Abstract
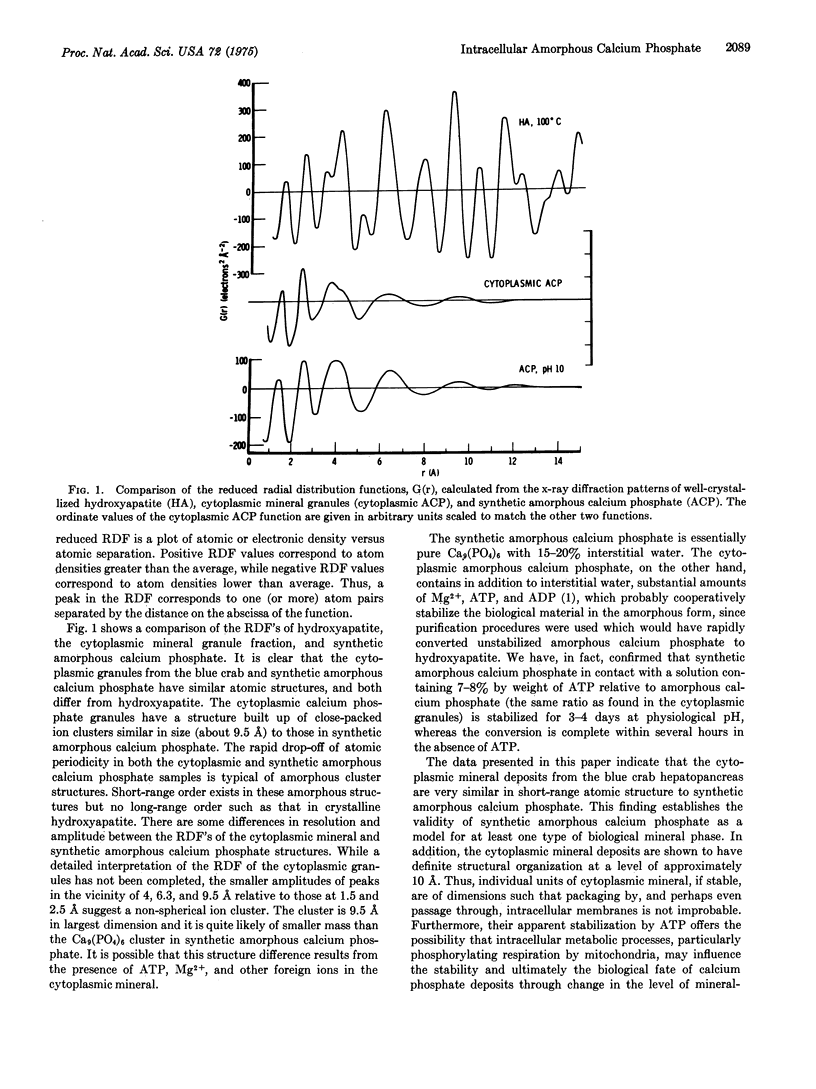
The radial distribution function calculated from x-ray diffraction of mineralized cytoplasmic structures isolated from the hepatopancreas of the blue crab (Callinectes sapidus) is very similar to that previously found for synthetic amorphous calcium phosphate. Both types of mineral apparently have only short-range atomic order, represented as a neutral ion cluster of about 10 A in longest dimension, whose probable composition is expressed by the formula Ca9(PO4)6. The minor differences observed are attributed to the presence in the biological mineral of significant amounts of Mg-2+ and ATP. Synthetic amorphous calcium phosphate in contact with a solution containing an amount of ATP equivalent to that of the biological mineral failed to undergo conversion to the thermodynamically more stable hydroxyapatite. The amorphous calcium phosphate of the cytoplasmic mineral granules is similarly stable, and does not undergo conversion to hydroxyapatite, presumably owing to the presence of ATP and Mg-2+, known in inhibitors of the conversion process. The physiological implications of mineral deposits consisting of stabilized calcium phosphate ion clusters are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHRA B. N. Precipitation of calcium carbonates and phosphates from metastable solutions. Ann N Y Acad Sci. 1963 May 31;109:251–255. doi: 10.1111/j.1749-6632.1963.tb13469.x. [DOI] [PubMed] [Google Scholar]
- Becker G. L., Chen C. H., Greenawalt J. W., Lehninger A. L. Calcium phosphate granules in the hepatopancreas of the blue crab Callinectes sapidus. J Cell Biol. 1974 May;61(2):316–326. doi: 10.1083/jcb.61.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARAFOLI E., ROSSI C. S., LEHNINGER A. L. UPTAKE OF ADENINE NUCLEOTIDES BY RESPIRING MITOCHONDRIA DURING ACTIVE ACCUMULATION OF CA++ AND PHOSPHATE. J Biol Chem. 1965 May;240:2254–2261. [PubMed] [Google Scholar]
- Eanes E. D., Gillessen I. H., Posner A. S. Intermediate states in the precipitation of hydroxyapatite. Nature. 1965 Oct 23;208(5008):365–367. doi: 10.1038/208365a0. [DOI] [PubMed] [Google Scholar]
- Eanes E. D., Posner A. S. Intermediate phases in the basic solution preparation of alkaline earth phosphates. Calcif Tissue Res. 1968 Jul 15;2(1):38–48. doi: 10.1007/BF02279192. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Role of nucleation and inhibition in calcification. Clin Orthop Relat Res. 1964 Jan-Feb;32:170–180. [PubMed] [Google Scholar]
- Francis M. D. The inhibition of calcium hydroxypatite crystal growth by polyphosphonates and polyphosphates. Calcif Tissue Res. 1969;3(2):151–162. doi: 10.1007/BF02058658. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]


