Key Points
HCT recipients have increased susceptibility to herpes zoster, but live-attenuated vaccines are not appropriate for highly immunocompromised people.
An adjuvanted subunit vaccine against herpes zoster elicits strong immune responses with an acceptable safety profile in adult autologous HCT recipients.
Abstract
Recombinant herpes zoster (HZ) vaccines may be an alternative to the live-attenuated HZ vaccine for immunocompromised individuals. This was a phase 1/2, randomized, observer-blind, placebo-controlled study in adults with multiple myeloma, non-Hodgkin lymphoma (B- or T-cell), Hodgkin lymphoma, or acute myeloid leukemia who had undergone autologous hematopoietic stem-cell transplant 50 to 70 days earlier. Subjects (N = 121) were randomized 1:1:1:1 to receive (at months 0, 1, 3) three doses of 50 μg varicella-zoster virus glycoprotein E (gE) adjuvanted with AS01B, 3 doses of gE adjuvanted with AS01E, 1 dose of saline followed by 2 doses of gE/AS01B, or 3 doses of saline. One month after the last dose (6 months after transplant), frequencies of CD4+ T cells expressing ≥2 activation markers after induction with gE and anti-gE antibody concentrations were higher with all gE/AS01 regimens than with saline. Both responses persisted up to 1 year in subjects vaccinated with gE/AS01. Immune responses were higher in the gE/AS01B 3-dose group than in the gE/AS01B 2-dose group but not higher than in the gE/AS01E 3-dose group. One serious adverse event (pneumonia) was considered vaccine related. Both formulations and both schedules were immunogenic and well tolerated in this population. This study was registered at www.clinicaltrials.gov as #NCT00920218.
Introduction
Herpes zoster (HZ), or shingles, is a painful vesicular cutaneous eruption typically restricted to 1 or 2 contiguous dermatomes. HZ results from reactivation of latent varicella-zoster virus (VZV) in nerve-root ganglia, usually many years after a primary VZV infection.1 VZV reactivation is associated with decreased cell-mediated immunity (CMI),2 usually because of aging or immunosuppression.1,3
Hematopoietic cell transplant (HCT) recipients have profoundly diminished T-cell immunity, increasing their susceptibility to infectious diseases such as HZ. Accordingly, allogeneic and autologous HCT recipients have HZ rates of 15% to 30% during the first year after transplantation.4-8 In addition, HCT recipients are at increased risk for visceral dissemination during HZ.3,6 Long-term prophylaxis with antivirals such as acyclovir is partially effective against VZV reactivation after HCT; however, no standards exist on the dose or the duration of therapy, and HZ can occur, even at increased incidence, after discontinuation.9-12
Vaccination offers an alternative to prophylaxis with antivirals. A live-attenuated HZ vaccine (Zostavax, Merck & Co, Inc., Whitehouse Station, NJ), containing a high dose of the varicella vaccine strain, is licensed for adults aged ≥50 years.3,13 In immunocompetent persons aged ≥60 years, this vaccine reduced HZ incidence by 51% and postherpetic neuralgia incidence, the most frequent complication of HZ, by 67%.14 However, live-attenuated vaccines are contraindicated for immunocompromised people, including those undergoing HCT, because of the potential to cause disease.3,12,15 A vaccine for the prevention of HZ in HCT recipients is not currently available. Two studies that evaluated heat-inactivated varicella vaccines for the prevention of HZ in adult HCT recipients showed that VZV-specific CMI increased after vaccination and suggested that vaccination could prevent HZ in HCT recipients.16,17
Recombinant subunit vaccines are an alternative to live-attenuated vaccines for immunocompromised individuals because they circumvent the risk of vaccine-induced disease.18 VZV glycoprotein E (gE) is an attractive candidate antigen because it is the most abundant glycoprotein in VZV viral particles and infected cells19,20; plays a central role in virus infectivity, cell-to-cell spread, and the progression of infection20-22; and is the main target of VZV-specific CD4+ T-cell responses.19,23-26 A previous study showed that an adjuvanted gE subunit vaccine candidate was well tolerated and more immunogenic than a live-attenuated VZV vaccine in young (18-30 years) and older (50-70 years) immunocompetent adults.27 Here, we describe the results of a phase 1/2 clinical trial examining the safety and immunogenicity of this adjuvanted gE subunit candidate vaccine in adult autologous HCT recipients.
Patients and methods
Study design and subjects
This was a phase 1/2a, randomized, observer-blind, placebo-controlled, multicenter study performed in the United States. The study was approved by site-associated institutional review boards and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all subjects before enrollment.
Subjects aged ≥18 years were eligible if they had multiple myeloma, non-Hodgkin lymphoma (B- or T-cell), Hodgkin lymphoma, or acute myeloid leukemia, and had undergone autologous HCT in the previous 50 to 70 days. Subjects born in 1980 or later and subjects born in a tropical or subtropical region outside the United States before 1980 had to have serological evidence of VZV infection. Women had to be of non–child-bearing potential. Subjects were excluded if they had previously received a VZV or HZ vaccine, had a HZ history within the previous 12 months, had known exposure to VZV since transplantation, had received immunoglobulins or vaccinations (other than inactivated influenza vaccine) since transplantation, had received an investigational product within 30 days or were to receive one during the study period, had any contraindications to vaccination such as allergies, or had acute infection at enrollment.
Randomization and blinding
A randomization list was generated using a standard SAS (SAS Institute, Cary, NC) program with random blocks of four and stratification by site. Treatment allocation (1:1:1:1) was determined using a central Internet-based computerized randomization system. The randomization algorithm used a minimization procedure to account for subjects’ underlying disease. Subjects, investigators, and study team members were blinded to treatment allocation until the last patient completed the active study phase (month 4), and subjects remained blinded thereafter.
Procedures
At study months 0, 1, and 3 (ie, 2, 3, and 5 months after HCT), subjects received 3 doses of 50 μg VZV gE combined with the liposome-based adjuvant AS01B (50 µg monophosphoryl lipid A [MPL], 50 µg Quillaja saponaria Molina 21 [QS21]; Antigenics Inc., a wholly owned subsidiary of Agenus Inc., Lexington, MA)28; 3 doses of 50 µg gE combined with the liposome-based adjuvant AS01E (25 µg MPL, 25 µg QS21); 1 dose of saline followed by 2 doses of gE/AS01B; or 3 doses of saline (all administered by intramuscular injection in the deltoid area of the nondominant arm) (Table 1). Blood samples were collected at months 0 (prevaccination), 1, 2, 3, 4, and 15 for immunogenicity analyses.
Table 1.
