Abstract
An 8-year-old spayed female domestic shorthair cat was presented with a 4- to 5-month history of a progressively growing mass above her anus and an inability to defecate for 3 to 4 wk. External perianal and internal regional masses were subsequently identified and diagnosed as tumors of neuroendocrine origin through surgical excision and histopathologic evaluation. The cat was treated with 2 courses of chemotherapy and radiation therapy.
Résumé
Tumeur neuroendocrinienne périanale avec une métastase suspectée des ganglions lymphatiques causant une compression du côlon et un mégacôlon subséquent. Une chatte commune domestique stérilisée âgée de 8 ans a été présentée avec une anamnèse de 4 ou 5 mois d’une masse à croissance progressive au-dessus de l’anus et l’incapacité de déféquer depuis 3 ou 4 semaines. Les masses périanales externes et régionales internes ont été subséquemment identifiées et diagnostiquées comme des tumeurs d’origine neuro-endocrinienne suite à l’excision chirurgicale et une évaluation histopathologique. La chatte a été traitée à l’aide de deux séries de traitement et d’une radiothérapie.
(Traduit par Isabelle Vallières)
Neuroendocrine (NE) tumors are a heterogenous group of tumors, classified together based on their similar histologic features and their ability to take up and decarboxylate biogenic amines. However, this feature of amine-handling is not consistent among the entire family of neuroendocrine tumors, which makes identification sometimes difficult (1). Neuroendocrine tumors are often named based on the organ or cell-type from which they arise, making the diagnosis of metastatic tumors difficult without identification of the primary lesion.
Neuroendocrine tumors can arise from any cell of the NE system, which is located throughout the body (2). These cells are embryologically derived from the gut, and then become distributed into various tissues including the tracheobronchial tree, liver, pancreas, and genitourinary system (3). Tumors of the diffuse NE system are uncommonly reported in domestic animals. Reported locations in dogs and cats include the intestines, bile duct, lungs, liver, gall bladder, esophagus, skin, nostril, nasal cavity, nasopharynx, cauda equina, and tracheobronchial tree (3–13). However, NE tumors can also occur in tissues and organs that do not normally contain NE cells (1).
This case report describes the physical examination, radiographic, cytopathologic, magnetic resonance imaging (MRI), surgical, and histopathologic findings in a cat suffering from a perianal neuroendocrine tumor.
Case description
An 8-year-old spayed female domestic shorthair cat was evaluated for a progressively growing mass above her anus. The mass was first noticed 4 to 5 mo prior to presentation and difficulty defecating was noticed 3 to 4 wk before presentation. According to the owner the cat was hyporexic but was drinking normally. She was an indoor/outdoor cat, was up-to-date on vaccinations, and had no previous health concerns.
Initial physical examination of the cat revealed a poor body condition score (3/9); large firm feces in the transverse and descending colon; and a large 8 × 8 cm round, firm, subcutaneous, fixed external perianal mass dorsal to the anus on the midline (Figure 1, Mass 1). A rectal examination following sedation revealed normal anal sacs and a large, firm mass dorsal to the colon, approximately 8 cm orally from the external anal orifice (Figure 1, Mass 2).
