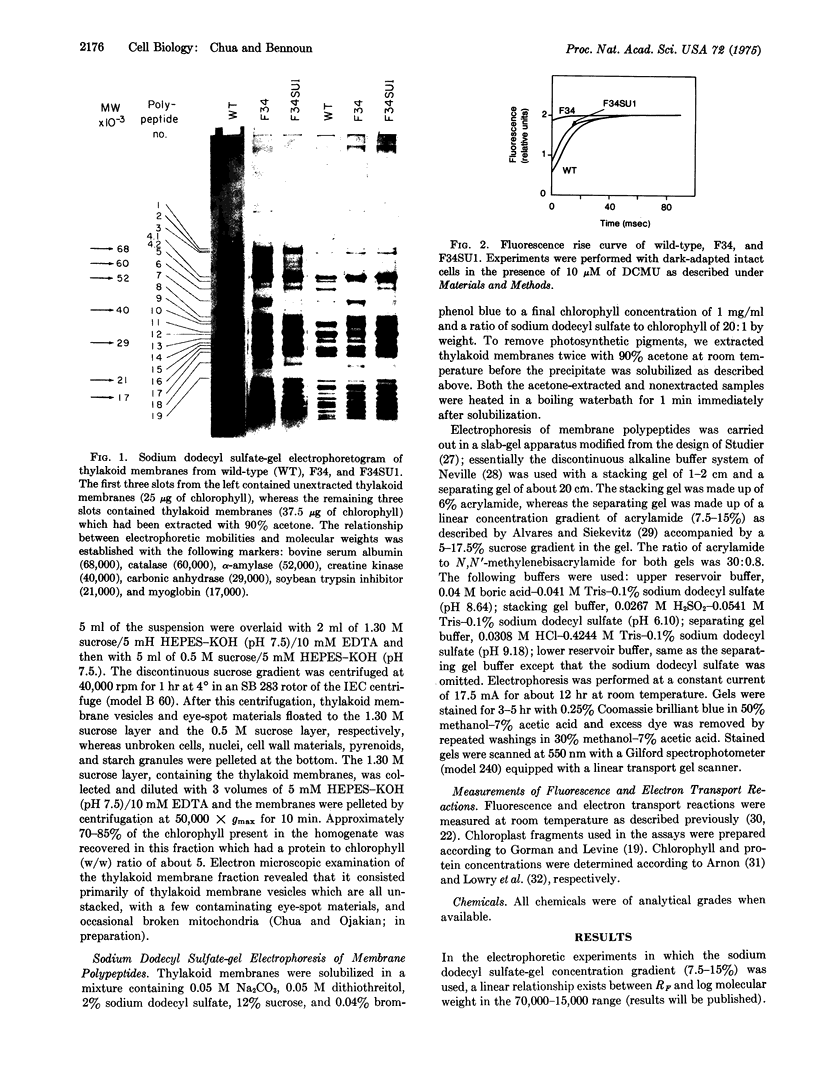
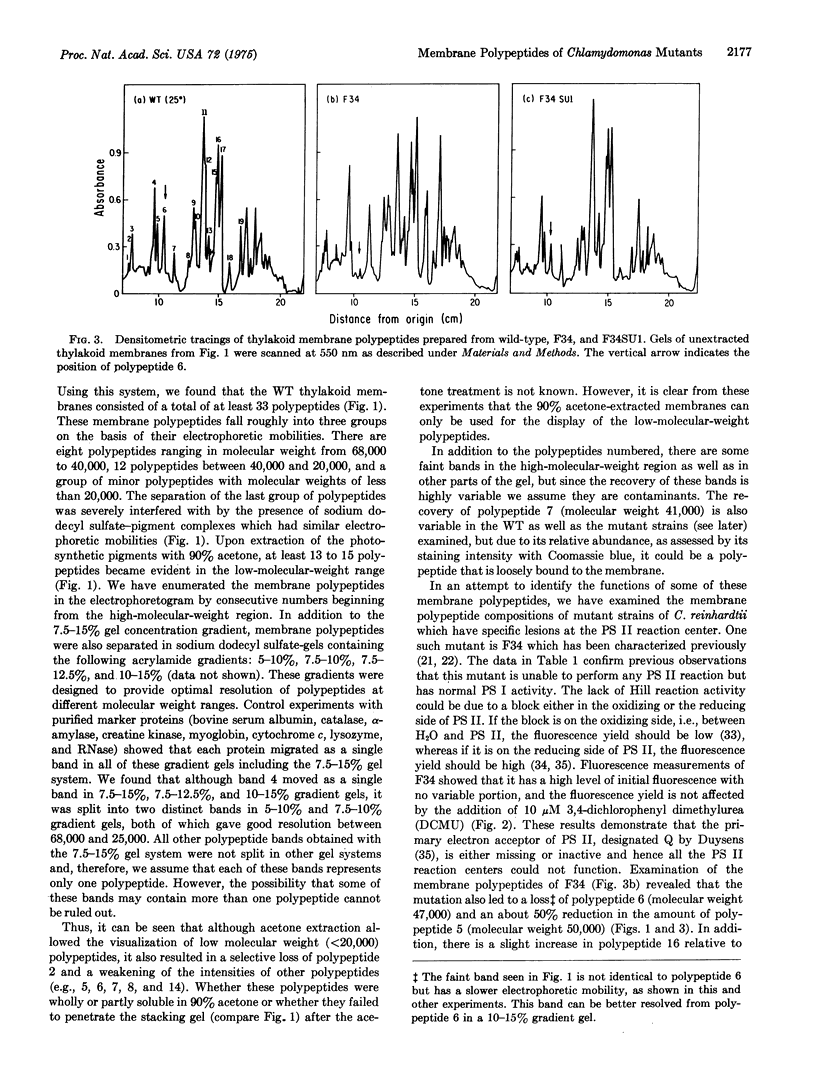
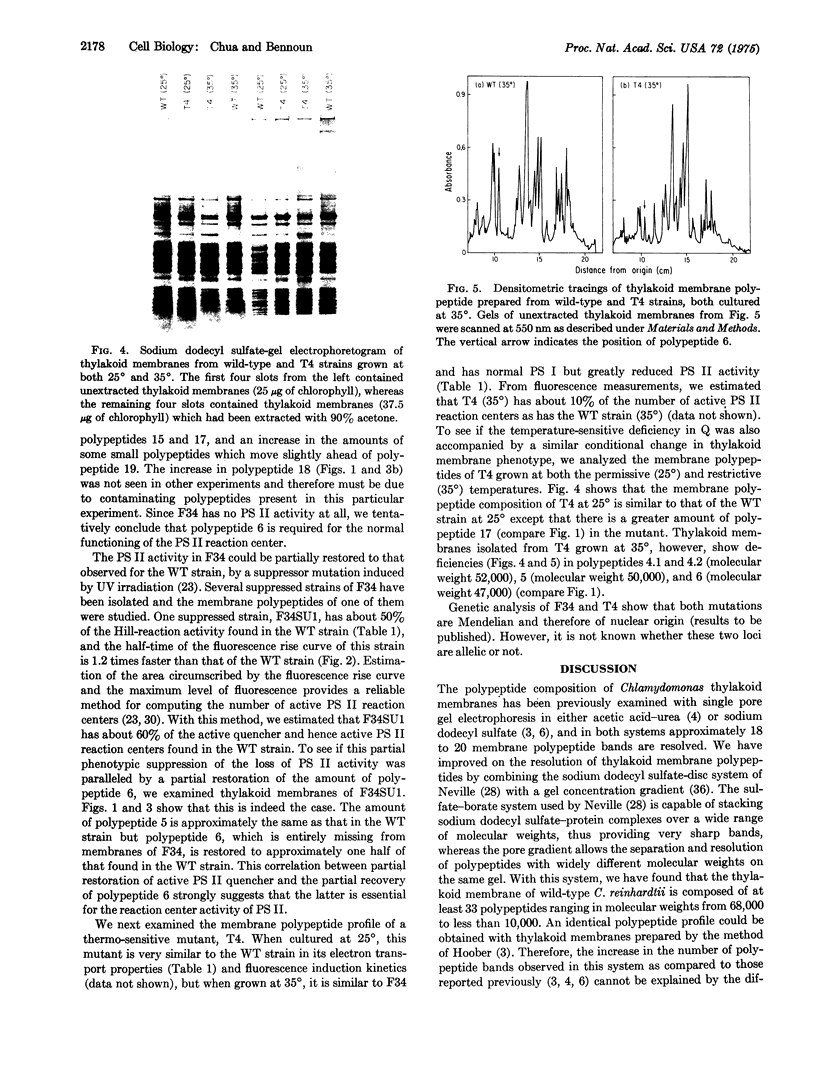
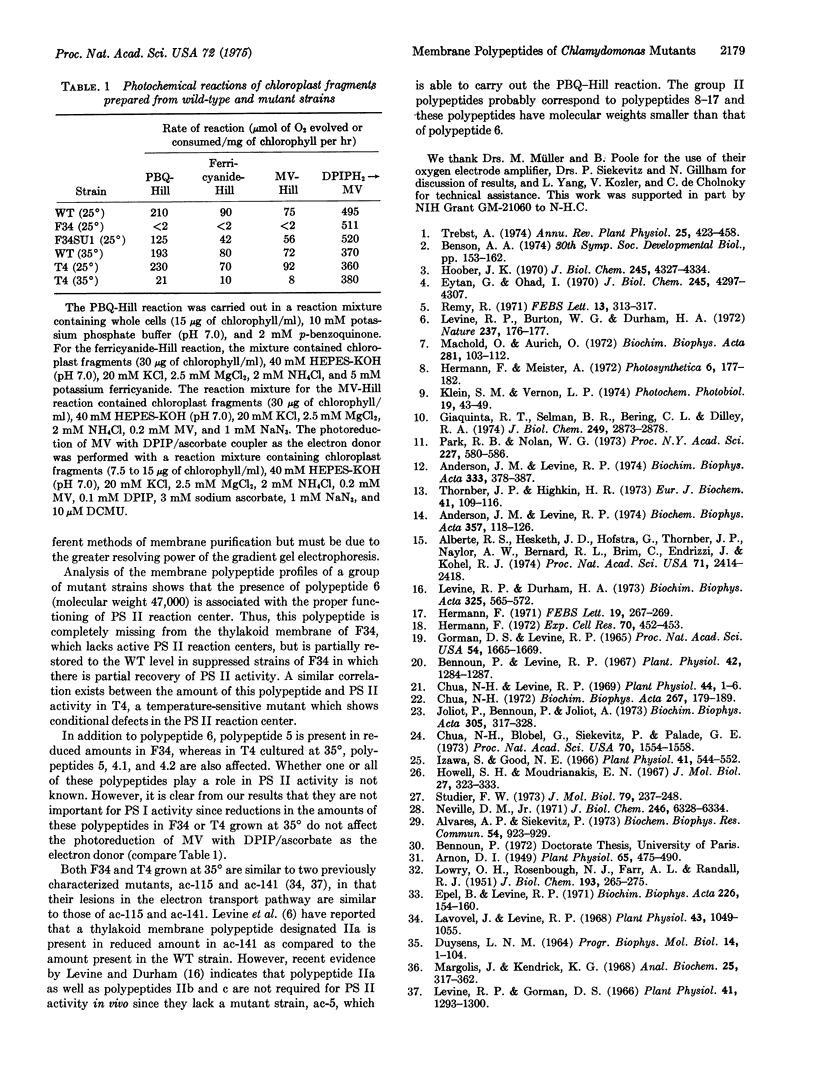
Abstract
Unstacked thylakoid membrane vesicles were obtained from a homogenate of Chlamydomonas reinhardtii by flotation in a 1.8 M sucrose layer containing 5 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid)-10 mM EDTA (pH 7.5). Sodium dodecyl sulfate-gradient gel electrophoresis showed that the wildtype membranes have a total of at least 33 polypeptides ranging in molecular weights from 68,000 to less than 10,000. The wild-type and three non-photosynthetic mutant strains were studied with respect to their photosynthetic electron transport properties, their fluorescence rise kinetics, and their membrane polypeptide compositions. The results showed a strong correlation between the presence of a membrane polypeptide (molecular weight = 47,000) and the activity of the photosystem II reaction center. This polypeptide is missing from F34 (a mendelian mutant lacking Q, the primary electron acceptor of photosystem II), but is partially restored in a suppressed strain of F34 in which there is an incomplete recovery of photosystem II activity. In a thermosensitive mutant, T4, the same polypeptide is present in reduced amount only in cells grown at 35 degrees but not in those grown at 25 degrees. Evidence from fluorescence rise kinetics and partial photochemical reactions show that the cells grown at 25 degree are similar to wild-type cells but the cells grown at 35 degrees are greatly deficient in Q.