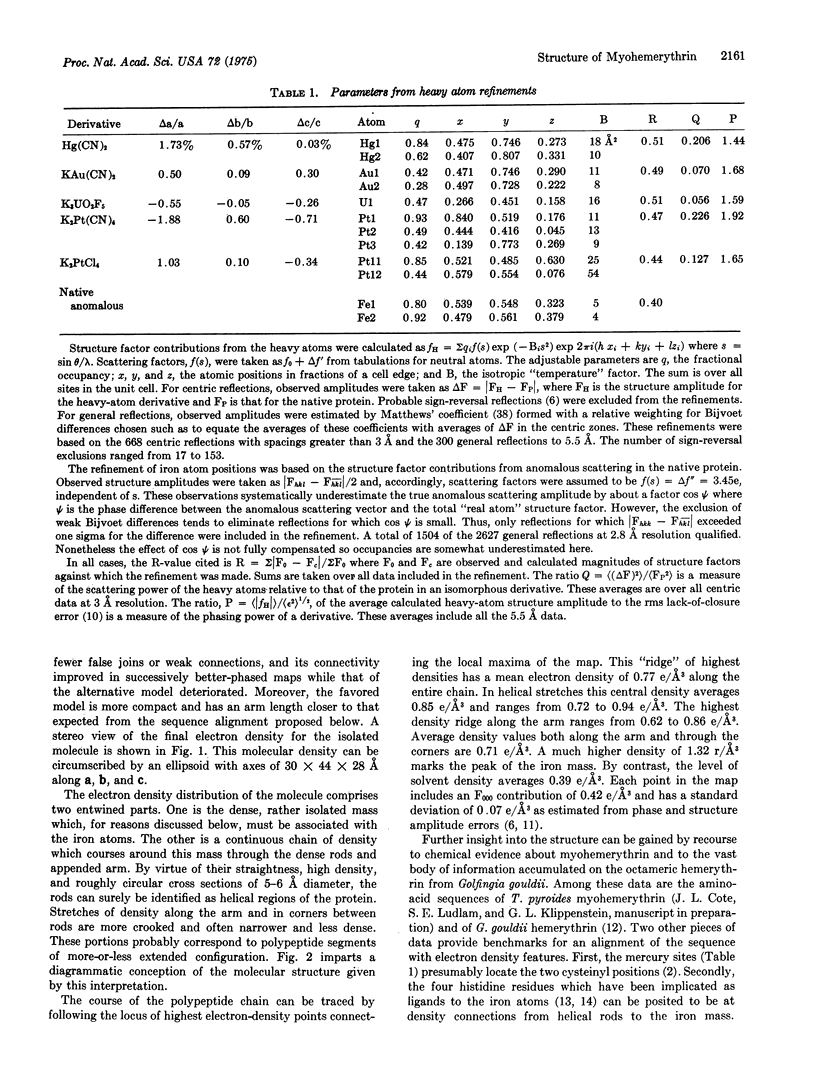
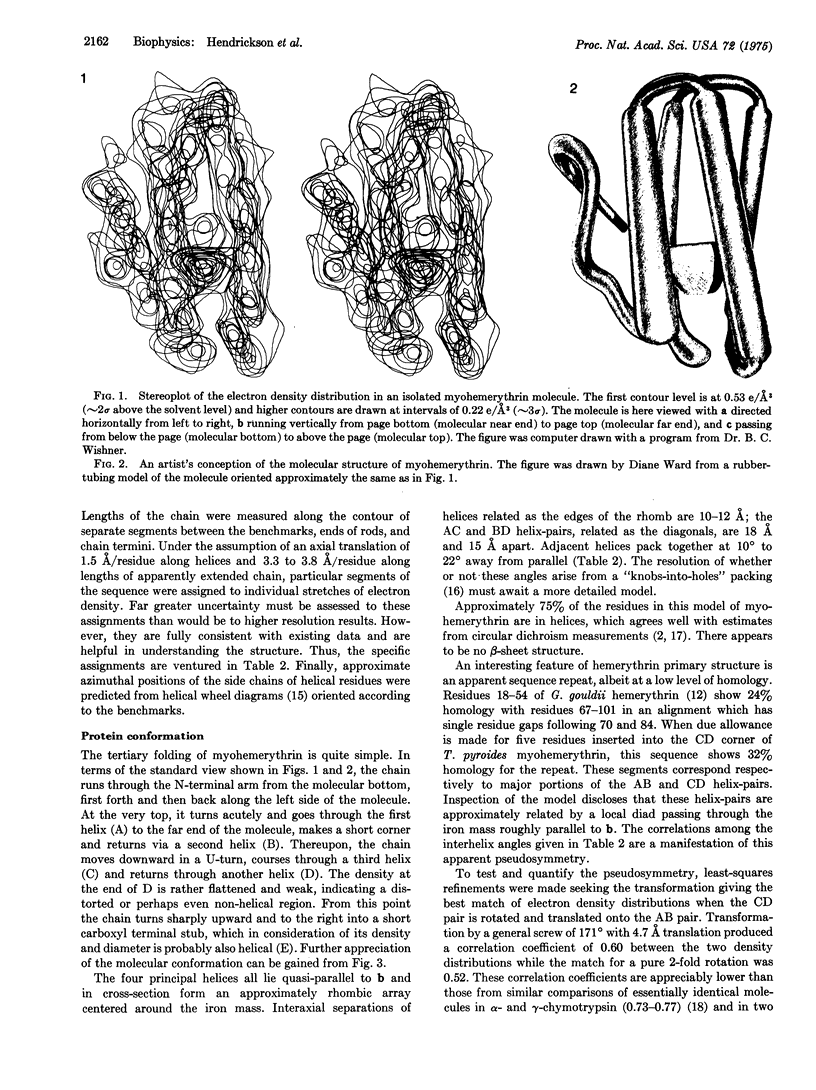
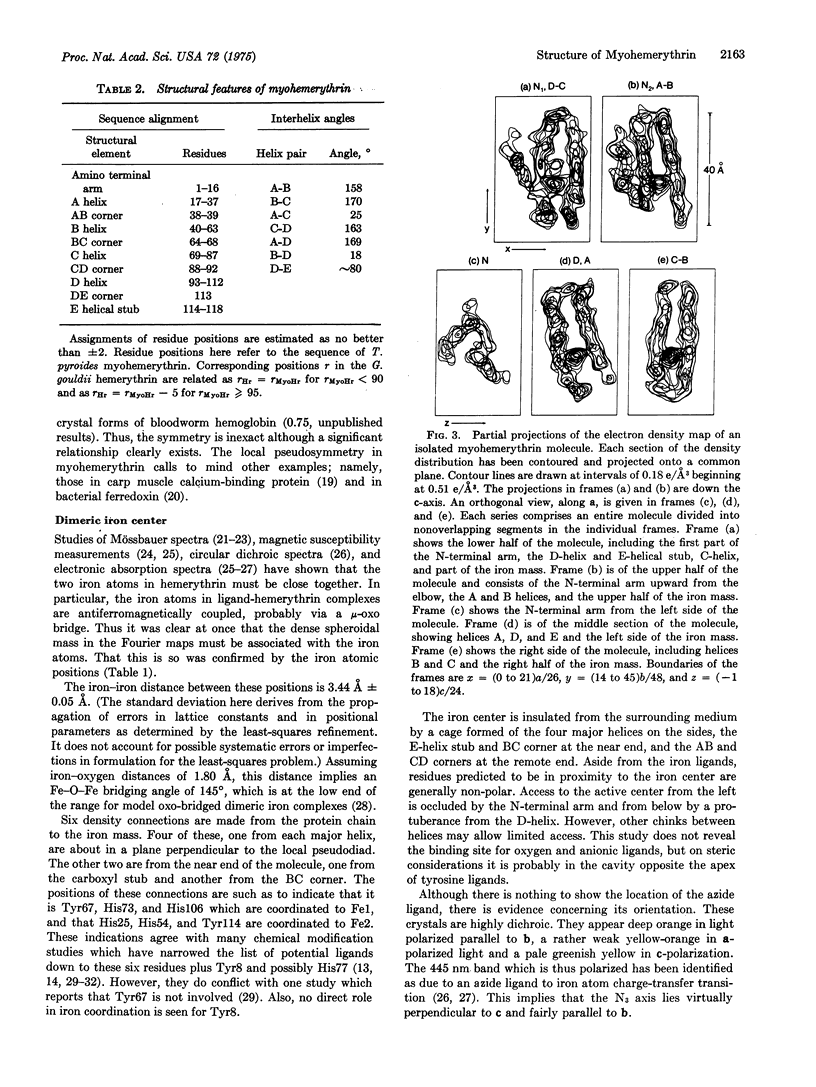
Abstract
X-ray diffraction studies have produced a low resolution image and also located the iron atoms of a monomeric hemerythrin from muscles of a sipunculan worm. These results reveal the course of the polypeptide chain and some details of the active center.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Cohen G. H., Matthews B. W., Davies D. R. The relation between gamma-and alpha-chymotrypsin. II. Direct comparison of the electron densities at 5-5 Angstrom resolution. Acta Crystallogr B. 1970 Aug 15;26(8):1062–1069. doi: 10.1107/s056774087000362x. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Garbett K., Klotz I. M., Aktipis S., Keresztes-Nagy S. Optical rotatory properties of hemerythrin in the ultraviolet range. Arch Biochem Biophys. 1969 Aug;133(1):103–107. doi: 10.1016/0003-9861(69)90492-5. [DOI] [PubMed] [Google Scholar]
- Dawson J. W., Gray H. B., Hoenig H. E., Rossman G. R., Schredder J. M., Wang R. H. A magnetic susceptibility study of hemerythrin using an ultrasensitive magnetometer. Biochemistry. 1972 Feb 1;11(3):461–465. doi: 10.1021/bi00753a026. [DOI] [PubMed] [Google Scholar]
- Fan C. C., York J. L. Implication of histidine at the active site of hemerythrin. Biochem Biophys Res Commun. 1969 Aug 7;36(3):365–372. doi: 10.1016/0006-291x(69)90573-7. [DOI] [PubMed] [Google Scholar]