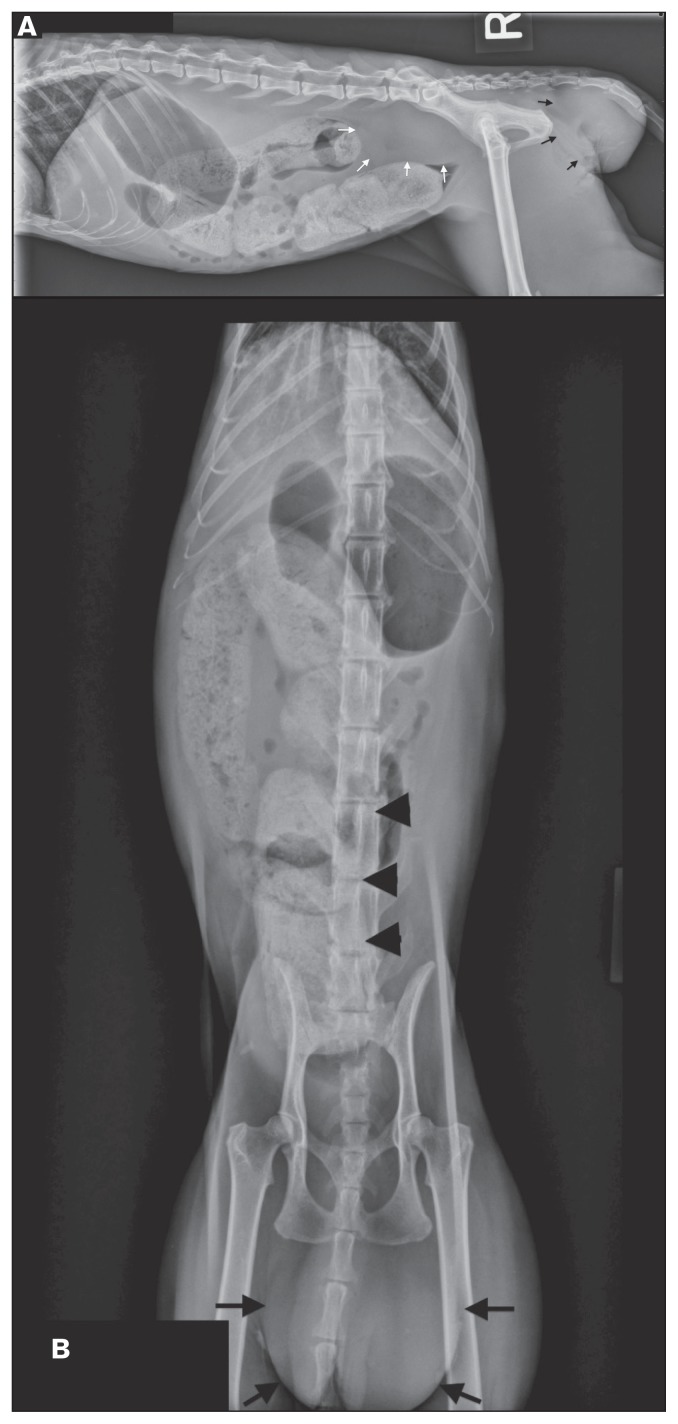
Figure 1.
Right lateral (A) and ventrodorsal (B) radiographic views of the abdomen. A soft tissue opaque mass (Mass 1) is present at the perianal region (black arrows). The distal descending colon is ventrally displaced with compression cranial to the pelvic canal by a soft tissue opacity mass (Mass 2) in the sublumbar area (white arrows). There is generalized loss of serosal detail. The entire colon is markedly distended with mineral opaque fecal content. The stomach is distended with gas.
Results of a complete blood (cell) count (CBC) and serum biochemical profile were within normal reference values with the exception of a mild leukocytosis [11.6 × 109/L; reference interval (RI): 6 to 10 × 109/L], mild hypoalbuminemia (24 g/L; RI: 27 to 41 g/L), mild hypocalcemia (2.10 mmol/L; RI: 2.27 to 2.70 mmol/L), and mild hyperchloremia (124 mmol/L; RI 110 to 122 mmol/L). A urinalysis revealed a mild proteinuria (2+ dipstick reaction) and hematuria (20 to 50/high-power field). Three-view thoracic radiographs were within normal limits.