Vaccine formulations and schedules
| Vaccine name | Antigen | Adjuvant | Schedule |
|---|---|---|---|
| gE/AS01B | VZV gE (50 µg) | AS01B liposome-based adjuvant: 50 µg monophosphoryl lipid A (MPL) and 50 µg Quillaja saponaria Molina 21 (QS21) | 3 doses at months 0, 1, and 3; or 2 doses at months 1 and 3 (1 dose of saline at month 0) |
| gE/AS01E | VZV gE (50 µg) | AS01E liposome-based adjuvant: 25 µg monophosphoryl lipid A (MPL) and 25 µg Quillaja saponaria Molina 21 (QS21) | 3 doses at months 0, 1, and 3 |
| Saline (placebo) | None | None | 3 doses at months 0, 1, and 3 |
AS, adjuvant system; gE, glycoprotein E; VZV, varicella-zoster virus.
Reactogenicity and safety assessments
Subjects reported solicited local reactions (pain, redness, and swelling at the injection site) and general reactions (fever, headache, fatigue, myalgia, and gastrointestinal symptoms) on diary cards for 7 days after each vaccination. Intensity of the solicited reactions was scored on a 0 to 3 rating scale (supplemental Table 1). Unsolicited adverse events (AEs) were reported from the study start to 30 days after the last vaccination. Recurrence of underlying malignancy, SAEs, and new onset of autoimmune diseases and other immune-mediated inflammatory disorders were recorded throughout the study. Causality was assessed by the investigator, except for solicited local reactions, which were all considered vaccination related. At the start of study, subjects were educated to recognize the signs and symptoms of HZ. They were to contact the study physician immediately for clinical evaluation and sample collection for VZV-specific polymerase chain reaction if they suspected HZ.
Serum anti-gE antibody concentrations
Serum anti-gE antibody concentrations were measured by noncommercial enzyme-linked immunosorbent assay with a cutoff of 18 mIU/mL. A subject with antibody concentration greater than or equal to the cutoff value was considered seropositive.
Intracellular cytokine staining
Frequencies of gE- and VZV-specific CD4+ and CD8+ T cells expressing at least 2 activation markers among interferon-γ, interleukin-2, tumor necrosis factor-α, and CD40 ligand, hereafter referred to as CD4(2+) and CD8(2+) cells, respectively, were measured after induction with gE or a whole inactivated lysate of VZV Oka (Varilrix; GlaxoSmithKline, Rixensart, Belgium) by intracellular cytokine staining followed by flow cytometry as previously described.27
Statistical analysis
Statistical analyses were performed using SAS version 9.2. The coprimary objectives of this study were (1) to evaluate the safety and reactogenicity of gE/AS01B and gE/AS01E in adult autologous HCT recipients and (2) to compare gE-specific humoral and cellular immune responses at month 4 (1 month post–final vaccination) between subjects who received 3 doses of gE/AS01B, 3 doses of gE/AS01E, 2 doses of gE/AS01B (at months 1 and 3), and saline alone. Secondary objectives of the study included comparison of gE-specific humoral, and gE- and VZV-specific cellular immune responses at months 1, 2, 3, and 15 among subjects of the different groups and evaluation of the incidence, duration, and severity of HZ and its complications during the entire study period. All statistical analyses were done on the total vaccinated cohort (subjects who received at least 1 dose), except for immunogenicity analysis at month 15, where confirmed HZ cases were excluded.
The number and percentage of subjects reporting a given adverse event (AE), geometric mean concentrations (GMCs) of anti-gE antibodies, percentages of subjects with concentrations equal to or above the cutoff, and percentage of subjects with a humoral or cell-mediated immune response were calculated with exact 95% confidence interval (CI). The 95% CIs for the geometric mean frequency of CD4(2+) after induction with gE and VZV were adjusted for the frequency of CD4(2+) in media only and prevaccination conditions. A humoral vaccine response was defined as greater than or equal to a fourfold increase in antibody concentration in subjects who were seropositive before vaccination or a concentration greater than or equal to four times the cutoff value in subjects seronegative before vaccination. A CMI response was defined as at least a twofold increase in CD4(2+) T-cell frequency after induction with antigen over prevaccination.
A sample size of 30 patients per group was estimated to show a greater than or equal to twofold increase in the gE-specific frequency of CD4(2+) T cells in at least 1 gE/AS01B vaccine group compared with saline, with ∼94% power at a 1-sided 15% significance level after Dunnett P value adjustment for 2 comparisons (gE/AS01B 3-dose and 2-dose groups) vs control, and assuming a 25% mortality risk at month 4.
The type-one error for primary objective at interim (8.46% 1-sided) and final (11.92% 1-sided) analysis has been adjusted according to the Wang-Tsiatis δ-class29 using Addplan 5.0.2 software (AptivSolutions, Reston, VA).
A repeated measurement analysis of covariance model was used to assess the confirmatory primary and secondary objectives. A vaccine group was considered to have a significantly higher immune response than saline when the lower limit of the CI of the fold increase was >3 (anti-gE GMCs) or 2 (CD4[2+] frequency after induction with gE) after Dunnett adjustment of the significance level of 2 and 3 doses of gE/AS01B vs saline (76.16% CI). A joint analysis of CMI and humoral immunity was performed using multivariate repeated measurement analysis. The superiority of gE/AS01B over gE/AS01E was confirmed if the multivariate F test 1-sided P value was <.25. The superiority of 3 doses of gE/AS01B over 2 doses of gE/AS01B at month 4 was confirmed if the multivariate F test 1-sided P value was <.15.
Results
Demographics
Between July 14, 2009 and March 21, 2012, 121 subjects were enrolled at 10 study centers (Figure 1). One subject was not vaccinated because of acute illness on the day of first vaccination. Of the vaccinated subjects, 110 (91.7%) completed the study up to month 4 (active phase) and 98 (81.7%) completed the study up to month 15. Throughout the whole study period, the most common reason for withdrawal was recurrence of underlying malignancy (n = 6). Five additional withdrawals were attributed to a SAE.
Figure 1.
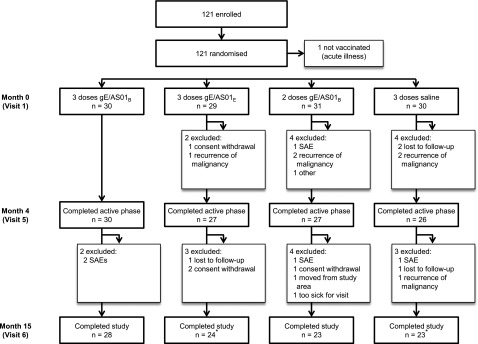
Subject disposition. gE/AS01B, glycoprotein E/liposome-based adjuvant (50 µg MPL, 50 µg QS21); gE/AS01E, glycoprotein E/liposome-based adjuvant (25 µg MPL, 25 µg QS21); SAEs, serious adverse events. *Four subjects (2 in the gE/AS01E and 2 in the saline groups) with confirmed HZ were included in the safety analysis but were excluded from the immunogenicity analyses at month 15.
