Abstract
The purpose of this study was to analyze the bone repair around commercially pure titanium implants with rough and porous surface, fabricated using powder metallurgy technique, after their insertion in tibiae of rabbits. Seven male rabbits were used. Each animal received 3 porous-surface implants in the left tibia and 3 rough-surface implants in the right tibia. The rabbits were sacrificed 4 weeks after surgery and fragments of the tibiae containing the implants were submitted to histological and histomorphometric analyses to evaluate new bone formation at the implant-bone interface. Means (%) of bone neoformation obtained in the histomorphometric analysis were compared by Student's t-test for paired samples at 5% significance level.. The results of the histological analysis showed that osseointegration occurred for both types of implants with similar quality of bone tissue. The histomorphometric analysis revealed means of new bone formation at implant-bone interface of 79.69 ± 1.00% and 65.05 ± 1.23% for the porous- and rough-surface implants, respectively. Statistically significant difference was observed between the two types of implants with respect to the amount new bone formation (p<0.05). In conclusion, the porous-surface implants contributed to the osseointegration because they provide a larger contact area at implant-bone interface.
Keywords: Osseointegration, Implants, Porous, Rough, Surface, Bone
Abstract
Opropósito deste estudo foi avaliar a reparação óssea ao redor de implantes de superfície porosa comparados com implantes de superfície rugosa, ambos confeccionados de titânio puro grau 2 por meio da técnica de metalurgia do pó. Os implantes foram inseridos em tíbias de coelhos. Foram utilizados neste estudo 7 coelhos machos, sendo que cada um recebeu 3 implantes de superfície porosa na tíbia esquerda e 3 implantes de superfície rugosa na tíbia direita. Os animais foram sacrificados 4 semanas após a cirurgia e os fragmentos das tíbias contendo os implantes foram submetidos à análise histológica e histomorfométrica, visando analisar a neoformação óssea na interface osso-implante. As médias (%) obtidas na análise histomorfométrica foram avaliadas por meio do teste estatístico t-student de amostras pareadas com nível de significância de 5%. Os resultados da análise histológica mostraram que a osseointegração foi obtida nos dois tipos de implantes com similar qualidade de tecido ósseo. Na análise histomorfométrica, verificaram-se médias de neoformação óssea na interface osso-implante de 79,69% ± 1,00 e 65,05 ± 1,23 para os implantes de superfície porosa e rugosa, respectivamente, e foi observada diferença estatisticamente significante entre os dois tipos de implantes com relação à quantidade de neoformação óssea. Concluiu-se que os implantes de superfície porosa contribuíram para a osseointegração devido à sua maior superfície de contato na interface osso-implante.
INTRODUCTION
Pure titanium and titanium alloys are the most used biomaterials for fabrication of surgical implants due to their excellent mechanical properties, biocompatibility15 and resistance to corrosion16. They are considered ideal materials because they have shown better acceptability by human tissues than other metals under diverse circumstances16.
The high biocompatibility of titanium derives partially from the stable and protective oxide layer, which apparently aids in connecting extracellular matrix to the implant surface16. The knowledge of the biomaterial-bone tissue interface is extremely important to define which material would promote a better tissue response and which kind of surface would be more adequate for the proliferation of bone cells21. After placement of an implant in the surgical cavity, several cellular events take place. Ideally, these events should lead to wound healing by intimate apposition of the bone to the biomaterial, i.e., osseointegration6. Osseointegration is a direct structural and functional connection between living bone and the surface of an implant5.
Surgical implants can be either cylindrical, conical or screw-shaped12,25. Regardless of their external shape, microscopically they can present smooth, porous or textured surfaces1,4,5,10,19,26. Several studies have shown that the success or failure of surgical implants can be related to chemical3,12,19 and biological12 properties of their surfaces as well as to their micromorphology13,16,26. The differences in the microstructure of implant surfaces seem to influence stress distribution, bone retention, cellular response on its surface and consequently the osseointegration3,12,23.