Abdominal radiographs (Figure 1) showed the cat to be very thin with generalized loss of serosal detail. The stomach was distended with gas. There was a poorly marginated soft tissue opaque mass in the perianal region (Figure 1, Mass 1) and ventral to the lumbosacral spine (Figure 2, Mass 2) that caused severe ventral and rightward displacement of the distal descending colon resulting in the colonic lumen abruptly terminating. The entire colon was markedly distended with mineral opaque fecal content, presumed to be due to the ventral lumbosacral mass (Mass 2) causing a colonic obstruction. Differentials for the perianal mass included neoplasia, perianal hernia, abscess or granuloma with extension of disease into the retroperitoneal space dorsal to the colon or enlargement of the medial iliac, hypogastric, or sacral lymph nodes. The markedly decreased serosal detail was likely due to poor body condition (lack of fat) and unlikely due to peritoneal effusion based on the lack of abdominal distention. A focused abdominal ultrasound examination to screen for free peritoneal fluid was negative.
Figure 2.
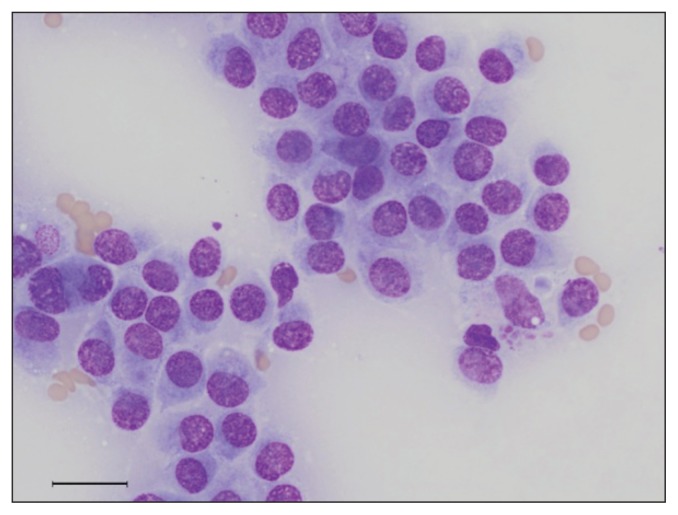
Photomicrograph of a cytology smear from a fine-needle aspiration of the perianal mass. The aspirate contains clusters of round to polygonal cells with moderate light blue cytoplasm and indistinct cell borders that exhibit mild anisocytosis and anisokaryosis. Wright-Giemsa stain; bar = 50 μm; 500×.
A fine-needle aspirate of the perianal mass (Mass 1) was stained with Wright-Giemsa (Figure 2). The aspirate contained clusters of round to polygonal cells with moderate light blue cytoplasm and indistinct cell borders that exhibited mild anisocytosis and anisokaryosis. The results were suspicious for a neuroendocrine neoplasm, although due to the pleomorphism of the neoplastic cells, a poorly differentiated carcinoma of another origin or a sarcoma could not be ruled out.
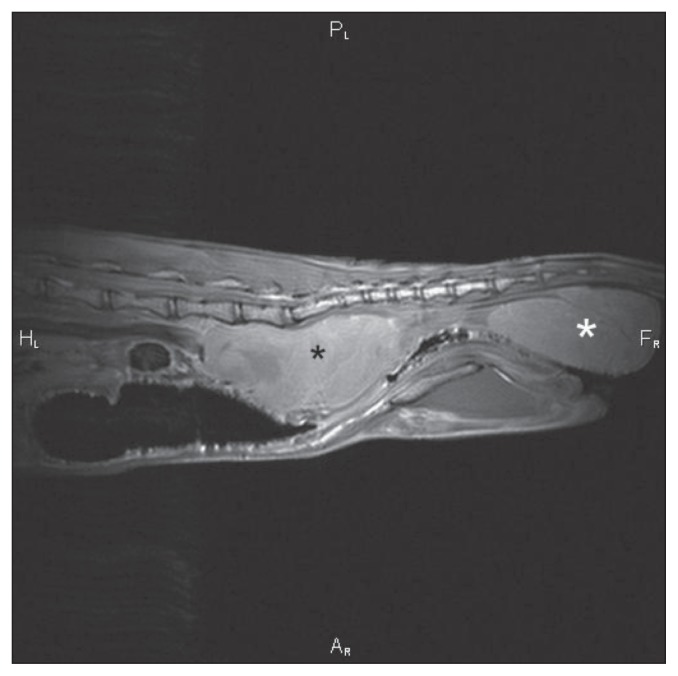
An MRI examination of the caudal abdomen was performed (Figure 3) using a 1.5T magnet (Echelon; Hitachi Medical Systems America, Twinsburg, Ohio, USA). There was a 6.5 × 3.5 × 3.5 cm sized ovoid heterogeneously intense mass in the sublumbar region (Mass 2) between the 6th lumbar and the 2nd caudal vertebrae that caused severe ventral displacement and extramural compression of the colon and rectum. The aorta was severely displaced ventrally by the same dorsal sublumbar mass but the adjacent musculature was not affected. The mass was hyperintense