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Hesketh J. D., Hofstra G., Thornber J. P., Naylor A. W., Bernard R. L., Brim C., Endrizzi J., Kohel R. J. Composition and activity of the photosynthetic apparatus in temperature-sensitive mutants of higher plants. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2414–2418. doi: 10.1073/pnas.71.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares A. P., Siekevitz P. Gel electrophoresis of partially purified cytochromes P450 from liver microsomes of variously-treated rats. Biochem Biophys Res Commun. 1973 Oct 1;54(3):923–929. doi: 10.1016/0006-291x(73)90782-1. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Bennoun P., Levine R. P. Detecting mutants that have impaired photosynthesis by their increased level of fluorescence. Plant Physiol. 1967 Sep;42(9):1284–1287. doi: 10.1104/pp.42.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. A. Lipids and membrane structure. Symp Soc Dev Biol. 1974;30(0):153–162. doi: 10.1016/b978-0-12-612973-1.50013-9. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Attachment of chloroplast polysomes to thylakoid membranes in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1973 May;70(5):1554–1558. doi: 10.1073/pnas.70.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Levine R. P. The photosynthetic electron transport chain of Chlamydomonas reinhardi. 8. The 520 nm light-induced absorbance change in the wild-type and mutant strains. Plant Physiol. 1969 Jan;44(1):1–6. doi: 10.1104/pp.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H. Photooxidation of 3,3'-diaminobenzidine by blue-green algae and Chlamydomonas reinhardii. Biochim Biophys Acta. 1972 Apr 20;267(1):179–189. doi: 10.1016/0005-2728(72)90149-1. [DOI] [PubMed] [Google Scholar]