The porous surface has been considered as a good alternative to rough coating11. Biomaterials with porous structure are aimed at optimizing the interfacial resistance between the material and the bone, leading to a more effective fixation of the implant. Porous implants allow an interdigitation of the bone tissue to the implant, thus increasing the interfacial resistance18. Additionally, a porous surface provides a more efficient fixation, shorter initial healing time19 and increased cellular adhesion potential. Otherwise, screw implants with rough surface present only juxtaposition of bone11,12,21. Porous implants have been developed to be stabilized by bone ingrowth into the pores11,19. Pilliar, et al.19 (1998) reviewed and compared several designs and surfaces of surgical implants and concluded that the surface with pores of 100 μm in diameter would be ideal for the neoformation of a three-dimensional osseointegration net inside the implant. Oliveira, et al.18 (2002) determined that, although the fabrication process parameters have been optimized, the ideal porous requirements for surgical implants have not yet been reached. These authors reported that some changes are necessary in order to increase porosity and advocated that an analysis of pore size distribution along the sample has been performed to indicate more efficiently which porous fraction would better meet implant requirements17.
Therefore, the purpose of this study was to analyze, by histological and histomorphometric methods, the bone repair around cylindrical grade-2 commercially pure titanium implants with rough and porous surface, fabricated using powder metallurgy technique, after their insertion in tibiae of rabbits.
MATERIAL AND METHODS
Seven male New Zealand albino rabbits aged 6 to 8 months and weighing 3.5 kg on average were used in this study. The animals were provided by the Vivarium of São Jose dos Campos Dental School and were kept in individual cages and fed a commercial pet chow (Coelhil R; Socil, Belo Horizonte, MG, Brazil) and water ad libitum.
The commercially pure titanium implants with either rough or porous surface were fabricated at the Department of Materials of the Air and Space Institute at CTA (Brazilian Air and Space Technical Center, São José dos Campos, SP, Brazil) in an association with the National Institute of Technology of Rio de Janeiro (Rio de Janeiro, RJ, Brazil). The implants had 3.0 mm in diameter and pore diameters ranging from 250 and 350 μm.
The implants were cleaned, wrapped and sterilized in autoclave at 121°C for 15 min. Prior to surgery, the animals were weighed and injected intramuscularly with 13 mg/kg of aqueous solution of 2% hydrochloride of 2-(2,6-xylidine)-5,6-dihydro-4H-1,3-thiazin (Rompum; Bayer, São Paulo, SP, Brazil), which is an analgesic, sedative and muscular relaxant substance. General anesthesia was obtained with administration of 33 mg/Kg of ketamine (Dopalen; Agribrands do Brasil Ltda, Paulinia, SP, Brazil).). A local anesthetic composed of 3% octapressin combined with prilocaine hydrochloride and felypressin (3%Citanest R – Dentsply) was also used.
The implant was removed from the wrap, placed in the perforation and pressed into the surgical cavity until it was fixed to the cortical bone. In order to standardize the procedure, the right tibia received a rough surface implant (control) group, while the left tibia received a porous-surface implant. The muscular tissue was sutured with absorbable thread and the skin with mononylon 4-0 surgical thread. After that, all animals were given penicillin and were monitored until sacrifice 30 days after surgery.
After euthanasia, the surgical segments with the implants were removed and the implants were tested for mobility using a clinical clamp. The segments were placed in 10% formalin solution for at least 48 h.
Next, the specimens were dehydrated in an increasing ethanol series (50%, 75%, 90% and 100%), embedded in polyester resin (Orto cristal T 208; Valglass com. e ind. Ltda. São Paulo, SP, Brazil) and sectioned longitudinally in a sectioning machine for hard tissues (Labcut 1010; Extec Technologies, Inc., Enfield, CT, USA) providing 80-μm-thick serial sections on average. The sections were ground in a polishing machine (Labpol 8-12; Extec Technologies, Inc.) using sandpapers of decreasing abrasiveness (#400, #600 and #1200) until reaching a thickness of 30-40 μm. The cuts were analyzed under optical microscopy and scanning electron microscopy (SEM). Images were taken with a digital camera (DSC-S85, Cyber-shot, Sony) coupled to the optical microscope and evaluated on a television monitor (Panasonic).
