Abstract
Background:
Many laboratories currently diagnose Blastocystis spp. infections by looking for the presence of vacuolar forms in faeces and the amoeboid form in diarrheal stools.
Objectives:
To investigate the best direct method in diagnosis of Blastocystis spp. and to study different morphological forms of the parasite.
Materials and Methods:
The study was carried out on one thousand and two hundred diarrheic stool samples. All samples were examined using direct smear, iodine stained smear, formalin-ether concentration techniques, trichrome stained smear and in vitro cultivation using Jones' medium.
Results:
Using direct smear, Blastocystis spp was detected in 42 cases (3.5%) with a sensitivity (28.4%) and specificity (100%). Iodine stained smear detected 72 positive cases (6%) with a sensitivity (48.7%), specificity (100%). Formol ether concentration technique detected 120 positive cases (10%) with a sensitivity (81.1%) and specificity (100%). Trichrome stained smear detected 148 positive cases (12.3%). In vitro cultivation using Joni's medium detected 274 positive cases (22.8%) which was the highest number among all different diagnostic methods with a sensitivity (100%) ,specificity (88%), PPV (54.1%) and NPV (100%). It was found that, 49 blastocystosis cases had mixed infection with other intestinal parasites. Giardia lamblia was the most frequently associated parasite with Blastocystis spp.
Conclusion:
In vitro cultivation is more sensitive in detection of B. hominis than simple smear and concentration technique. Blastocystis spp. vacuolar form was the most common form that was found by all methods used in this study G. lamblia was the most frequent parasite associated with Blastocystis spp .
KEY WORDS: Blastocystis spp., cultivation and morphological forms, diagnosis diarrhea
INTRODUCTION
Blastocystis spp. is an enteric protozoan; it is a common cause of gastrointestinal complaints throughout the world.[1] The recognized forms of Blastocystis spp. are vacuolar, granular, multi vacuolar, amoeboid and cystic forms. As other intestinal parasites, transmission occurs by fecal oral route, although this has not been confirmed experimentally.[2]
Direct microscopic examination of faecal material, with or without addition of Lugol's iodine solution, had been suggested for diagnostic purposes. Permanent smear stained with trichrome had also been recommended for the diagnosis of Blastocystis spp. infection.[3] Techniques for concentration using formalin–ether may be suitable because preservative liquids are used for storage and dilution of the feces.[4] Previous epidemiological study of Blastocystis spp. suggested that Blastocystis spp. rapidly multiplies in culture medium supplemented with serum after 24-48 h of cultivation and considered it the most sensitive method available for diagnosis.[5]
This study aimed to investigate the best direct method in the diagnosis of Blastocystis spp. and to study different morphological forms of the parasite.
MATERIALS AND METHODS
Ethics statement
Before the study began, the study objective was explained to the participants and if they were children objective was explained to their mothers. Oral consent was obtained. The study protocol was reviewed and approved by the ethical review board of the Faculty of Medicine, Benha University.
Study design
Study type
A cross-sectional study.
Study place
This study was conducted in six centers and six villages representing urban and rural areas of the Qualyobia Governorate, Egypt. Samples were examined in Department of Parasitology, Faculty of Medicine, Benha University.
Study group
The study included 1200 diarrheic cases; 694 cases (57.8%) were males, and 506 cases (42.2%) were females. The patients were attending Ministry health hospitals, out-patient clinics or referred to diagnostic laboratories in the study area. They were asked about other symptoms of gastroenteritis (vomiting, nausea, abdominal pain, and flatulence), skin rash, joint pain and Immunosuppression (hepatic illness, renal illness, and steroids intake).
Stool samples were collected and examined all over the period of the study from April 2012 to December 2013. Each sample was collected in a sterile, disposable clean, plastic container labeled with the patient's sex, name, age, residence, and date of collection.
Laboratory investigations in the form of: Parasitological examination of stool:
The following techniques were done
Macroscopic examination
By naked eyes to determine consistency, color, odor and presence of blood or mucous.