- Epel B. L., Levine R. P. Mutant strains of Chlamydomonas reinhardi with lesions on the oxidizing side of photosystem II. Biochim Biophys Acta. 1971 Jan 12;226(1):154–160. doi: 10.1016/0005-2728(71)90187-3. [DOI] [PubMed] [Google Scholar]
- Eytan G., Ohad I. Biogenesis of chloroplast membranes. VI. Cooperation between cytoplasmic and chloroplast ribosomes in the synthesis of photosynthetic lamellar proteins during the greening process in a mutant of Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4297–4307. [PubMed] [Google Scholar]
- Giaquinta R. T., Selman B. R., Bering C. L., Dilley R. A. Inhibition of coupling factor activity of chloroplast membranes by diazonium compounds. J Biol Chem. 1974 May 10;249(9):2873–2878. [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F. Chloroplast lamellar proteins of the plastid mutant en: viridis-1 of Antirrhinum majus having impaired photosystem II. Exp Cell Res. 1972 Feb;70(2):452–453. doi: 10.1016/0014-4827(72)90162-0. [DOI] [PubMed] [Google Scholar]
- Herrmann F. Genetic control of pigment-protein complexes I and Ia of the plastid mutant en:alba-1 of Antirrhinum majus. FEBS Lett. 1971 Dec 15;19(3):267–269. doi: 10.1016/0014-5793(71)80530-6. [DOI] [PubMed] [Google Scholar]
- Hoober J. K. Sites of synthesis of chloroplast membrane polypeptides in Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4327–4334. [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Hill reaction site in chloroplast membranes: non-participation of the quantasome particle in photoreduction. J Mol Biol. 1967 Jul 28;27(2):323–333. doi: 10.1016/0022-2836(67)90023-x. [DOI] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966 Mar;41(3):544–552. doi: 10.1104/pp.41.