Abstract
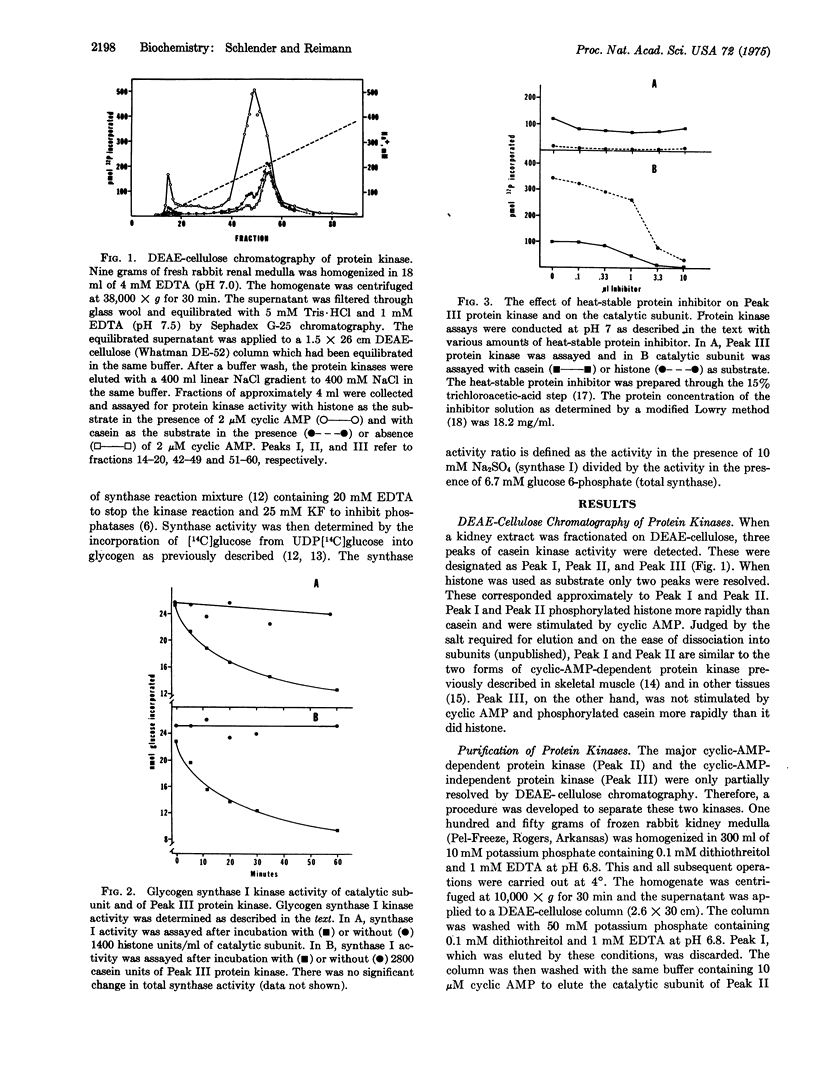
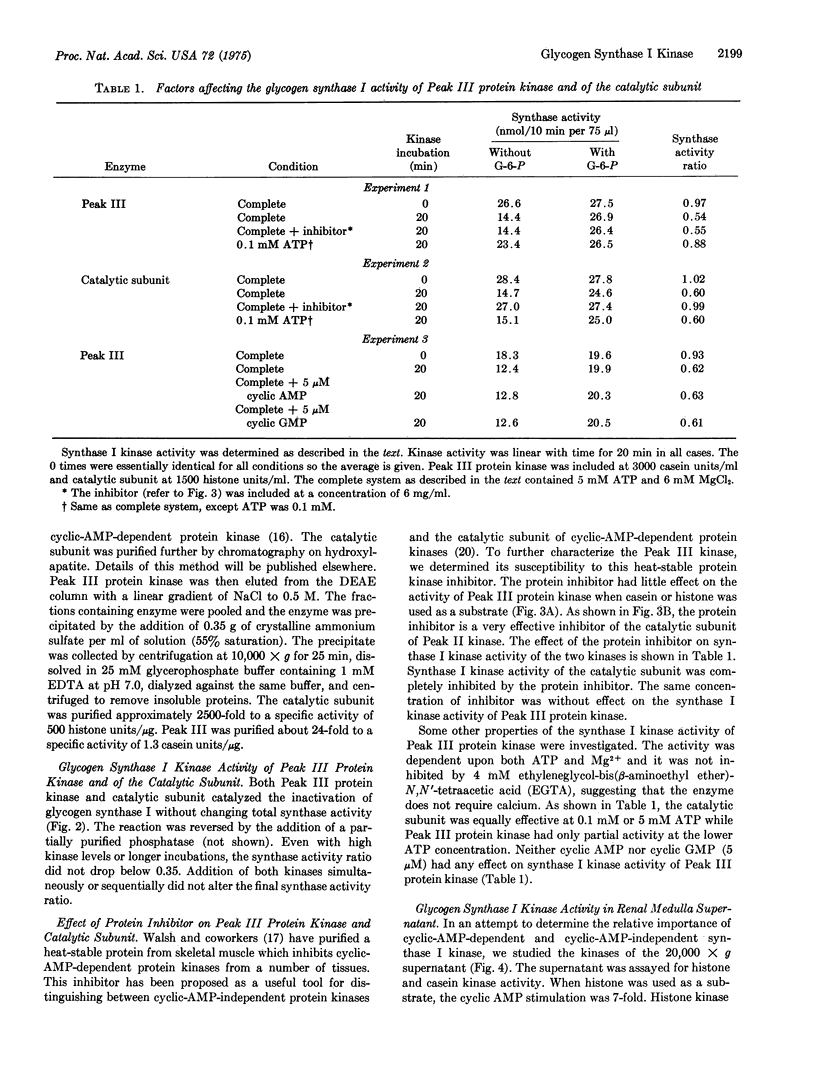
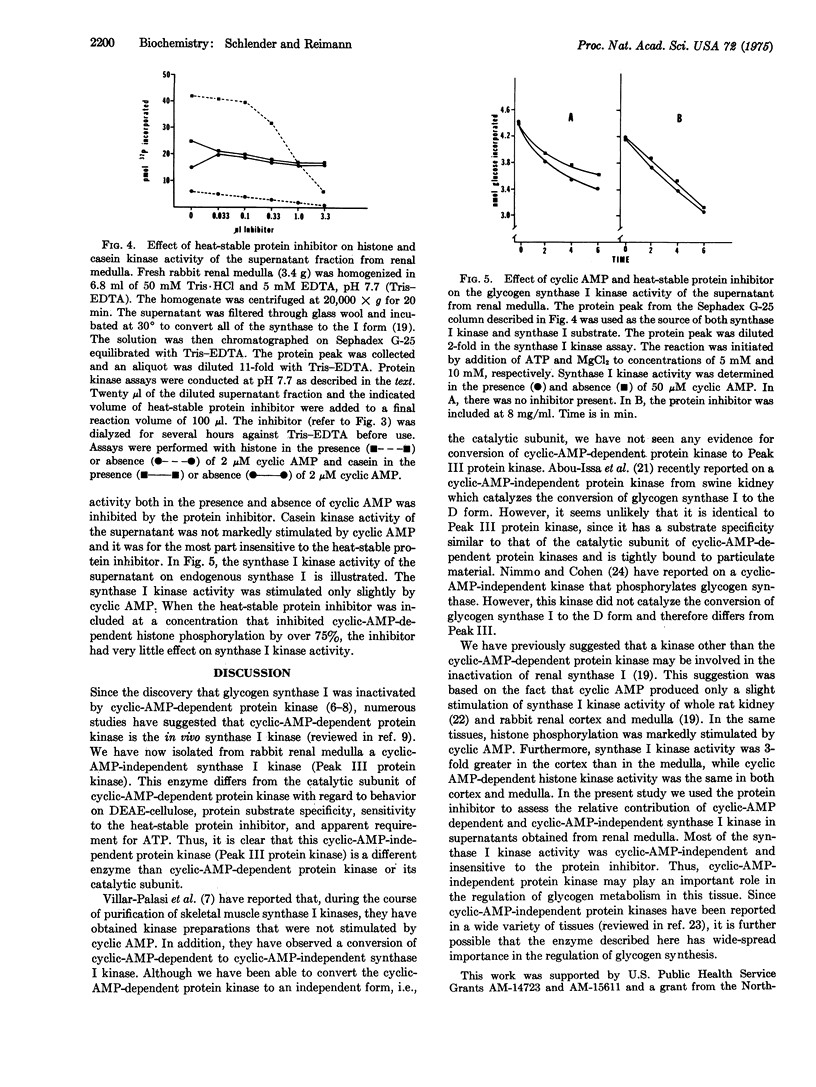
Three protein kinases (ATP:protein phosphotransferase, EC 2.7.1.37) were detected when the soluble fraction of rabbit kidney medulla was chromatographed on DEAE-cellulose with a linear NaC1 gradient. The first two kinases eluted (Peak 1 and Peak II) were cyclic-AMP-dependent, wheras Peak III was cyclic-AMP-independent. A procedure was developed to separate the catalytic subunit of Peak II cyclic-AMP-dependent protein kinase (representing the bulk of the histone kinase activity) from Peak III protein kinase. In contrast to the catalytic subunit, Peak III protein kinase phosphorylated casein more rapidly than histone. Peak III was insensitive to the heat-stable protein inhibitor of cyclic-AMP-dependent protein kinases and appeared to have a higher requirement for ATP than did the catalytic subunit. Peak III catalyzed the conversion of glycogen synthase (UDPglucose:glycogen alpha-4-glucosyltransferase, EC 2.4.1.11) from the I (glucose-6-phosphate-independent) to the D (glucose-6-phosphate-dependent) form. This conversion was dependent on Mg-2+ and ATP and was unaffected by cyclic AMP, cyclic GMP, or the protein