Three sections of each implant were evaluated for the percentage of bone neoformation at implant-bone interface. Two areas of each section were digitized (X100), representing the medial and distal interfaces of the implant. Therefore, 124 sections were analyzed for each type of implant. Bone neoformation rate and bone ingrowth into the pores were calculated using the Image J software (NIH, USA). The final data were submitted to statistical analysis using the Minitab software version 12.3 (Minitab Inc, USA) and Student's t-test at 5% significance level.
RESULTS
All animals presented satisfactory postoperative results, without any evidence of inflammation or infection of the surgical site. No adverse reaction was observed during the procedure. During the clinical evaluation, performed with a clinical clamp, none of the implants presented mobility.
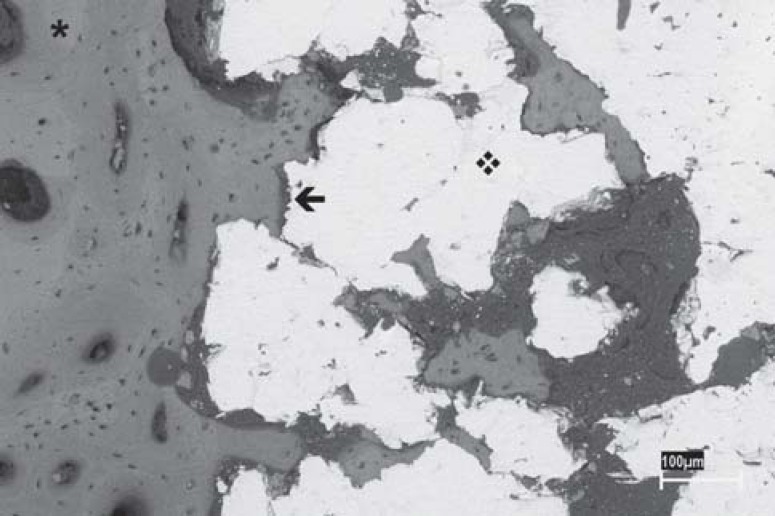
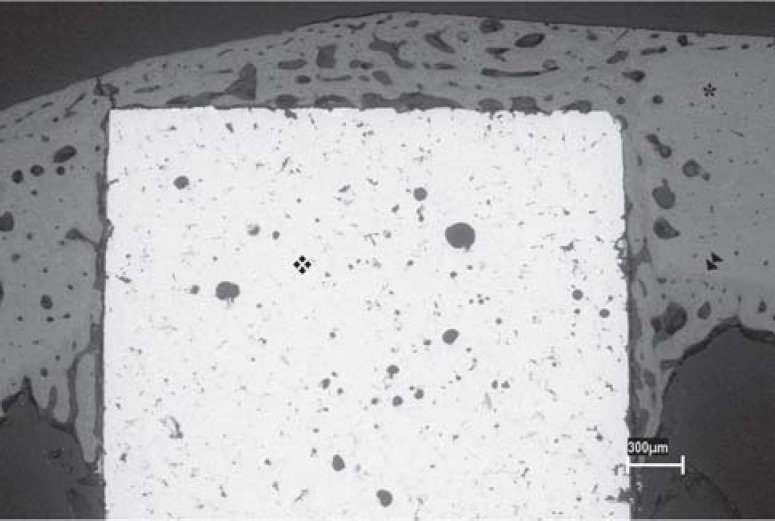
In both groups (rough- and porous-surface implants), there was bone neoformation around the implant leading to osseointegration (Figures 1 and 2). This new bone formation was similar in both groups and was constituted by mature bone trabeculae that presented lamellar arrangement and by medullar spaces of different sizes. In several specimens, regardless of the analyzed group, bone neoformation above the implants and at their bottom surface was observed, sometimes contacting the internal cortical face on the opposite side. A clear delimitation between the new bone formation and the preexistent cortical bone was also noticed (Figures 3, 4 and 5).
FIGURE 1. SEM micrograph of a cylindrical implant with porous surface (❖); bone (✱); bone-implant interface (←). (Original magnification X50).
FIGURE 2. SEM micrograph of a cylindrical implant with rough surface (❖); bone (✱); bone-implant interface (►►). (Original magnification X50).