Microscopic examination of stools by
Direct wet mount smear,[6] Iodine-stained smear,[7] Formol-éther concentration technique,[8] Modified trichrome stain[9] and Stool culture on Jones' medium.[10]
Inoculation of culture media procedure
1.244 g of Na2 HPO4 was dissolved in 131.25 ml of distilled water.O.397 g of KH2 PO4 was dissolved in 43.75 ml of distilled water.7.087 g of NaCl was dissolved in 787.50 ml of distilled water. All the former were mixed, and the total volume was 962.5 ml. From the solution 15.2 ml was discarded (so 950 ml left), 100 ml of the solution was taken, and 1 g of yeast extract was added (so 850 ml left). Then 100 ml of 1% yeast (5.2) was added back to 850 ml of the solution. Then, the final volume reaches 950 ml with approximately 0.01% yeast (usually pH 7).The solution was aliquoted into 100 ml bottles and autoclaved at 121°C for 15 min and left to cool. 10 ml of horse serum was added to 90 ml of above sterile medium. A volume of 5 ml of the medium was aliquoted into sterile 7 ml culture tube using a sterile pipette and was kept at 4°C until use. Before inoculation of the stool sample, the tube containing culture medium was wormed in autoclave at 37°C. The tubes were incubated at 37°C. On the 3rd day of culture, a drop of sediment was taken using sterile pipette and examined by microscopy for the presence of Blastocystis spp.
Statistical analysis
The statistical analysis was performed using statistical analysis system (SPSS); version 16. (SPSS Inc. Chicago IL. USA). Frequency and percentage were used with qualitative data. Z-test and Chi-square were used to compare frequencies.
RESULTS
In the present study, different techniques used in the detection of Blastocystis spp. showed that culture detected 274 positive cases (22.8%). The overall relations were statistically significant (P <0.05) [Table 1]. Using trichrome stained smear as a standard test, the sensitivity of culture was 100%, while the specificity was 88%.
Table 1.
Techniques used for diagnosis of Blastocystis hominis
In the present study, the relation between mixed infected cases with Blastocystis spp. and those infected by the parasite only showed that out of 274 positive patients, 225 patients had Blastocystis spp. alone in their stool, while the remaining 49 patients had mixed infection with other intestinal parasites, G. lamblia was the most frequent parasite associated with Blastocystis spp [Table 2].
Table 2.
Mixed Blastocystis hominis infection and other intestinal parasite
In the present work, the vacuolar form is the most commonly found by all methods used in this study followed by cyst form then granular form and the amoeboid form which was detected only in culture [Table 3 and Figures 1–5].
Table 3.
Forms of Blastocystis hominis as recovered by different techniques
Figure 1.
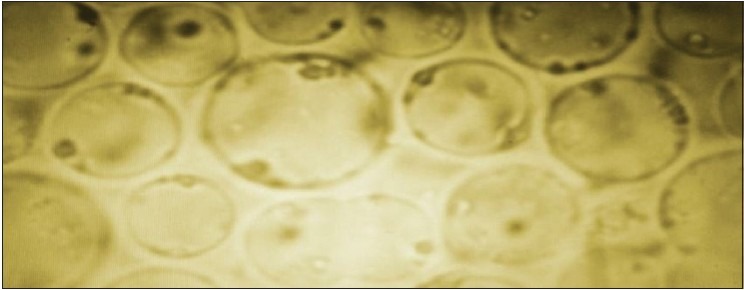
Blastocystis spp. after in vitro cultivation using Joni's medium showing predominance of the vacuolar forms by ×1000 objective
Figure 5.