The baseline characteristics were similar for the 4 vaccination groups (Table 2). Autologous HCT was used most frequently for treatment of myeloma (63.3%). Before HCT, most subjects (88.3%) had received myeloablative treatment without total body irradiation. Five (4.2%) had received total body irradiation. Most subjects (97.5%) received prophylactic antiviral therapy during the study.
Table 2.
Demographic and baseline characteristics
| Characteristics | 3 doses gE/AS01B (N = 30) | 3 doses gE/AS01E (N = 29) | 2 doses gE/AS01B (N = 31) | 3 doses saline (N = 30) | Total (N = 120) |
|---|---|---|---|---|---|
| Age, years | |||||
| Median (range) | 56.5 (20-70) | 58.0 (24-70) | 58.0 (42-68) | 59.5 (30-70) | 59.0 (20-70) |
| Sex, n (%) | |||||
| Male | 18 (60.0) | 20 (69.0) | 21 (67.7) | 19 (63.3) | 78 (65.0) |
| Ethnicity, n (%) | |||||
| White | 24 (80.0) | 24 (82.8) | 30 (96.8) | 22 (73.3) | 100 (83.3) |
| African/African American | 2 (6.7) | 2 (6.9) | 1 (3.2) | 1 (3.3) | 6 (5.0) |
| Asian | 1 (3.3) | 0 (0.0) | 0 (0.0) | 3 (10.0) | 4 (3.3) |
| Other | 3 (10.0) | 3 (10.3) | 0 (0.0) | 4 (13.3) | 10 (8.3) |
| Source of stem cells, n (%) | |||||
| Bone marrow | 3 (10.0) | 0 (0.0) | 2 (6.5) | 2 (6.7) | 7 (5.8) |
| Peripheral blood* | 27 (90.0) | 29 (100) | 29 (93.5) | 28 (93.3) | 113 (94.2) |
| Diagnosis leading to HCT, n (%) | |||||
| Myeloma | 18 (60.0) | 19 (65.5) | 20 (64.5) | 19 (63.3) | 76 (63.3) |
| Non-Hodgkin B-cell lymphoma | 8 (26.7) | 7 (24.1) | 7 (22.6) | 6 (20.0) | 28 (23.3) |
| Hodgkin lymphoma | 4 (13.3) | 2 (6.9) | 2 (6.5) | 1 (3.3) | 9 (7.5) |
| Non-Hodgkin T-cell lymphoma | 0 (0.0) | 1 (3.4) | 1 (3.2) | 4 (13.3) | 6 (5.0) |
| Acute myeloid leukemia | 0 (0.0) | 0 (0.0) | 1 (3.2) | 0 (0.0) | 1 (0.8) |
| Regimen before the current HCT, n (%) | |||||
| Myeloablative† | 28 (93.3) | 27 (93.1) | 28 (90.3) | 28 (93.3) | 111 (92.5) |
| Nonmyeloablative‡ | 2 (6.7) | 2 (6.9) | 3 (9.7) | 2 (6.7) | 9 (7.5) |
gE/AS01B, glycoprotein E/liposome-based adjuvant (50 µg MPL, 50 µg QS21); gE/AS01E, glycoprotein E/liposome-based adjuvant (25 µg MPL, 25 µg QS21); HCT, hematopoietic cell transplant.
Including apheresis.
With or without total body irradiation.
Including one patient who received lenalidomide, zoledronic acid, and plerixafor.
Reactogenicity and safety
Most subjects experienced solicited local and general reactions of mild or moderate intensity (Table 3 and supplemental Table 1). The most common local reaction was pain at the injection site. This reaction was more common in subjects receiving gE/AS01B (85.7%-90.0% per group) or gE/AS01E (75.9%) than in subjects receiving saline (13.3%-23.3%). A grade 3 pain level was reported only in subjects receiving gE/AS01 vaccines (3.6%-17.2%) and grade 3 redness or swelling was not reported by any subject. The most common general reactions were fatigue and myalgia. Myalgia was also the most frequent grade 3 general reaction and was most commonly related to the gE/AS01 vaccines (3.6%-26.7%). Vaccine-related grade 3 fever was reported for only 1 subject in the gE/AS01B 3-dose group and 1 subject in the gE/AS01B 2-dose group after the saline dose.
Table 3.
Percentage of solicited local and general symptoms reported during the 7-day postvaccination period overall by subject
| Symptom | Type | 3 doses gE/AS01B (N = 30) | 3 doses gE/AS01E (N = 29) | 2 doses gE/AS01B (N = 28) | 1 dose saline* (N = 30) | 3 doses saline (N = 30) |
|---|---|---|---|---|---|---|
| Local reactions, (%) 95% CI | ||||||
| Pain | Any | 90.0 (73.5-97.9) | 75.9 (56.5-89.7) | 85.7 (67.3-96.0) | 13.3 (3.8-30.7) | 23.3 (9.9-42.3) |
| Grade 3† | 10.0 (2.1-26.5) | 17.2 (5.8-35.8) | 3.6 (0.1-18.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Redness | Any | 20.0 (7.7-38.6) | 24.1 (10.3-43.5) | 25.0 (10.7-44.9) | 3.3 (0.1-17.2) | 3.3 (0.1-17.2) |
| Grade 3‡ | 0.0 (0.0-11.6) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Swelling | Any | 20.0 (7.7-38.6) | 13.8 (3.9-31.7) | 14.3 (4.0-32.7) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) |
| Grade 3‡ | 0.0 (0.0-11.6) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| General reactions, (%) 95% CI | ||||||
| Fatigue | Any | 60.0 (40.6-77.3) | 62.1 (42.3-79.3) | 71.4 (51.3-86.8) | 50.0 (31.3-68.7) | 33.3 (17.3-52.8) |
| Grade 3† | 13.3 (3.8-30.7) | 6.9 (0.8-22.8) | 3.6 (0.1-18.3) | 3.3 (0.1-17.2) | 3.3 (0.1-17.2) | |
| Grade 3 related | 10.0 (2.1-26.5) | 3.4 (0.1-17.8) | 3.6 (0.1-18.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Gastrointestinal§ | Any | 33.3 (17.3-52.8) | 24.1 (10.3-43.5) | 21.4 (8.3-41.0) | 30.0 (14.7-49.4) | 13.3 (3.8-30.7) |
| Grade 3† | 0.0 (0.0-11.6) | 0.0 (0.0-11.9) | 3.6 (0.1-18.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Grade 3 related | 0.0 (0.0-11.6) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Headache | Any | 43.3 (25.5-62.6) | 10.3 (2.2-27.4) | 32.1 (15.9-52.4) | 23.3 (9.9-42.3) | 20.0 (7.7-38.6) |
| Grade 3† | 10.0 (2.1-26.5) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Grade 3 related | 3.3 (0.1-17.2) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) | |
| Myalgia | Any | 70.0 (50.6-85.3) | 62.1 (42.3-79.3) | 78.6 (59.0-91.7) | 30.0 (14.7-49.4) | 26.7 (12.3-45.9) |
| Grade 3† | 26.7 (12.3-45.9) | 6.9 (0.8-22.8) | 3.6 (0.1-18.3) | 0.0 (0.0-11.6) | 3.3 (0.1-17.2) | |
| Grade 3 related | 26.7 (12.3-45.9) | 6.9 (0.8-22.8) | 3.6 (0.1-18.3) | 0.0 (0.0-11.6) | 3.3 (0.1-17.2) | |
| Fever | Any | 40.0 (22.7-59.4) | 27.6 (12.7-47.2) | 28.6 (13.2-48.7) | 23.3 (9.9-42.3) | 13.3 (3.8-30.7) |
| Grade 3‖ | 3.3 (0.1-17.2) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 6.7 (0.8-22.1) | 0.0 (0.0-11.6) | |
| Grade 3 related | 3.3 (0.1-17.2) | 0.0 (0.0-11.9) | 0.0 (0.0-12.3) | 3.3 (0.1-17.2) | 0.0 (0.0-11.6) | |
gE/AS01B, glycoprotein E/liposome-based adjuvant (50 µg MPL, 50 µg QS21); gE/AS01E, glycoprotein E/liposome-based adjuvant (25 µg MPL, 25 µg QS21); HCT, hematopoietic cell transplant.