- Fan C. C., York J. L. The role of tyrosine in the hemerythrin active site. Biochem Biophys Res Commun. 1972 Apr 28;47(2):472–476. doi: 10.1016/0006-291x(72)90738-3. [DOI] [PubMed] [Google Scholar]
- Ferrell R. E., Kitto G. B. Structural studies on Dendrostomum pyroides hemerythrin. Biochemistry. 1971 Jul 20;10(15):2923–2929. doi: 10.1021/bi00791a020. [DOI] [PubMed] [Google Scholar]
- Garbett K., Darnall D. W., Klotz I. M., Williams R. J. Spectroscopy and structure of hemerythrin. Arch Biochem Biophys. 1969 Dec;135(1):419–434. doi: 10.1016/0003-9861(69)90559-1. [DOI] [PubMed] [Google Scholar]
- Garbett K., Johnson C. E., Klotz I. M., Okamura M. Y., Williams R. J. Hemerythrin: further studies of Mössbauer spectra. Arch Biochem Biophys. 1971 Feb;142(2):574–583. doi: 10.1016/0003-9861(71)90521-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Klippenstein G. L. Crystals of myohemerythrin. J Mol Biol. 1974 Jul 25;87(1):147–149. doi: 10.1016/0022-2836(74)90567-1. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Love W. E., Karle J. Crystal structure analysis of sea lamprey hemoglobin at 2 angstrom resolution. J Mol Biol. 1973 Mar 5;74(3):331–361. doi: 10.1016/0022-2836(73)90377-x. [DOI] [PubMed] [Google Scholar]
- KERESZTES-NAGY S., KLOTZ I. M. INFLUENCE OF COORDINATING LIGANDS ON STRUCTURE AND SPECTRA OF HEMERYTHRIN. Biochemistry. 1965 May;4:919–931. doi: 10.1021/bi00881a019. [DOI] [PubMed] [Google Scholar]