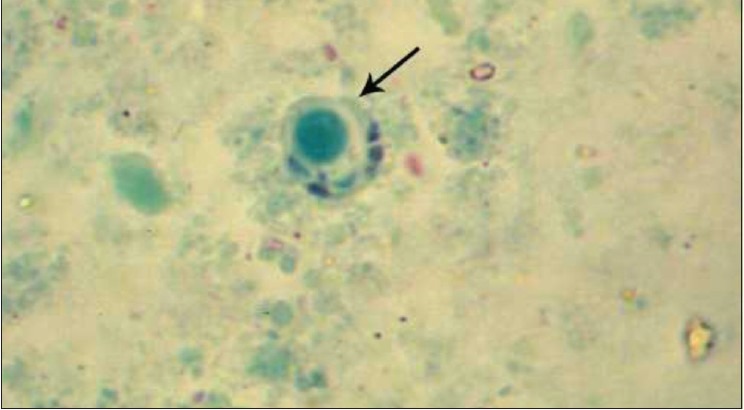
Cyst form of Blastocystis spp. stained with trichrome by ×1000 objective
Figure 2.

Amoeboid form of Blastocystis spp. in culture stained with iodine by ×1000 objective
Figure 3.
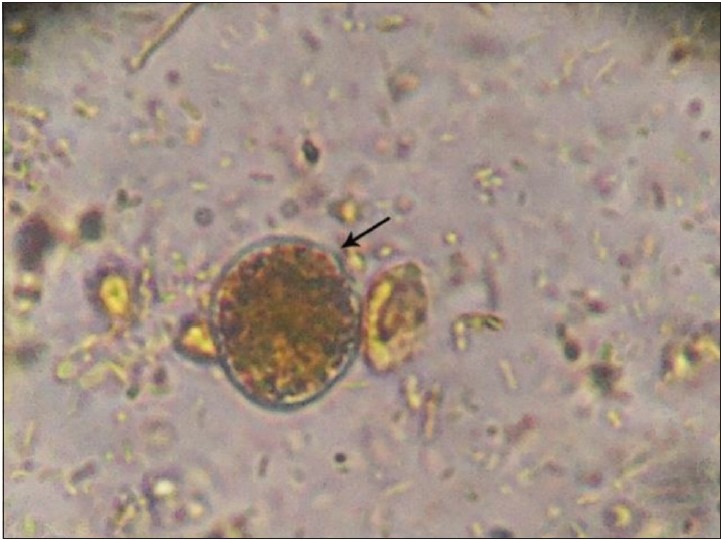
Granular form of Blastocystis spp. in culture by ×1000 objective
Figure 4.
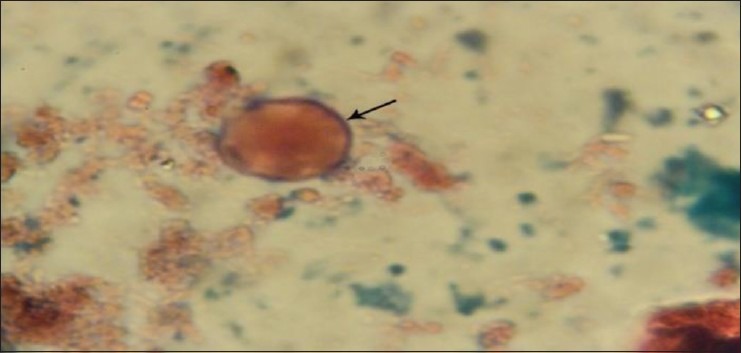
Vacuolar form of Blastocystis spp. stained with trichrome by ×1000 objective
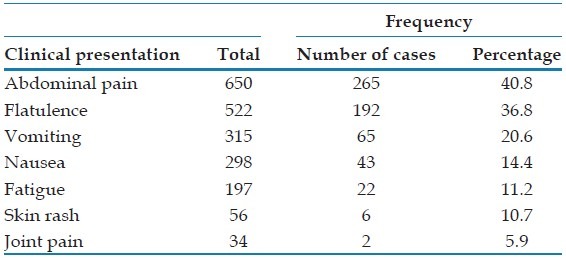
The major clinical presentation of the patients with blastocystosis [Table 4] was abdominal pain, 265 cases (40.8%) while the minor one was joint pain, 2 cases (5.9%).
Table 4.