FIGURE 3. SEM micrograph showing a panoramic view of a cylindrical implant with porous surface (❖) inserted into rabbit tibia (♦); osseointegration at the bottom of the implant (►►). (Original magnification X16).
FIGURE 4. SEM micrograph of a cylindrical implant with porous surface (❖); bone interface; bone ingrowth (✱); delimitation between the new bone formation and the preexistent cortical bone (←). (Original magnification X35).
FIGURE 5. SEM micrograph of a cylindrical implant with rough surface (❖); bone interface (✱); delimitation between the new bone formation and the preexistent cortical bone (►►). (Original magnification X35).
The results showed that the types of implant used presented statistically significant difference to each other regarding the amount of new bone formation on the implant-bone interface, the porous-surface implants showing a larger amount neoformed bone. Table 1 shows the means of bone neoformation (%) at bone-implant interface of rough- vs. porous-surface implants.
TABLE 1. Means of bone neoformation (%) at bone-implant interface for rough-surface and porous-surface implants. Result of the Student's t-test.
| Statistical Analysis | Implant surface | |
|---|---|---|
| Porous | Rough | |
| Mean ± SD | 79.69 ± 1.00 | 65.05 ± 1.23 |
| Difference | 14.64 | |
| t (df) | 32.75 | |
| p value | 0.0001* | |
| CI (95%) | 13.54 to 15.73 | |
statistically significant difference.
DISCUSSION
In our study, cylindrical implants with porous surface were compared to cylindrical implants with rough surface regarding the quantity and quality of new bone formation on the implant-bone interface after implantation in rabbit tibiae. The results showed no difference of new bone quality between both types of implants. However, when the quantity of bone neoformation at implant-bone interface was evaluated, a larger formation of bone tissue was observed for the porous-surface implants, and this difference was statistically significant.
The most important factors to dental implant osseointegration are related to the characteristics of its surfaces, which include topography and chemical and electric properties of the material19, since bone-implant interaction is mainly related to the most external layers of the implants13. Important factors to a more successful osseointegration are: implant material, implant shape, surgical technique20, quantity of bone tissue20,25, load6 and implant resistance4. However, some other factors such as surface energy, sterilization techniques and chemical and topographic properties of the implant surface are extremely important for the final outcome of osseointegration2,8,12,16.
In the present study, the results showed that all implants were well tolerated when placed in rabbit tibiae, thus corroborating the findings of previous studies that indicated titanium as the best biomaterial for surgical implants15,16.
Bone growth is also dependent on factors such as percentage of surface porosity, stability and degree of micromovement of the implant and the presence of gaps between the implant and the bone at the time of placement. Implants placed under pressure inside the surgical cavity help minimizing the gaps and implant micromovement8. Therefore, in order to obtain osseointegration, the surgical cavity must be prepared with the least injury possible15,20. In order to cause minimal damage to the surrounding bone tissues, in the present study, bone perforation was performed using burs of increasingly larger diameters, and under constant saline irrigation. After that, the implants were gently pressed into the surgical cavity, which diminished the gap between the implant and the bone and promoted efficient stability. Additionally, all implants were evaluated with a clinical clamp at the moment of sacrifice, 30 days after surgery, and were stable.
Some previous studies used a 4-week healing period to evaluate the biocompatibility of metal materials7,14,17,22. Deporter, et al.10 (1986) reported that healing periods (i.e., between implant placement and euthanasia of the animals) longer than 4 weeks added no benefits to increase the quantity of bone tissue ingrowth into porous-surface implants, and observed that only bone tissue maturation took place after this period. Thus, in the present study, a 4-week period was used to evaluate the biocompatibility of porous-surface grade 2 commercially pure titanium implants fabricated by means of powder metallurgy technique.
The purpose of studying and developing porous-surface implants is to promote a more stable and biocompatible fixation of titanium implants. The creation of a porous geometry surface aims not only at increasing contact area but also at allowing bone ingrowth into the pores, including those located more centrally. Such ingrowth is due to pore intercommunication, which produces a three-dimensional net and allows a mechanical entanglement7,8,9,18.