Saline for dose 1 in the gE/AS01B 2-dose group.
Symptom that prevented normal activity.
Diameter >100 mm.
Nausea, vomiting, diarrhea, or abdominal pain.
Oral temperature >39.0°C.
Within 30 days of vaccination, 75.9% to 83.3% of subjects per gE/AS01 group and 51.6% to 70.0% of subjects receiving saline reported unsolicited AEs. Only 13.8% to 20.0% of subjects per gE/AS01 group and 6.5% to 13.3% of subjects receiving saline reported unsolicited AEs causally related to vaccination, without apparent differences between the groups. The most common vaccination-related unsolicited AE was chills (reported in 4 subjects after injection of gE/AS01 vaccines and in 1 subject after injection of saline in the gE/AS01B 2-dose group). Only 2 subjects reported a vaccination-related grade 3 unsolicited AE (both chills): one in the gE/AS01B 3-dose group and one after injection of saline in the gE/AS01B 2-dose group.
Through study month 15, 54 SAEs were reported in 33 subjects (6 in the gE/AS01B 3-dose group, 9 in the gE/AS01E 3-dose group, 10 in the gE/AS01B 2-dose group, and 8 in the saline group). One SAE was considered possibly related to vaccination by the investigator: pneumonia 105 days after a second dose of vaccine in the gE/AS01B 2-dose group. Underlying malignancy recurred in 32 subjects (6 in the gE/AS01B 3-dose group, 7 in the gE/AS01E 3-dose group, 11 in the gE/AS01B 2-dose group, and 8 in the saline group). Nine subjects died during the study: 2 from recurrence of underlying malignancy and 2 from unknown reasons. No deaths were considered vaccination related.
Four HZ cases were confirmed during the study: 2 in the gE/AS01E 3-dose group and 2 in the saline group. The 2 cases in the gE/AS01E group occurred after progression of the underlying malignancy (non-Hodgkin B-cell lymphoma and myeloma), whereas the 2 cases in the saline group were in patients with nonprogressive myeloma. No new onset of autoimmune diseases or other immune-mediated inflammatory disorders was reported.
Humoral immune response
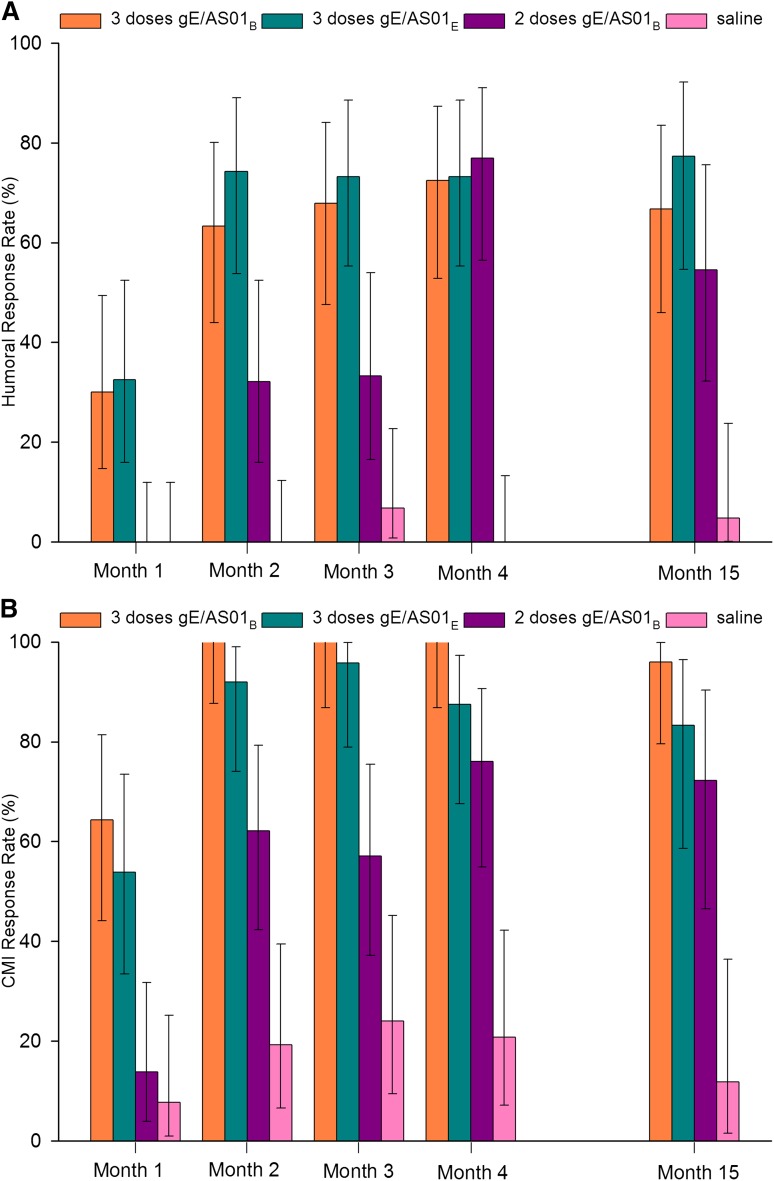
All subjects except one in the gE/AS01E 3-dose group were seropositive for anti-gE antibodies before vaccination, and all subjects were seropositive after the second vaccination (Table 4). One month after the final dose, anti-gE GMCs were higher in all gE/AS01 groups than in the saline group (all P < .0001). However, GMCs were not significantly higher in the gE/AS01B 3-dose group than in the gE/AS01E 3-dose group. GMCs peaked at month 4 in the 3 gE/AS01 groups and were 25- to 55-fold higher than before vaccination. These GMCs remained high for at least 1 year after the last vaccination, although they decreased 29% to 46% from month 4 to month 15. Approximately one-third of subjects had an anti-gE response after the first dose of gE/AS01B or gE/AS01E (Figure 2). At month 4, response rates were similar for the 3 vaccine groups (72.4%-76.9%). Also, in the 2 groups receiving 3 doses of gE/AS01B or gE/AS01E, response rates after the second and third doses were similar. At month 15, ≥54.5% of subjects in all gE/AS01 groups remained above the response rate threshold.