Other intestinal parasite as recovered by different techniques
The highest infection rate was recorded [Table 5] in cases with moderate diarrhea 169 cases (61.7%), followed by those with mild diarrhea 90 cases (32.8%) and lastly those with severe diarrhea 15 cases (5.5%). The overall relations were statistically significant where Chi-square test =72.01, P < 0.05.
Table 5.
Severity of diarrhea among blastocystosis patients
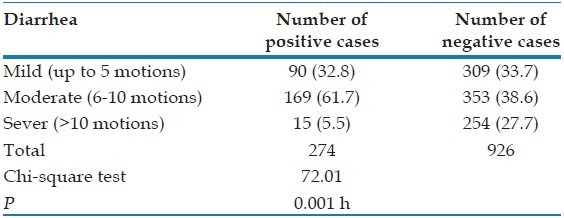
Table 6.
Frequency of clinical presentation of the patients
DISCUSSION
In the present study, different diagnostic techniques were employed to found out the most reliable one. A total of 1200 stool samples were collected from diarrheic patients and examined by direct microscopy using saline and iodine wet mounts with ×40 and ×100 objectives. Direct smears detected 42 positive cases only (3.5%) and iodine-stained smear detected 72 positive cases (6%). This was in agreement with[11] who concluded that it was difficult to identify the organism in wet mounts due to its variation in size and shape.
In the present work, concentration technique using formalin-ether sedimentation identified 120 positive cases (10%). This number was higher than those were detected by direct smears (3.5%) and iodine-stained smear (6%). This was in agreement with[12] who reported that concentration technique were useful. This was in contrary with[5] who reported that, out of the 48 positive samples 36 (75%) were identified by direct iodine wet mount; 33 (68.8%) by direct saline wet mount, and 34 (70.8%) samples were detected by formalin-ether sedimentation. Miller and Minshew, 1988[13] suggested that concentration techniques were unsuitable to detect Blastocystis spp. from fecal specimens because the parasite could be easily disturbed. Stensvold et al., 2007[14] confirmed the previous studies and reported that diagnosis using formalin-ether sedimentation method alone was apparently associated with false negative results, although the reason for this result is currently unknown. These finding are in agreement with the obtained results from the present study,[15] reported that, fecal concentration and direct smears have been used by several authors to detect the presence of Blastocystis spp. although those methods have been shown to have a low sensitivity.
In the current work, trichrome stain detected 148 positive cases (12.3%) while direct smears detected 42 positive cases only (3.5%) and iodine-stained smear detected 72 positive cases (6%). This was in agreement with[15] who concluded that, trichrome stain was more sensitive for the detection of intestinal protozoa than iodine-stained wet mounts. On the other hand,[16] reported that, staining with trichrome stain proved to be as efficient as direct examination for confirming the presence of this protozoon in the feces. Dogruman-Al et al., 2010 reported that using culture as a standard, the sensitivity of iodine-stained smear was 91% and trichrome stain was 100%.
Another study showed that 82% of Blastocystis spp. positive specimens were detected by simple Trichrome stain, 100% were detected by Trichrome stain from concentration technique, and reported that concentration technique were suitable for the diagnosis of Blastocystis spp. infection.[17] This was in agreement with[7] who found that 3.2% of samples were positive for Blastocystis spp. using formol-ether concentration and trichrome staining.
In the present work, in vitro cultivation using Jones' medium detected significantly higher positive Blastocystis spp. cases than other different diagnostic methods. This was in agreement with[18] who conducted a study to compare between methods for detection of Blastocystis spp. showed that microscopy following formol ether concentration was thus ineffective in detecting infection compared to in vitro culture, indicating that either the organism was present in low numbers in the stool samples tested or less likely were confused with fat globules. A previous study in Turkey reported that from a set of 105 stool specimens submitted for routine parasitological analysis, 30 were identified as positive for Blastocystis spp. by the culture method, from that group of 30 positives, Lugol's iodine-stain and trichrome staining identified 11, 15 samples as positive respectively.[19]
A previous study in Thailand reported that Blastocystis spp. positive cases were found as high as 13.5% and 9.6% with culture method using Jones medium and formalin ethyl acetate, respectively.[20]
Eida and Eida, 2008[21] conducted another study for detection of Blastocystis spp. in patients with irritable bowel syndrome using microscopy and culture, from 83 patients with irritable bowel syndrome 25 (30.1%) were found positive by microscopy and 34 (41%) by culture. This was in agreement with[22] who concluded that, Blastocystis spp. was isolated from 46% and 32% of 95 stool samples from irritable bowel syndrome patients using culture and microscopic examination respectively.