to muscle on T1W, T2W and fast inversion recovery sequences and was situated between the left and right external iliac arteries and veins, which it displaced laterally. Another mass (Mass 1) with the same intensity characteristics and approximately the same size was located ventral to the tail in the perianal region but had no visible connection to the sublumbar mass. A 3rd small round mass (Mass 3), 2 cm in diameter, also with the same MRI intensity as the other masses, was present ventral to the tail base, left, parasagittal. There was no contrast enhancement visible in any of the masses or surrounding tissues in the post contrast T1W images. The bony structures of the spine were unremarkable. Left inguinal lymph-adenomegaly was noted and both anal sacs were unremarkable. The adductor muscle group on the left was smaller than on the right and had striated hyperintensities throughout. The urinary bladder was displaced to the right of the rightwards displaced descending colon. No peritoneal effusion was observed. An MRI diagnosis of a perianal mass of unknown origin with extension of disease to the sublumbar and inguinal lymph nodes and distal colonic extramural obstruction was made. Mass 3 was thought to be either extension of this disease or an additional aspect of primary disease. Neoplasia or granulomatous diseases were considered the most likely cause.
Figure 3.
Sagittal MRI T1 weighted image of the caudal abdomen. A large, heterogeneously intense mass is observed in the sublumbar region (black *, Mass 2) between the 6th lumbar vertebrae and the 2nd caudal vertebrae, which severely displaces the colon and rectum ventrally and severely compresses them. A similar appearing mass is present in the perianal region (white *, Mass 1).
The patient was anesthetized and a ventral midline exploratory celiotomy was performed. At least 4 separate masses were observed dorsal to the descending colon (originally referred to as Mass 2), suspected to be medial iliac and hypogastric lymph nodes, and were 4 × 3 cm, 3 × 3 cm, 2 × 2 cm, and 2 × 3 cm in size. These were all removed with marginal excision. After routine closure, the patient was then placed in sternal recumbency and a 6 × 4.5 × 4.5 cm midline perianal mass (Mass 1) dorsal to the rectum was removed with marginal excision. On palpation of both anal sacs, no masses or thickenings were noted. The smallest mass (Mass 3), which was palpated dorsal to the left anal sac and measured 1.5 × 2.5 × 1.5 cm, was removed without margins.