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P., Bennoun P., Joliot A. New evidence supporting energy transfer between photosynthetic units. Biochim Biophys Acta. 1973 May 30;305(2):317–328. doi: 10.1016/0005-2728(73)90179-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavorel J. Fluorescence Properties of Wild-Type Chlamydomonas reinhardi and Three Mutant Strains Having Impaired Photosynthesis. Plant Physiol. 1968 Jul;43(7):1049–1055. doi: 10.1104/pp.43.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. P., Burton W. G., Duram H. A. Membrane polypeptides associated with photochemical systems. Nat New Biol. 1972 Jun 7;237(75):176–177. doi: 10.1038/newbio237176a0. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Duram H. A. The polypeptides of stacked and unstacked Chlamydomonas reinhardi chloroplast membranes and their relation to photsystem II activity. Biochim Biophys Acta. 1973 Dec 14;325(3):565–572. doi: 10.1016/0005-2728(73)90216-8. [DOI] [PubMed] [Google Scholar]
- Machold O., Aurich O. Sites of synthesis of chloroplast lamellar proteins in Vicia faba. Biochim Biophys Acta. 1972 Sep 29;281(1):103–112. doi: 10.1016/0005-2787(72)90192-x. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Park R. B., Nolan W. G. The chemical composition and photochemical activity of chloroplast grana and stroma lamellae. Ann N Y Acad Sci. 1974 Feb 18;227:580–586. doi: 10.1111/j.1749-6632.1974.tb14420.x. [DOI] [PubMed] [Google Scholar]
- Remy R. Resolution of chloroplast lamellar proteins by electrophoresis in polyacrylamide gels. Different patterns obtained with fractions enriched in either chlorophyll a or chlorophyll b. FEBS Lett. 1971 Apr 2;13(6):313–317. doi: 10.1016/0014-5793(71)80249-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]