In the current study, in vitro culture detected more Blastocystis spp. cases than trichrome staining. This was in agreement with[23] who reported that in vitro culture detected more Blastocystis spp. cases than modified Trichrome staining, though the difference was statically insignificant. Trichrome staining was much more time consuming, which make it unsuitable for use in the survey study. The prevalence of Blastocystis spp. using in vitro cultivation was 30.3% that was six times higher than those detected by simple smears and two times than those detected by trichrome staining.[29] This was in agreement with[11] who reported that the culture was more sensitive in the detection of Blastocystis spp. than the simple smear and concentration techniques. In vitro culture increased the size and number of the parasites.
In the present study, the examination of diarrheic stool by all methods used revealed that the vacuolar form was the most predominant and the most common form found, and it was as follow: There were 36, 54, 100, 123, 222 positive vacuolar form were detected by direct smear, iodine-stained smear, formol ether concentration technique, trichrome stain and in vitro culture respectively.
This was in agreement with[24] who considered that the vacuolar form was the typical Blastocystis cell form, and it was the most frequently used microbiological evidence for the diagnosis of Blastocystis spp. infection.
In the present study, the cyst form was found in 4, 13, 15, 19, 27 positive samples and the granular form was found in 2, 5, 5, 6, 14 positive samples by direct smear, iodine-stained smear, formol ether concentration technique, trichrome stain and in vitro culture respectively. Abu El Naga and Negm, 2001[25] reported that the cyst forms of Blastocystis spp. were found in fecal material particularly that stored for several days before being fixed and occasionally in laboratory cultures.
Tan and Suresh, 2006 and Katsarou-Katsari et al., 2008[26,27] reported that, granular forms mostly appeared in older cultures of isolates. Also the granular form that was detected in culture samples shares many similarities with the vacuolar form except that numerous granules found within the central vacuole and this agrees with[28] who suggested that the granular form arises from the vacuolar form and the transition induced by a varieties of factors like increased serum concentrations in the culture medium.
The amoeboid form was detected only by culture technique as it was detected in 11 positive cases out of 274 positive cases (4%). This was in agreement with[29] who reported that direct-mount examination and permanent staining of feces disclosed vacuolar and granular forms but no amoeboid forms, amoeboid forms found only in the culture of the symptomatic isolates. Furthermore, amoeboid forms probably arise from the vacuolar forms. It was evidenced by seeing vacuolar forms when cultured in agar and after incubation; the resultant colonies contain numerous amoeboid forms.[30] An epidemiological research of Blastocystis spp. was done and reported that the distribution of 778 patients infected by Blastocystis spp. due to parasite form was determined as follow: Vacuolar form in 525 (67.49%), granular in 115 (14.78%), both vacuolar and granular in 138 (17.73%) cases.[31]
In the present study about, mixed infected cases, G. lamblia was found to be the most frequent parasite associated with Blastocystis hominis and this association is in accordance with that reported by.[5] On the contrary,[32] reported that Endolimax nana was the most common protozoan parasite found in conjunctions with Blastocystis spp. followed by G. lamblia and Entamoeba histolytica.