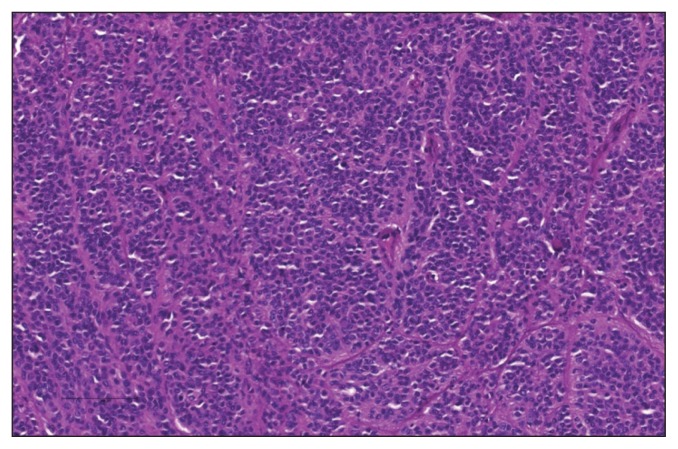
Microscopically, the normal architecture of the perianal soft tissues was effaced by an unencapsulated, well-circumscribed, densely cellular neoplasm (Mass 1). The neoplasm was composed of nest, packets, and trabeculae of polygonal cells supported on a fine fibrovascular stroma (Figure 4). The neoplastic cells had round to oval nuclei with finely stippled to vesicular chromatin and prominent nucleoli; frequently nuclei contained large, round, to oval eosinophilic inclusions interpreted as cytoplasmic invaginations. The neoplastic cells had a small amount of finely granular eosinophilic cytoplasm with indistinct cell borders. There was marked anisocytosis and anisokaryosis, and the mitotic index was 8 mitoses per 10 high power fields. There were multifocal areas of necrosis scattered throughout the mass. Occasionally neoplastic cells were noted to palisade around blood vessels. Immunohistochemical staining revealed that the neoplastic cells exhibited strong vimentin and S100 positivity and weak chromogranin positivity, and were negative for cytokeratin, smooth muscle actin, synaptophysin, and melan A.
Figure 4.
Photomicrograph of a section of the perianal mass. The neoplasm consists of cords of polygonal cells supported on a fibrovascular stroma. H&E stain; bar = 100 μm; 200×.
Effacing > 95% of the medial iliac and hypogastric lymph nodes (Mass 2) was an unencapsulated, densely cellular neoplasm consistent with the previously described neoplasm. Similar neoplastic cells were found to efface and replace > 95% of the tissue examined from the mass dorsal to the left anal sac (Mass 3).
Neoplastic cells were seen by electron microscopy to contain a heterogenous population of secretory granules. Some of the granules were large, approximately 500 μm in diameter, and were homogenous, moderately electron dense (zymogen-like granules), whereas others were membrane bound with an eccentric electron dense-core (norepinephrine-like granules). These granules were typical of dense-core neurosecretory granules (DCNG), and therefore suggestive of neuroendocrine differentiation in these neoplastic cells.
On the basis of the histologic findings and electron microscopy, the diagnosis was that of a poorly differentiated carcinoma with neuroendocrine differentiation in the perianal region, with metastasis to the medial iliac and hypogastric lymph nodes. The top differentials included a carcinoid, Merkel cell tumor, neuroendocrine cell tumor of the apocrine glands of the anal sac, or a paraganglioma.
Discussion
Histologically, the diagnosis of NE tumors is based on the presence of a typical growth pattern characterized by the formation of cords and packets (organoid growth pattern), dense-core neurosecretory granules (DCNG), and immunohistochemical reactivity to neuroendocrine markers such as neuron-specific enolase (NSE), synaptophysin, and chromogranin A (4). Dense-core neurosecretory granules represent the cellular capacity to convert exogenously administered amine precursors into dopa-mine or serotonin, which are stored in these granules. Therefore, the presence of these granules is related to the degree of tumor cell differentiation (4).
Neuroendocrine tumors can be divided into 2 broad categories, those with epithelial differentiation and those with neural differentiation (1). Due to their similar histologic features, identification or diagnosis is often achieved with the help of immunohistochemistry, where cytokeratin expression (positive with broad-spectrum cytokeratin antibodies) is indicative of epithelial differentiation and expression of neurofilaments is indicative of neuronal differentiation (1). Most of those with neural differentiation will be negative for cytokeratins and positive for neurofilament triplet proteins and the extent of staining is proportional to the degree of differentiation (1).