The porous-surface implant have an ideal diameter for the proliferation of bone cells2,12, and most studies report that pore diameter ranges from 100 to 400 μm. In addition, the pores have been shown to be interconnected, so that bone interdigitation into the porous structure may occur, thus reaching maximum interfacial resistance18,24. However, Nguyen, et al.17 (2004) reported that small pores measuring only 45 μm in diameter also allow bone ingrowth. Furthermore Frosch, et al.14 (2002) found that 300 to 600 μm pores showed a three-dimensional, reticular osteoblast-like cell development within 4 weeks, and that in 1000 μm pores cell ingrowth was incomplete. In the present study, the diameter of the pores varied between 250 and 350 μm, corroborating the findings of other studies9,19,23.
Svehla, et al.23 (2000) compared five different implant surfaces and verified that porous surfaces promoted an excellent substrate for the ingrowth of bone tissue and consequent implant fixation, whereas smooth surfaces resulted in the formation of a fibrous tissue and improper fixation. In the present study, when the cylindrical porous-surface implants were compared to the cylindrical rough-surfaces implants, osseointegration was observed in both groups. No formation of fibrous tissue was observed at bone-implant interface. However, significantly more bone formation was observed in the porous-surface implants.
Deporter, et al.11 (1990) concluded that a smaller segment of porous-surface implants allowed a more effective contact with the bone, due to a bigger contact area, determined by its topography. Similar findings were observed in the present study in which a statistically significant difference was observed between the groups in relation to the quantity of bone neoformation at titanium-bone interface. Therefore, smaller porous-surface implants could be developed to be used in complex clinical situations such as regions with low-quality bone9,19.
The results of this study showed that because of the larger contact surface promoted by the presence of pores, there was more bone ingrowth on the implant-bone interface. Such results are consistent with those of Cook and Rust-Dawicki7 (1995), Deporter, et al.9 (2002), Deporter, et al.10 (1986), Deporter, et al.11 (1990), Frosch, et al.14 (2002), Karabuda, et al.15 (1999), Story, et al.22 (1998), Svehla, et al.23 (2000), Vidigal Junior, et al.25 (1999), Zinger, et al.26 (2005) who also observed more effectiveness of the porous-surface implants compared to other types of implants.
CONCLUSION
Based on the results of this study, it may be concluded that the cylindrical porous-surface implants yielded greater bone neoformation than the cylindrical rough-surface implants because of their larger area in contact with the bone tissue and the presence of an intercommunicating porous structure that allowed the formation of a three-dimensional osseointegration network.
REFERENCES
- 1.Bagno A, Bello CD. Surface treatments and roughness properties of Ti-based biomaterials. J Mater Sci Mater Med. 2004;15:935–949. doi: 10.1023/B:JMSM.0000042679.28493.7f. [DOI] [PubMed] [Google Scholar]
- 2.Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992;7:302–310. [PubMed] [Google Scholar]
- 3.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 4.Braceras I, Alava JI, Oñate JI, Brizela M, Garcia-Luis A, Garagorri N, et al. Improved osseointegration in ion implantation-treated dental implants. Surface e coating Tecnology. 2002;158:28–32. [Google Scholar]
- 5.Branemark PI. Osseointegration and its experimental background. J Prosth Dent. 1983;50(3):399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 6.Brunski BJ. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv Dent Res. 1999;13:99–119. doi: 10.1177/08959374990130012301. [DOI] [PubMed] [Google Scholar]
- 7.Cook SD, Rust-Dawicki AM. In vivo evaluation of a CSTi dental implant: a healing time course study. J Oral Implantology. 1995;2(3):182–190. [PubMed] [Google Scholar]
- 8.Cornell CN, Lane JM. Current understanding of osteoconduction in bone regeneration. Clin Orthop. 1998;1(355S):267–273. doi: 10.1097/00003086-199810001-00027. [DOI] [PubMed] [Google Scholar]