ACKNOWLEDGMENTS
We would like to acknowledge all members of Parasitology Lab, Department of Parasitology, Benha Faculty of Medicine, Benha University for their help in the competition of this work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Ahmed A, Surin J, Mak JW. Drinking water is a significant predictor of Blastocystis infection among rural Malaysian primary schoolchildren. Parasitology. 2012;139:1014–20. doi: 10.1017/S0031182012000340. [DOI] [PubMed] [Google Scholar]
- 2.Coyle CM, Varughese J, Weiss LM, Tanowitz HB. Blastocystis: To treat or not to treat. Clin Infect Dis. 2012;54:105–10. doi: 10.1093/cid/cir810. [DOI] [PubMed] [Google Scholar]
- 3.Amato Neto V, Rodriguez RS, Gakiya E. Blastocystosis: Controversy and indefinedness. Rev Soc Bras Med Trop. 2003;36:515–7. doi: 10.1590/s0037-86822003000400014. [DOI] [PubMed] [Google Scholar]
- 4.Bogoch II, Raso G, Goran EK, Marti HP, Utzinger J. Differences in microscopic diagnosis of helminths and intestinal protozoa among diagnostic centres. Eur J Clin Microbiol Infect Dis. 2006;25:344–7. doi: 10.1007/s10096-006-0135-x. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento SA, Moitinho Mda L. Blastocystis hominis and other intestinal parasites in a community of Pitanga City, Paraná State, Brazil. Rev Inst Med Trop Sao Paulo. 2005;47:213–7. doi: 10.1590/s0036-46652005000400007. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Basic Laboratory Methods in Medical Parasitology. Reagents and Solutions and their Preparation. 1991:100–5. [Google Scholar]
- 7.King M. A Medical Laboratory for Developing Countries. Vol. 15. ELBS, ELBS, Oxford University Press; 1975. pp. 45–55. [Google Scholar]
- 8.Cheesbrough M. Medical laboratory manual for tropical countries. Tropical Health Technology, Butterworth-Heinemann. 1987;88:365–89. [Google Scholar]
- 9.Garcia LS. Diagnostic Medical Parasitology. 5th ed. Washington: ASM Press; 2007. Intestinal protozoa: Amoebae, Blastocystis hominis; pp. 81–94. [Google Scholar]
- 10.Leelayoova S, Taamasri P, Rangsin R, Naaglor T, Thathaisong U, Mungthin M. In-vitro cultivation: A sensitive method for detecting Blastocystis hominis. Ann Trop Med Parasitol. 2002;96:803–7. doi: 10.1179/000349802125002275. [DOI] [PubMed] [Google Scholar]
- 11.Garcia LS, Bruckner DA. Diagnostic Medical Parasitology. 2nd ed. Washington: ASM Press; 1997. Intestinal protozoa flagellates and ciliates; pp. 13–39. [Google Scholar]
- 12.Hussein SM, Al-okaili GA, Al-Dayel F. Clinical significance of Blastocystis hominis. J Clin Microbiol. 1989;27:2407–9. doi: 10.1128/jcm.27.11.2407-2409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller RA, Minshew BH. Blastocystis hominis: An organism in search of a disease. Rev Infect Dis. 1988;10:930–8. doi: 10.1093/clinids/10.5.930. [DOI] [PubMed] [Google Scholar]
- 14.Stensvold CR, Suresh GK, Tan KS, Thompson RC, Traub RJ, Viscogliosi E, et al. Terminology for Blastocystis subtypes - A consensus. Trends Parasitol. 2007;23:93–6. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Tan KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. 2008;21:639–65. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miné JC, Rosa JA. Frequency of Blastocystis hominis and other intestinal parasites in stool samples examined at the Parasitology Laboratory of the School of Pharmaceutical Sciences at the São Paulo State University, Araraquara. Rev Soc Bras Med Trop. 2008;41:565–9. doi: 10.1590/s0037-86822008000600004. [DOI] [PubMed] [Google Scholar]