In the current case, the presence of advanced metastatic disease at the time of presentation made the identification of the primary site of neoplasia difficult and inhibited the elucidation of the histogenesis of the tumor. Cytology of the perianal mass (Mass 1) was highly suggestive of a neuroendocrine neoplasm. Differentials for the perianal neuroendocrine neoplasm from cytology included a melanoma, carcinoid, paraganglioma, Merkel cell tumor, and anal sac adenocarcinoma. Histopathologically, the neoplasm exhibited some characteristic features of a neuroendocrine tumor, such as organoid growth pattern, and expression of chromogranin A; however, neoplastic cells failed to express other common neuroendocrine markers such as NSE and synaptophysin, yielding equivocal results. With electron microscopy we identified membrane bound dense-core secretory granules confirming the neuroendocrine origin of this tumor. Based on the histopathological findings, the top differentials included a carcinoid, Merkel cell tumor, neuroendocrine cell tumor of the apocrine glands of the anal sac, or a paraganglioma.
The origin of the perianal masses could not be determined. Microscopically, there was no normal tissue remaining to indicate the tissue of origin. Both anal sacs appeared normal on the MRI and the masses did not show any association with either anal sac. Therefore, Masses 1 and 3 may have been a primary tumor of the perianal soft tissues, or one may have been the primary tumor with metastasis forming the other. Mass 2 was undoubtedly metastatic in origin and this conclusion is supported by the invasion of the regional lymph nodes. A multicentric cutaneous neuroendocrine tumor has been previously reported in a dog (14), but metastasis to lymph nodes was not noted in that case report. A necropsy was not performed in that case, so metastasis from an internal source could not be ruled out.
No specific treatments have been evaluated in domestic animals with NE tumors. Surgical excision followed by radiation and chemotherapy was elected in this case. In humans, surgical excision is the principle therapy for localized disease while for patients with advanced disease, the somatostatin analogue octreotide or single agent or combination chemotherapy regimens are also efficacious (2). In a case report of a dog with multicentric cutaneous neuroendocrine (Merkel Cell) carcinoma, therapy consisted of an alternating regimen of carboplatin and doxorubicin/cyclophosphamide every 3 wk, followed by an antiangiogenic regimen of doxycycline, cyclophosphamide, and piroxicam when it continued to develop disseminated and progressive cutaneous tumors (14). This dog survived 8 mo after diagnosis before dying of progressive disease. Another treatment option reported in cats and dogs for various tumors, including those of neuroendocrine origin, included tyrosine kinase inhibitors (15,16). Due to the inability to administer oral medication to this patient, this was not considered a viable treatment option. In this case, the cat was re-evaluated 2 wk after surgery and large masses could be palpated on rectal examination, indicating recurrence. Treatment with radiation therapy was initiated (24 Gy to her pelvic canal) in addition to chemotherapy with carboplatin (160 mg/m2 IV every 4 wk for 5 treatments) and the patient’s disease remained stable. Six months following initial diagnosis, slightly progressive disease was diagnosed based on enlargement of the pelvic masses, and treatment consisted of 1 dose of doxorubicin (1 mg/kg body weight IV) followed by 1 treatment with lomustine (12.5 mg IV) 3 wk later. However, further progression of the masses was evident and additional treatment with 5 fractions of 4 Gy was initiated (7 mo after initial presentation). The masses were minimally responsive. At this point, the patient’s care was transferred to a veterinary oncology facility closer to the client’s home. According to that veterinary facility as of the last follow-up, approximately 13 mo after original presentation, the patient was receiving supportive care weekly including intravenous and subcutaneous fluids and appetite stimulants, but was suffering from worsening progressive disease.
