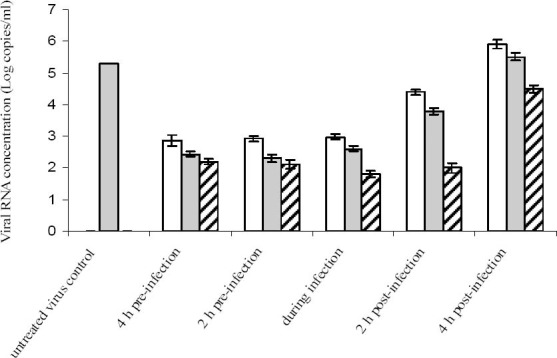
Time of addition effect of kaempferol (white bar) and kaempferol-7-O-glucoside (gray bar) obtained from S. securidaca on HIV replication in Vero cells. The extracts was added either before (−2 and −4), during (0) and after (2 and 4) virus infection. The extracts added before virus infection was rinsed off prior to the virus exposure. Each value is the result of mean ± SD of three independent experiments. Zidovudine has been used as positive control (hatched bar).
The strongest HIV proliferation inhibition was achieved when the extract was added before and during the initial stages of infection. Similar result was also accomplished when 50 μg/ml of the kaempferol was also added before and during the initial stages of infection The result showed that viral DNA during and 2 h before infection was 10 to 50 folds lower than the viral DNA present in the untreated virus control. No viral DNA was detected in non-infected cells.
Reverse transcriptase assay
The results indicated that kaempferol and kaempferol-7-O-glucoside inhibit reverse transcriptase activity. In the present study, anti-HIV1 activity of kaempferol-7-O-glucoside was significantly higher than kaempferol (Fig. 4). Kaempferol-7-O-glucoside and AZT inhibit reverse transcriptase activity rather than 60 %.
Fig. 4.

HIV-1 reverse transcriptase inhibition by kaempferol and kaempferol-7-O-glucoside isolated from S. securigera The data represent the mean HIV-1 reverse transcriptase inhibition of extracts. The reverse transcriptase inhibitions of extracts are the mean of three separate experiments.
DISCUSSION
Medicinal plants are used for the treatment of a variety of human viral infections. Some of them appear to pose the broad spectrum antiviral activity without extensive toxic effects as ideal candidates in antiviral therapy (15). Kaempferol and kaempferol-7-O-glucoside isolated from medicinal plant S. securidaca have been reported to have potent anti-HSV-1 activity (10). These compounds inhibited HIV-1 replication when added concurrently and before infection. According to literature kaempferol and 3-O-glycoside derivatives are natural flavonols which have been identified in various plants, vegetables, fruits, and beverages(16,17). These flavonols have been reported as antitumor, anti-oxidative, anti-allergic and antivirus activities (18). The anti-HIV1 activity of some flavonols such as quercetin, kaempferol, and luteolin have been reported (19). Some researchers reported that Kaempferol isolated from Rosa damascene and rhizome of Dryopteris crassirhizoma can inhibit the enzyme reverse transcriptase. This activity, along with its ability to inhibit viral proteases and binding of gp 120 to lymphocytes CD4, may account for the anti-HIV activity of this flavonoid (20,21). The anti-HIV and anti-reverse transcriptase activity of derivatives of kaempferol have not been investigated yet. In the present study, anti-HIV1 activity of kaempferol-7-O-glucoside was significantly more than kaempferol. The glycoside derivates are more water soluble than respective aglycons. Therefore they can be actively transported into the tissues, using the glucose-transport system and led to a decrease of the anti-viral activity (22). These observations indicated that kaempferol and kaempferol-7-O-glucoside acts at an early stage of HIV-1 infection and inhibits reverse transcription in target cells. Nevertheless, further studies are needed to verify the mechanism of isolated compounds from S. securidaca extract.
CONCLUSION
Based on the findings of this study, it can be concluded that S. securidaca seeds have an effect on inhibition of HIV-1 activity. The results of detection on the bioactive compounds signified that kaempferol-7-O-glucoside constituted the most active fraction in the crude methanolic extract from S. securidaca displayed an effective inhibitory mechanism on the initial stage of HIV-1 infection.
ACKNOWLEDGMENTS
This work was supported by the grant from Faculty of Biotechnology, University of Isfahan, Iran.