Table 4.
Seropositivity rate, geometric mean concentrations, and ratio above saline of anti-gE antibodies
| Group | Timing | N | Seropositivity rate (>18 mIU/mL), % (95% CI) | GMCs, % (95% CI) | N | Adjusted GMC fold increase over saline (95% CI) | P for the ratio over saline |
|---|---|---|---|---|---|---|---|
| 3 doses gE/AS01B | Month 0 (Pre) | 30 | 100 (88.4-100) | 531.2 (402.6-700.8) | — | — | — |
| Month 1 | 30 | 100 (88.4-100) | 1 489.7 (847.3-2 619.0) | 30 | 2.99 (1.73-5.16) | .0003 | |
| Month 2 | 30 | 100 (88.4-100) | 11 963.0 (4 739.4-30 196.6) | 30 | 26.66 (9.97-71.29) | <.0001 | |
| Month 3 | 28 | 100 (87.7-100) | 13 412.9 (4 967.1-36 219.7) | 28 | 26.87 (9.07-79.56) | <.0001 | |
| Month 4 | 29 | 100 (88.1-100) | 29 192.9 (11 004.7-77 442.1) | 29 | 74.41 (25.74-215.09) | <.0001 | |
| Month 15 | 27 | 100 (87.2-100) | 8 052.7 (3 333.4-19 453.7) | 27 | 28.70 (10.92-75.46) | <.0001 | |
| 3 doses gE/AS01E | Month 0 (Pre) | 28 | 96.4 (81.7-99.9) | 536.2 (304.0-945.7) | — | — | — |
| Month 1 | 28 | 96.4 (81.7-99.9) | 1 288.1 (684.9-2 422.5) | 28 | 2.53 (1.6-23.94) | .0002 | |
| Month 2 | 28 | 100 (87.7-100) | 11 628.8 (5 196.6-26 022.8) | 27 | 22.26 (9.03-54.91) | <.0001 | |
| Month 3 | 27 | 100 (87.2-100) | 13 385.4 (5 506.5-32 537.7) | 26 | 20.51 (7.14-58.90) | <.0001 | |
| Month 4 | 26 | 100 (86.8-100) | 22 503.0 (8 432.7-60 050.2) | 26 | 52.11 (17.36-156.41) | <.0001 | |
| Month 15 | 22 | 100 (84.6-100) | 9 165.5 (3 796.0-22 130.1) | 22 | 21.98 (7.87-61.37) | <.0001 | |
| 2 doses gE/AS01B | Month 0 (Pre) | 30 | 100 (88.4-100) | 448.0 (289.7-692.8) | — | — | — |
| Month 1 | 30 | 100 (88.4-100) | 376.1 (239.7-590.1) | 29 | 0.96 (0.80-1.14) | .6240 | |
| Month 2 | 29 | 100 (88.1-100) | 1 047.8 (564.8-1 943.9) | 28 | 2.94 (1.78-4.85) | <.0001 | |
| Month 3 | 28 | 100 (87.7-100) | 972.6 (520.6-1 817.2) | 27 | 2.31 (1.20-4.42) | .0126 | |
| Month 4 | 27 | 100 (87.2-100) | 11 064.6 (4 361.2-28 071.8) | 26 | 42.20 (16.07-110.82) | <.0001 | |
| Month 15 | 23 | 100 (85.2-100) | 2 968.0 (1 184.7-7 436.1) | 22 | 8.81 (3.41-22.80) | <.0001 | |
| 3 doses saline | Month 0 (Pre) | 30 | 100 (88.4-100) | 659.9 (470.5-925.4) | — | — | — |
| Month 1 | 29 | 100 (88.1-100) | 619.3 (424.2-904.0) | 29 | — | — | |
| Month 2 | 28 | 100 (87.7-100) | 520.2 (349.3-774.8) | 28 | — | — | |
| Month 3 | 29 | 100 (88.1-100) | 585.2 (310.0-1 104.9) | 29 | — | — | |
| Month 4 | 26 | 100 (86.8-100) | 406.0 (244.2-674.9) | 26 | — | — | |
| Month 15 | 21 | 100 (83.9-100) | 362.3 (170.7-768.7) | 21 | — | — |
Analysis was done on the total vaccinated cohort at months 0 to 4 and on the total vaccinated cohort excluding 4 HZ cases at month 15.
gE/AS01B, glycoprotein E/liposome-based adjuvant (50 µg MPL, 50 µg QS21); gE/AS01E, glycoprotein E/liposome-based adjuvant (25 µg MPL, 25 µg QS21); GMC, geometric mean concentration.
Figure 2.
Antiglycoprotein E humoral and cell-mediated immune response rates. Shown are the percentages of subjects with humoral (A) and cell-mediated (B) immune responses against VZV glycoprotein E. A humoral response was defined as a greater than or equal to fourfold increase in GMC in subjects who were seropositive before vaccination or a GMC ≥4 times the cutoff value in subjects seronegative before vaccination. A cell-mediated immune response was defined as a greater than or equal to twofold increase in CD4(2+) T-cell frequency over prevaccination. Bars indicate means, and errors bars indicate 95% confidence intervals. gE/AS01B indicates glycoprotein E/liposome-based adjuvant (50 µg MPL, 50 µg QS21); and gE/AS01E, glycoprotein E/liposome-based adjuvant (25 µg MPL, 25 µg QS21).
We also analyzed the humoral responses by underlying malignancy. Anti-gE GMCs after vaccination with gE/AS01B or gE/AS01E did not increase in subjects with non-Hodgkin B-cell lymphoma, although the GMCs increased for all other diagnoses (supplemental Table 2).