- KERESZTES-NAGY S., KLOTZ I. M. MERCAPTAN INVOLVEMENT IN DISSOCIATION AND RECONSTITUTION OF HEMERYTHRIN. Biochemistry. 1963 Sep-Oct;2:923–927. doi: 10.1021/bi00905a005. [DOI] [PubMed] [Google Scholar]
- Klippenstein G. L. Complete chemical modification of the carboxyl groups in hemerythrin: effect on the active center and subunit interactions. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1474–1479. doi: 10.1016/0006-291x(72)90505-0. [DOI] [PubMed] [Google Scholar]
- Klippenstein G. L., Holleman J. W., Klotz I. M. The primary structure of Golfingia gouldii hemerythrin. Oder of peptides in fragments produced by tryptic digestion of succinylated hemerythrin. Complete amino acid sequence. Biochemistry. 1968 Nov;7(11):3868–3878. doi: 10.1021/bi00851a012. [DOI] [PubMed] [Google Scholar]
- Klippenstein G. L. Molecular variants of Golfingia gouldii hemerythrin. The primary structure of the variants arising from five amino acid interchanges. Biochemistry. 1972 Feb 1;11(3):372–380. doi: 10.1021/bi00753a011. [DOI] [PubMed] [Google Scholar]
- Klippenstein G. L., Van Riper D. A., Oosterom E. A. A comparative study of the oxygen transport proteins of Dendrostomum pyroides. Isolation and characterization of hemerythrins from muscle, the vascular system, and the coelom. J Biol Chem. 1972 Sep 25;247(18):5959–5963. [PubMed] [Google Scholar]
- Kretsinger R. H. Gene triplication deduced from the tertiary structure of a muscle calcium binding protein. Nat New Biol. 1972 Nov 15;240(98):85–88. doi: 10.1038/newbio240085a0. [DOI] [PubMed] [Google Scholar]