- 9.Deporter DA, Todescan R, Riley N. Porous-surfaced dental implants in the partially edentulous maxilla: assessment for subclinical mobility. Int J Period Rest Dent. 2002;22(2):184–192. [PubMed] [Google Scholar]
- 10.Deporter DA, Watson PA, Pilliar RM, Melcher AH, Winslow J, Howley TP. A histological assessment of the initial healing response adjacent to porous-surfaced, titanium alloy dental implants in dogs. J Dent Res. 1986;65(8):1064–1070. doi: 10.1177/00220345860650080501. [DOI] [PubMed] [Google Scholar]
- 11.Deporter DA, Watson PA, Pilliar RM, Chipman ML, Valiquete N. A histological comparison in the dog of porous coated vs: threaded dental implants. J Dent Res. 1990;69(5):1138–1145. doi: 10.1177/00220345900690050401. [DOI] [PubMed] [Google Scholar]
- 12.Ellingsen JE. Surface configurations of dental implants. Periodontoly 2000. 1998;17:36–46. doi: 10.1111/j.1600-0757.1998.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 13.Fini M, Savarino L, Nicoli Aldini N, Martini L, Giavaresi G, Rizzi G, et al. Biomechanical and histomorphometric investigations on two morphologically differing titanium surfaces with and without fluorhydroxyapatite coating: an experimental study in sheep tibiae. Biomaterials. 2003;24(19):3183–3192. doi: 10.1016/s0142-9612(03)00164-9. [DOI] [PubMed] [Google Scholar]
- 14.Frosch KH, Barvencik F, Lohmann CH, Viereck V, Siggelkow H, Breme J, et al. Migration, matrix production and lamellar bone formation of human osteoblast-like cells in porous titanium implants. Cells Tissues Organs. 2002;170:214–227. doi: 10.1159/000047925. [DOI] [PubMed] [Google Scholar]
- 15.Karabuda C, Sandalli P, Yalcin S, Steflik DE, Parr GR. Histologic and histomorphometric comparison of immediately placed hydroxyapatite-coated and titanium plasma-sprayed implants: a pilot study in dogs. Int J Oral Maxillofac Implants. 1999;14:510–515. [PubMed] [Google Scholar]
- 16.Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosth Dent. 1983;49(6):832–837. doi: 10.1016/0022-3913(83)90359-1. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen HQ, Deporter DA, Pilliar RM, Valiquette N, Yakubovich R. The effect of sol-gel formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials. 2004;25(5):865–876. doi: 10.1016/s0142-9612(03)00607-0. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira MV, Pereira LC, Cairo CAA. Porous structure characterization in titanium coating for surgical implants. Mater Res. 2002;5(3):269–273. [Google Scholar]
- 19.Pilliar RM. Overview of surface variability of metallic endosseous dental implants: textured and porous surface-structured designs. Implant Dentistry. 1998;7(4):305–314. doi: 10.1097/00008505-199807040-00009. [DOI] [PubMed] [Google Scholar]
- 20.Roberts WE, Smith RK, Zilberman Y, Mozsary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod. 1984;86(2):95–111. doi: 10.1016/0002-9416(84)90301-4. [DOI] [PubMed] [Google Scholar]
- 21.Shenk RK, Buser D. Osseointegration: a reality. Periodontology 2000. 1998;17:22–35. doi: 10.1111/j.1600-0757.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 22.Story JB, Wagner WR, Gaisser DM, Cook SD, Rust-Dawicki AM. In vivo performance of a modified CSTi dental implant coating. J Oral Maxillofac Implants. 1998;13:749–757. [PubMed] [Google Scholar]
- 23.Svehla J, Morberg P, Zicat B, Bruce W, Sonnabend D, Walsh WR. Morphometric and mechanical evaluation of titanium implant integration: comparison of five surface structures. Biomed Mater Res. 2000;51:15–22. doi: 10.1002/(sici)1097-4636(200007)51:1<15::aid-jbm3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Vasconcellos LMR, Momose DR, Brentel AS, Oliveira MV, Cairo CAA, Carvalho YR. Surgical Tecnique to place porous surface dental implants. Acta Microscopic. 2003;12(SuplC):265–266. [Google Scholar]
- 25.Vidigal MG, Junior, Aragones LCA, Campos A, Junior, Groisman M. Histomorphometric analyses of hydroxyapatite-coated and uncoated titanium dental implants in rabbit cortical bone. Implant Dent. 1999;8(3):295–302. doi: 10.1097/00008505-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, et al. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]