Cell-mediated immune response
At month 4, gE-specific CMI, as measured by the CD4(2+) T-cell frequency after induction with gE, was significantly higher for all gE/AS01 groups than for saline (all P < .0001; Table 5). The CD4(2+) T-cell frequencies in the gE/AS01B 3-dose and 2-dose groups were superior to that of the saline group (data not shown). One month after the second dose of gE/AS01B, the CD4(2+) T-cell frequency was 2.3-fold higher after early vaccination (2 and 3 months post-HCT) than after delayed vaccination (3 and 5 months post-HCT; P = .0042). The CD4(2+) T-cell frequencies were not significantly higher in the gE/AS01B 3-dose group than in the gE/AS01E 3-dose group at any postvaccination time point.
Table 5.
Frequency of CD4(2+) T cells after induction with gE and fold increase over saline
| Descriptive statistics | Adjusted geometric mean frequency CD4(2+) | Adjusted fold increase over saline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Timing | N | Q1 | Median | Q3 | N | Value | 95% CI | Value | 95% CI | P |
| 3 doses gE/AS01B | Month 0 (Pre) | 28 | 121.9 | 197.8 | 324.5 | — | — | — | — | — | — |
| Month 1 | 30 | 225.5 | 626.3 | 981.9 | 28 | 588.70 | 434.77-797.13 | 2.75 | 1.80-4.20 | <.0001 | |
| Month 2 | 30 | 2717.6 | 5705.5 | 9 430.9 | 27 | 5131.29 | 3 461.04-7 607.57 | 20.70 | 11.94-35.91 | <.0001 | |
| Month 3 | 28 | 1750.8 | 4377.1 | 10 561.5 | 26 | 4233.37 | 2 977.64-6 018.66 | 16.65 | 10.17-27.25 | <.0001 | |
| Month 4 | 28 | 3888.7 | 7934.2 | 14 416.3 | 25 | 7540.57 | 4 960.60-11 462.36 | 32.31 | 17.78-58.71 | <.0001 | |
| Month 15 | 26 | 2308.8 | 4772.2 | 10 038.1 | 25 | 4104.59 | 2 758.10-6 108.44 | 15.15 | 8.33-27.54 | <.0001 | |
| 3 doses gE/AS01E | Month 0 (Pre) | 27 | 117.1 | 253.6 | 535.7 | — | — | — | — | — | — |
| Month 1 | 29 | 230.2 | 584.8 | 1 268.9 | 27 | 690.55 | 462.24-1 031.63 | 2.65 | 1.58-4.44 | .0004 | |
| Month 2 | 28 | 1829.5 | 3085.5 | 12 615.0 | 24 | 3409.24 | 2 067.87-5 620.73 | 11.54 | 6.01-22.14 | <.0001 | |
| Month 3 | 27 | 1608.6 | 3656.9 | 11 121.2 | 22 | 3602.31 | 2 290.50-5 665.41 | 11.74 | 6.55-21.02 | <.0001 | |
| Month 4 | 27 | 1801.5 | 5490.3 | 10 638.3 | 24 | 4510.41 | 2 801.47-7 261.83 | 16.49 | 8.87-30.68 | <.0001 | |
| Month 15 | 18 | 2127.2 | 3418.6 | 10 095.1 | 18 | 3193.39 | 2 119.71-4 810.91 | 11.39 | 6.68-19.42 | <.0001 | |
| 2 doses gE/AS01B | Month 0 (Pre) | 31 | 166.2 | 300.1 | 432.1 | — | — | — | — | — | — |
| Month 1 | 29 | 134.2 | 234.1 | 354.1 | 28 | 200.58 | 148.07-271.72 | 0.94 | 0.62-1.42 | .7586 | |
| Month 2 | 29 | 389.3 | 725.7 | 1 265.8 | 27 | 644.05 | 434.39-954.89 | 2.60 | 1.51-4.46 | .0007 | |
| Month 3 | 28 | 282.6 | 629.7 | 1 352.9 | 25 | 512.61 | 359.56-730.81 | 2.02 | 1.24-3.27 | .0050 | |
| Month 4 | 24 | 755.4 | 1990.8 | 7 223.2 | 22 | 2220.09 | 1 437.96-3 427.65 | 9.51 | 5.23-17.32 | <.0001 | |
| Month 15 | 18 | 946.1 | 2297.6 | 5 442.5 | 18 | 1363.51 | 886.13-2 098.06 | 5.03 | 2.74-9.26 | <.0001 | |
| 3 doses saline | Month 0 (Pre) | 29 | 229.8 | 356.6 | 693.7 | — | — | — | — | — | — |
| Month 1 | 29 | 178.2 | 340.9 | 594.6 | 25 | 213.99 | 156.04-293.48 | — | — | — | |
| Month 2 | 28 | 195.0 | 318.7 | 494.9 | 26 | 247.85 | 167.47-366.82 | — | — | — | |
| Month 3 | 28 | 176.8 | 394.8 | 573.3 | 24 | 254.30 | 178.44-362.41 | — | — | — | |
| Month 4 | 26 | 116.1 | 246.2 | 621.2 | 22 | 233.39 | 151.95-358.48 | — | — | — | |
| Month 15 | 18 | 221.0 | 292.5 | 389.4 | 17 | 270.97 | 173.08-424.22 | — | — | — | |
Analysis was done on the total vaccinated cohort at months 0 to 4 and on the total vaccinated cohort excluding 4 HZ cases at month 15.
CD4(2+), gE-specific CD4+ T cells expressing at least 2 activation markers among interferon-γ, interleukin-2, tumor necrosis factor-α, and CD40 ligand per 106 cells after induction with gE; gE/AS01B, glycoprotein E/liposome-based adjuvant (50 µg MPL, 50 µg QS21); gE/AS01E, glycoprotein E/liposome-based adjuvant (25 µg MPL, 25 µg QS21); Q1, first quartile; Q3, third quartile.
The highest CMI response rate after induction with gE was in the gE/AS01B 3-dose group, with 100% subjects having a CMI response starting at month 2 (Figure 2). This rate reached 75.0% at month 4 in the gE/AS01B 2-dose group and 84.0% in the gE/AS01E 3-dose group. In the 3 vaccine groups, the CMI response persisted up to month 15. There were no differences in CMI responses according to underlying disease (supplemental Table 3). Activation of gE-specific CD8+ T cells was not observed (data not shown).
VZV-specific CMI, as measured by the CD4(2+) T-cell frequency after induction with VZV, was significantly higher for all gE/AS01 groups than for saline at month 4, and persisted to month 15. In the adjuvanted vaccine groups, mean VZV-specific CMI values were lower than the corresponding gE-specific CMI values at all time points (data not shown).