- Loehr J. S., Meyerhoff K. N., Sieker L. C., Jensen L. H. An x-ray crystallographic study of hemerythrin. J Mol Biol. 1975 Feb 5;91(4):521–522. doi: 10.1016/0022-2836(75)90278-8. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Moleski C., York J. L. Magnetic susceptibility evidence for a binuclear iron complex in hemerythrin. Biochemistry. 1971 Mar 2;10(5):840–842. doi: 10.1021/bi00781a017. [DOI] [PubMed] [Google Scholar]
- North A. C., Stubbs G. J. Crystallography of hemerythrin. J Mol Biol. 1974 Sep 5;88(1):125–131. doi: 10.1016/0022-2836(74)90298-8. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Klotz I. M., Johnson C. E., Winter M. R., Williams R. J. The state of iron in hemerythrin. A Mössbauer study. Biochemistry. 1969 May;8(5):1951–1958. doi: 10.1021/bi00833a027. [DOI] [PubMed] [Google Scholar]
- Rill R. L., Klotz I. M. Tyrosine ligands to iron in hemerythrin. II. Specification of residues involved. Arch Biochem Biophys. 1971 Nov;147(1):226–241. doi: 10.1016/0003-9861(71)90330-4. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J. L., Bearden A. J. Active site of hemerythrin. Iron electronic states and the binding of oxygen. Biochemistry. 1970 Nov 10;9(23):4549–4554. doi: 10.1021/bi00825a014. [DOI] [PubMed] [Google Scholar]
- York J. L., Fan C. C. Implication of tyrosine in iron binding in hemerythrin. Biochemistry. 1971 Apr 27;10(9):1659–1665. doi: 10.1021/bi00785a025. [DOI] [PubMed] [Google Scholar]