This case report describes a perianal neuroendocrine tumor with lymph node metastasis, which is a location not previously described in the veterinary literature. Through surgery, chemotherapy, and radiation therapy, this patient achieved a prolonged survival, although progressive disease did ultimately occur. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.DeLellis The neuroendocrine system and its tumors. Am J Clin Pathol. 2001;115:S5–S16. doi: 10.1309/7GR5-L7YW-3G78-LDJ6. [DOI] [PubMed] [Google Scholar]
- 2.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340:858–868. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 3.Kuwata K, Shibutani M, Kemmochi Y, et al. A neuroendocrine carcinoma of undetermined origin in a dog. J Toxicol Pathol. 2010;23:151–155. doi: 10.1293/tox.23.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi G, Magi GE, Tarantino C, et al. Tracheobronchial neuroendocrine carcinoma in a cat. J Comp Pathol. 2007;137:165–168. doi: 10.1016/j.jcpa.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Birettoni F, Porciello F, Caivano D, Avcelli R, Sforna M, Antognoni M. Primary neuroendocrine carcinoma of the gallbladder in a dog. Vet Res Commun. 2008;32(Suppl 1):S239–242. doi: 10.1007/s11259-008-9151-x. [DOI] [PubMed] [Google Scholar]
- 6.Patnaik AK. A morphologic and immunocytochemical study of hepatic neoplasms in cats. Vet Pathol. 1992;29:405–415. doi: 10.1177/030098589202900506. [DOI] [PubMed] [Google Scholar]
- 7.Patnaik AK, Erlandson RA, Lieberman PH. Esophageal neuroendocrine carcinoma in a cat. Vet Pathol. 1990;27:128–130. doi: 10.1177/030098589002700211. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik AK, Hurvitz AI, Lieberman PH. Canine hepatic neoplasms: A clinicopathologic study. Vet Pathol. 1980;17:553–564. doi: 10.1177/030098588001700504. [DOI] [PubMed] [Google Scholar]
- 9.Patnaik AK, Ludwig LL, Erlandson RA. Neuroendocrine carcinoma of the nasopharynx in a dog. Vet Pathol. 2002;39:496–500. doi: 10.1354/vp.39-4-496. [DOI] [PubMed] [Google Scholar]
- 10.Patnaik AK, Newman SJ, Scase T, et al. Canine hepatic neuroendocrine carcinoma: An immunohistochemical and electron microscopic study. Vet Pathol. 2005;42:140–146. doi: 10.1354/vp.42-2-140. [DOI] [PubMed] [Google Scholar]
- 11.Patnaik AK, Post GS, Erlandson RA. Clinicopathologic and electron microscopic study of cutaneous neuroendocrine (Merkel cell) carcinoma in a cat with comparisons to human and canine tumors. Vet Pathol. 2001;38:553–556. doi: 10.1354/vp.38-5-553. [DOI] [PubMed] [Google Scholar]
- 12.Bagnasco G, Properzi R, Porto R, Nardini V, Poli A, Abramo F. Feline cutaneous neuroendocrine carcinoma (Merkel cell tumour): Clinical and pathological findings. Vet Dermatol. 2003;14:111–115. doi: 10.1046/j.1365-3164.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis WP, Watson GL, Koehler LK, Brown CA. Maignant cauda equina paraganglioma in a cat. Vet Pathol. 1997;34:243–246. doi: 10.1177/030098589703400313. [DOI] [PubMed] [Google Scholar]
- 14.Joiner KS, Smith AN, Henderson RA, Brawner WR, Spangler EA, Sartin EA. Multicentric cutaneous neuroendocrine (Merkel cell) carcinoma in a dog. Vet Pathol. 2010;47:1090–1094. doi: 10.1177/0300985810375945. [DOI] [PubMed] [Google Scholar]
- 15.London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia(®)) in solid tumours. Vet Comp Oncol. 2012;10:194–205. doi: 10.1111/j.1476-5829.2011.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly M, Sheppard S, Cohen N, et al. Safety of masitinib mesylate in healthy cats. J Vet Intern Med. 2011;25:297–302. doi: 10.1111/j.1939-1676.2011.0687.x. [DOI] [PubMed] [Google Scholar]