Combined vaccine humoral and cellular responses
Multivariate analysis showed that the combined humoral and cellular response was superior in the gE/AS01B 3-dose group than in the gE/AS01B 2-dose group from month 1 to month 4 (all P < .15). Furthermore, the gE/AS01B 3-dose group was superior to the gE/AS01E 3-dose group at month 4 for gE-specific responses (P < .25) (data not shown).
Discussion
In this study, we examined the safety and immunogenicity profiles of 2 formulations of an adjuvanted gE subunit candidate vaccine in adult autologous HCT recipients. We found that both gE/AS01 formulations and both schedules were well tolerated and immunogenic in this population.
Clinical studies using heat-inactivated varicella vaccine suggested that immunity, especially cell-mediated immunity, can be induced by vaccination in highly immunocompromised subjects.16,17 Our study showed that gE/AS01B and gE/AS01E were immunogenic even if given soon after HCT, and immune responses persisted up to 1 year, which offers the possibility of early and sustained HZ protection in this highly susceptible population. The levels of anti-gE GMCs and frequencies of CD4(2+) T cells were comparable with those in immunocompetent adults ≥50 years of age immunized with 2 doses of gE/AS01B.26 In that study, the levels of humoral and cellular immunity achieved with gE/AS01B were higher than those achieved with a live-attenuated VZV vaccine.27
gE-specific humoral and cellular immune responses were higher in the gE/AS01B 3-dose group than in the gE/AS01B 2-dose group at all time points; however, within the 3-dose group, the additional increase after the third dose was modest. When only 2 doses of gE/AS01B were given, the 0/1-month schedule (2 and 3 months after HCT) elicited higher CD4(2+) frequencies than the 1/3-month schedule (3 and 5 months after HCT) (Table 5). Interestingly, even the lower 2-dose responses induced by the 1/3-month schedule were similar to those seen in healthy older adults.27
Although gE-specific GMCs and CD4(2+) T-cell frequencies were not statistically different between gE/AS01B and gE/AS01E, gE/AS01B tended to be more immunogenic than gE/AS01E. Similar results were observed in a previous study in immunocompetent older adults.30 This suggests that a higher adjuvant dose improves immunogenicity.
Finally, the immune responses differed according to the underlying malignancy. Anti-gE antibody concentrations and humoral response rates were lower in subjects with non-Hodgkin B-cell lymphoma than in subjects with other malignancies. This is likely caused by B-cell depletion induced by the anti-CD20 monoclonal antibody rituximab, which is typically given to these patients before HCT as part of their initial chemotherapeutic regimen and during salvage therapy immediately before HCT.31,32 In addition, the initial and salvage regimens frequently contain chemotherapeutic agents such as fludarabine and bendamustine, which profoundly deplete lymphocytes. In contrast, the strong humoral response to the vaccine in patients with multiple myeloma may be a result of HCT being performed earlier in the course of the disease before extensive pretreatment with immunosuppressive agents.33,34 Although many of these patients have low normal immunoglobulin concentrations at diagnosis, the concentrations have often improved at the time of HCT because of remission induced by their initial therapy.
In the absence of an established immunologic correlate of protection for HZ, especially in immunocompromised subjects, the threshold of a twofold increase for determining CMI response was empirical. Correlation between the immune responses described in this study and the level of clinical protection against HZ should not be assumed. Also, immunogenicity was analyzed only up to one year after the final vaccination. However, because most HZ cases occur within 1 to 2 years after HCT,7,35 even short-term immune responses may provide a substantial benefit to this highly immunocompromised population.
Because the candidate vaccine does not contain any live virus, it can be given early after HCT, in contrast to live-attenuated vaccines.12,36,37 Although most patients reported AEs, most reactions were of mild to moderate intensity. Few grade 3 AEs and SAEs were reported, and there were no major differences between groups. Furthermore, immunization was not associated with increased transplant failure (or recurrence of malignancy) incidence. These results suggest that the HZ vaccine candidate may have a favorable benefit:risk ratio for these patients.
In conclusion, our study showed that the adjuvanted gE subunit vaccine candidate was immunogenic even if given shortly after HCT. Both humoral and cellular immune responses were strong and persisted for up to 1 year after final vaccination. Furthermore, both gE/AS01 vaccine formulations and both schedules had clinically acceptable safety profiles. Thus, immunization against HZ using an adjuvanted gE subunit vaccine candidate may be a suitable strategy to reduce HZ burden in adult autologous HCT recipients.
Acknowledgments
The authors thank Dr Mindy Schuster for her contributions to the trial. Figures were developed by Ashmita Ravishankar (GlaxoSmithKline Vaccines). Medical writing assistance was provided by Dr Julie Harriague (4Clinics, France), and manuscript coordination was provided by Dr Jarno Jansen (Keyrus Biopharma) on behalf of GlaxoSmithKline Vaccines.
This work was supported by GlaxoSmithKline Vaccines.
Footnotes
Presented in part at the 1st IDWeek, San Diego, CA, in October 2012; at the 2013 Bone Marrow Transplant Tandem Meetings, Salt Lake City, UT, in February 2013; and at the 38th Annual International Herpesvirus Workshop, Grand Rapids, MI, in July 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.C.H. and E.M.B. contributed to the study design and protocol writing; E.A.S., K.M.S., F.M.M., S.S.D., G.A.P., T.C.S., S.B.M., C.A., J.H.Y., and F.K.B. contributed to data collection; M.E.I. performed the statistical analyses; and all authors contributed to data analysis and interpretation, the writing or reviewing of the manuscript, and approving the final version. Medical writing assistance was used in the preparation of this manuscript.
Conflict-of-interest disclosure: E.A.S., K.M.S., F.M.M., S.S.D., G.A.P., T.C.S., S.B.M., C.A., J.H.Y., and F.K.B. received grant support from GlaxoSmithKline for this study. E.A.S. and K.M.S. received support for travel for the study from GlaxoSmithKline. E.A.S., K.M.S., F.M.M., and S.B.M. received compensation from GlaxoSmithKline for consultancy activities. F.M.M., G.A.P., and S.B.M. received consultancy fees from Chimerix. F.M.M. and T.C.S. have grants pending from GlaxoSmithKline. F.M.M. has grants pending from Chimerix. S.S.D. received a grant from Ansun Biopharma. S.B.M. received research funding from Roche and Chimerix. M.E.I., T.C.H., and E.M.B. are employees of GlaxoSmithKline Vaccines. T.C.H. and E.M.B. receive stock equity in GlaxoSmithKline.