REFERENCES
- 1.Bessong PO, Obi CL. Ethnopharmacology of human immunodeficiency virus in south Africa minireview. African j biotechnol. 2006;5:1693–1699. [Google Scholar]
- 2.Vermani K, Garg S. Herbal medicines for sexually transmitted diseases and AIDS. J Ethnopharm. 2005;80:49–66. doi: 10.1016/s0378-8741(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 3.Rates SM. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 4.Singh IP, Bharate SB, Bhutani KK. Anti-HIV natural products. Curr Sci. 2005;89:269–291. [Google Scholar]
- 5.Wang RR, Gu Q, Yang LM, Chen JJ, Li SY, Zheng YT. Anti-HIV-1 activities of extracts from the medical plant Rhus chinensis. Ethnopharm. 2006;105:269–273. doi: 10.1016/j.jep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 6.De Clerq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Ag. 2009;33:307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Pouramir M, Shahaboddin ME, Moghadamnia AA, Parastouie K. To study the effects of Securigera securidaca (L.) seed against alloxan-induced hyperglycemia. J Medi Plant Res. 2011;5:3188–3191. [Google Scholar]
- 8.Garjani A, Fathiazad F, Zakheri A, Akbari NA, Azarmie Y, Fakhrjoo A, et al. The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol. 2009;126:525–532. doi: 10.1016/j.jep.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Zatula VV, Kovalev IP, Kolesnikov DG. Configuration of securigenin and securigenol. Chem Nat Compd. 1969;5:111–112. [Google Scholar]
- 10.Behbahani M, Shanehsazzadeh M, Shokoohinia Y, Soltani M. Evaluation of anti-herpetic activity of methanol seed extract and fractions of Securigera securidacain vitro. J Antivir Antiretrovir. 2013;5:72–76. [Google Scholar]
- 11.Sadeghi-aliabadi H, Emami A, Saidi M, Sadeghi B, Jafarian A. Evaluation of in vitro cytotoxic effects of Juniperus foetidissima and juniperus sabina extracts against a panel of cancer cells. Iran J Pharm Res. 2009;8:281–286. [Google Scholar]
- 12.Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type1 Infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–2717. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legoff J, Bouhlal H, Grésenguet G, Weiss H, Khonde N, Hocini H, et al. Real-Time PCR quantification of genital shedding of herpes simplex virus (HSV) and human immunodeficiency virus (HIV) in women coinfected with HSV and HIV. J Clin Microbiol. 2006;44:423–432. doi: 10.1128/JCM.44.2.423-432.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harnett SM, Oosthuizen V, Van de Venter M. Anti-HIV activities of organic and aqueous extracts of Sutherlandia frutescens and Lobostemon trigonus. Ethnopharm. 2005;96:113–119. doi: 10.1016/j.jep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Mukhtar M, Arshad M, Ahmad M, Pomerantz RJ, Wigdahl B, Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008;2:111–120. doi: 10.1016/j.virusres.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YJ, Wu TD. Total synthesis of kaempferol and methylated kaempferol derivatives. J Chin Chem Soc. 2001;48:201–206. [Google Scholar]
- 17.Yarmoinsky L, Huleihel M, Zaccai M, Ben-Shabat S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia. 2012;83:362–367. doi: 10.1016/j.fitote.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim TH, Ku SK, Lee IC, Bae JS. Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells. Inflamm Res. 2012;61:217–224. doi: 10.1007/s00011-011-0403-9. [DOI] [PubMed] [Google Scholar]
- 19.Chu SC, Hsieh YS, Lin JY. Inhibitory effects of flavonoids on Moloney murine leukemia virus reverse transcriptase activity. J Nat Prod. 1992;55:179–183. doi: 10.1021/np50080a005. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood N, Piacente S, Pizza C, Burke A, Khan AI, Hay AJ. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem Biophy Res Commun. 1996;229:73–79. doi: 10.1006/bbrc.1996.1759. [DOI] [PubMed] [Google Scholar]
- 21.Min BS, Tomiyama M, Ma CM, Nakamura N, Hattori M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem Pharm Bull. 2001;49:546–550. doi: 10.1248/cpb.49.546. [DOI] [PubMed] [Google Scholar]
- 22.Yarmolinsky L, Huleihel M, Zaccai M, Ben-Shabat S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia. 2012;83:362–367. doi: 10.1016/j.fitote.2011.11.014. [DOI] [PubMed] [Google Scholar]