- 17.Ozçakir O, Güreser S, Ergüven S, Yilmaz YA, Topaloglu R, Hasçelik G. Characteristics of Blastocystis hominis infection in a Turkish university hospital. Turkiye Parazitol Derg. 2007;31:277–82. [PubMed] [Google Scholar]
- 18.Suresh K, Smith H. Comparison of methods for detecting Blastocystis hominis. Eur J Clin Microbiol Infect Dis. 2004;23:509–11. doi: 10.1007/s10096-004-1123-7. [DOI] [PubMed] [Google Scholar]
- 19.Dogruman-Al F, Simsek Z, Boorom K, Ekici E, Sahin M. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS One. 2010;5:e15484. doi: 10.1371/journal.pone.0015484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaicharoen R, Ngrenngarmlert W, Wongjindanon N, Sripochang S, Kiatfuengfoo R. Infection of Blastocystis hominis in primary schoolchildren from Nakhon Pathom province, Thailand. Trop Biomed. 2006;23:117–22. [PubMed] [Google Scholar]
- 21.Eida AM, Eida MM. Identification of Blastocystis hominis in patients with Irritable Bowel Syndrome using microscopy and culture compared to PCR. Parasitologist U J. 2008;1:87–92. [Google Scholar]
- 22.Yakoob J, Jafri W, Jafri N, Khan R, Islam M, Beg MA, et al. Irritable bowel syndrome: In search of an etiology: Role of Blastocystis hominis. Am J Trop Med Hyg. 2004;70:383–5. [PubMed] [Google Scholar]
- 23.Sumeth T, Saovanee L, Pote A, Umaporn T, Thirayost N, Paanjit T, et al. The usefulness of short-term in vitro cultivation for the detection and molecular study of Blastocystis hominis stool specimens. Parasitol Res. 2004;93:445–7. doi: 10.1007/s00436-004-1157-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Qiao JY, Zhou XJ, Yao FR, Wei ZC. Morphology and reproductive mode of Blastocystis hominis in diarrhea and in vitro. Parasitol Res. 2007;101:43–51. doi: 10.1007/s00436-006-0439-x. [DOI] [PubMed] [Google Scholar]
- 25.Abou El Naga IF, Negm AY. Morphology, histochemistry and infectivity of Blastocystis hominis cyst. J Egypt Soc Parasitol. 2001;31:627–35. [PubMed] [Google Scholar]
- 26.Katsarou-Katsari A, Vassalos CM, Tzanetou K, Spanakos G, Papadopoulou C, Vakalis N. Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm Venereol. 2008;88:80–1. doi: 10.2340/00015555-0338. [DOI] [PubMed] [Google Scholar]
- 27.Tan TC, Suresh KG. Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol Res. 2006;98:189–93. doi: 10.1007/s00436-005-0033-7. [DOI] [PubMed] [Google Scholar]
- 28.Soriano SV, Barbieri LM, Pierángeli NB, Giayetto AL, Manacorda AM, Castronovo E, et al. Intestinal parasites and the environment: Frequency of intestinal parasites in children of Neuquén, Patagonia, Argentina. Rev Latinoam Microbiol. 2001;43:96–101. [PubMed] [Google Scholar]
- 29.Vassalos CM, Spanakos G, Vassalou E, Papadopoulou C, Vakalis N. Differences in clinical significance and morphologic features of Blastocystis sp subtype 3. Am J Clin Pathol. 2010;133:251–8. doi: 10.1309/AJCPDOWQSL6E8DMN. [DOI] [PubMed] [Google Scholar]
- 30.Tikhonova DV, Fedianina LV, Pliushcheeva GL. The specific features of the clinical picture of blastocystosis and laboratory methods for its diagnosis. Med Parazitol (Mosk) 2012;3:44. [PubMed] [Google Scholar]
- 31.Inceboz T, Usluca S, Over L, Yalçin G, Tuncay S, Ozkoç S. The epidemiology research of Blastocystis hominis in the Dokuz Eylül University Medical Faculty Hospital between 2005 and 2009. Turkiye Parazitol Derg. 2011;35:72–6. doi: 10.5152/tpd.2011.19. [DOI] [PubMed] [Google Scholar]
- 32.Roberts T, Barratt J, Harkness J, Ellis J, Stark D. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am J Trop Med Hyg. 2011;84:308–12. doi: 10.4269/ajtmh.2011.10-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]