Correspondence: Edward A. Stadtmauer, Abramson Cancer Center, 2 West Perelman Center for Advanced Medicine, 34th and Civic Center Bldg, Philadelphia, PA 19104; e-mail: stadtmae@uphs.upenn.edu.
References
- 1.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg A, Zhang JH, Oxman MN, et al. US Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200(7):1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5) 1-30; quiz CE32-CE34. [PubMed] [Google Scholar]
- 4.Arvin AM. Varicella-Zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2000;6(3):219–230. doi: 10.1016/s1083-8791(00)70004-8. [DOI] [PubMed] [Google Scholar]
- 5.Leung TF, Chik KW, Li CK, et al. Incidence, risk factors and outcome of varicella-zoster virus infection in children after haematopoietic stem cell transplantation. Bone Marrow Transplant. 2000;25(2):167–172. doi: 10.1038/sj.bmt.1702119. [DOI] [PubMed] [Google Scholar]
- 6.Locksley RM, Flournoy N, Sullivan KM, Meyers JD. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152(6):1172–1181. doi: 10.1093/infdis/152.6.1172. [DOI] [PubMed] [Google Scholar]
- 7.Schuchter LM, Wingard JR, Piantadosi S, Burns WH, Santos GW, Saral R. Herpes zoster infection after autologous bone marrow transplantation. Blood. 1989;74(4):1424–1427. [PubMed] [Google Scholar]
- 8.Rogers JE, Cumpston A, Newton M, Craig M. Onset and complications of varicella zoster reactivation in the autologous hematopoietic cell transplant population. Transpl Infect Dis. 2011;13(5):480–484. doi: 10.1111/j.1399-3062.2011.00655.x. [DOI] [PubMed] [Google Scholar]
- 9.Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110(8):3071–3077. doi: 10.1182/blood-2007-03-077644. [DOI] [PubMed] [Google Scholar]
- 10.Sempere A, Sanz GF, Senent L, et al. Long-term acyclovir prophylaxis for prevention of varicella zoster virus infection after autologous blood stem cell transplantation in patients with acute leukemia. Bone Marrow Transplant. 1992;10(6):495–498. [PubMed] [Google Scholar]
- 11.Steer CB, Szer J, Sasadeusz J, Matthews JP, Beresford JA, Grigg A. Varicella-zoster infection after allogeneic bone marrow transplantation: incidence, risk factors and prevention with low-dose aciclovir and ganciclovir. Bone Marrow Transplant. 2000;25(6):657–664. doi: 10.1038/sj.bmt.1702190. [DOI] [PubMed] [Google Scholar]
- 12.Tomblyn M, Chiller T, Einsele H, et al. Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60(44):1528. [PubMed] [Google Scholar]
- 14.Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 15.Sartori AM. A review of the varicella vaccine in immunocompromised individuals. Int J Infect Dis. 2004;8(5):259–270. doi: 10.1016/j.ijid.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347(1):26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 17.Redman RL, Nader S, Zerboni L, et al. Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis. 1997;176(3):578–585. doi: 10.1086/514077. [DOI] [PubMed] [Google Scholar]
- 18.Clark TG, Cassidy-Hanley D. Recombinant subunit vaccines: potentials and constraints. Dev Biol (Basel) 2005;121:153–163. [PubMed] [Google Scholar]
- 19.Arvin AM. Immune responses to varicella-zoster virus. Infect Dis Clin North Am. 1996;10(3):529–570. doi: 10.1016/s0891-5520(05)70312-3. [DOI] [PubMed] [Google Scholar]
- 20.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 21.Ali MA, Li Q, Fischer ER, Cohen JI. The insulin degrading enzyme binding domain of varicella-zoster virus (VZV) glycoprotein E is important for cell-to-cell spread and VZV infectivity, while a glycoprotein I binding domain is essential for infection. Virology. 2009;386(2):270–279. doi: 10.1016/j.virol.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berarducci B, Rajamani J, Zerboni L, Che X, Sommer M, Arvin AM. Functions of the unique N-terminal region of glycoprotein E in the pathogenesis of varicella-zoster virus infection. Proc Natl Acad Sci USA. 2010;107(1):282–287. doi: 10.1073/pnas.0912373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arvin AM, Kinney-Thomas E, Shriver K, et al. Immunity to varicella-zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. J Immunol. 1986;137(4):1346–1351. [PubMed] [Google Scholar]
- 24.Brunell PA, Novelli VM, Keller PM, Ellis RW. Antibodies to the three major glycoproteins of varicella-zoster virus: search for the relevant host immune response. J Infect Dis. 1987;156(3):430–435. doi: 10.1093/infdis/156.3.430. [DOI] [PubMed] [Google Scholar]
- 25.Harper DR, Kangro HO, Heath RB. Antibody responses in recipients of varicella vaccine assayed by immunoblotting. J Med Virol. 1990;30(1):61–67. doi: 10.1002/jmv.1890300114. [DOI] [PubMed] [Google Scholar]
- 26.Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol. 2008;152(3):522–531. doi: 10.1111/j.1365-2249.2008.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroux-Roels I, Leroux-Roels G, Clement F, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis. 2012;206(8):1280–1290. doi: 10.1093/infdis/jis497. [DOI] [PubMed] [Google Scholar]
- 28.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10(4):471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 29.Wang SK, Tsiatis AA. Approximately optimal one-parameter boundaries for group sequential trials. Biometrics. 1987;43(1):193–199. [PubMed] [Google Scholar]
- 30.Chlibek R, Bayas JM, Collins H, et al. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis. 2013;208(12):1953–1961. doi: 10.1093/infdis/jit365. [DOI] [PubMed] [Google Scholar]
- 31.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 32.Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26(25):3603–3613. doi: 10.1038/sj.onc.1210376. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S. Stem cell transplantation for multiple myeloma. Curr Opin Oncol. 2009;21(2):162–170. doi: 10.1097/CCO.0b013e328324bc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23(3):449–456. doi: 10.1038/leu.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offidani M, Corvatta L, Olivieri A, et al. A predictive model of varicella-zoster virus infection after autologous peripheral blood progenitor cell transplantation. Clin Infect Dis. 2001;32(10):1414–1422. doi: 10.1086/320157. [DOI] [PubMed] [Google Scholar]
- 36.Ljungman P, Cordonnier C, Einsele H, et al. Center for International Blood and Marrow Transplant Research; National Marrow Donor Program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Disease Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Diseases Canada; Centers for Disease Control and Prevention. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44(8):521–526. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 37.Naidus E, Damon L, Schwartz BS, Breed C, Liu C. Experience with use of Zostavax(®) in patients with hematologic malignancy and hematopoietic cell transplant recipients. Am J Hematol. 2012;87(1):123–125. doi: 10.1002/ajh.22196. [DOI] [PubMed] [Google Scholar]