inhibitor. Glycogen synthase I in the soluble fraction of kidney medulla could be converted to the D form by endogenous glycogen synthase I kinase if Mg-2+ and ATP were added. Most of this glycogen synthase I kinase activity was unaffected by cyclic AMP or by the protein inhibitor, suggesting that Peak III may be of major importance in the regulation of glycogen synthase in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Issa H., Kratowich N., Mendicino J. Properties of soluble and bound protein kinases isolated from swine kidney. Eur J Biochem. 1974 Mar 1;42(2):461–473. doi: 10.1111/j.1432-1033.1974.tb03360.x. [DOI] [PubMed] [Google Scholar]
- Appleman M. M., Belocopitow E., Torres H. N. Factors affecting the activity of muscle glycogen synthetase. Biochem Biophys Res Commun. 1964;14:550–554. doi: 10.1016/0006-291x(64)90267-0. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Erlichman J., Hirsch A. H., Rosen O. M. Interconversion of cyclic nucleotide-activated and cyclic nucleotide-independent forms of a protein kinase from beef heart. Proc Natl Acad Sci U S A. 1971 Apr;68(4):731–735. doi: 10.1073/pnas.68.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN D. L., LARNER J. STUDIES ON UDPG-ALPHA-GLUCAN TRANSGLUCOSYLASE. III. INTERCONVERSION OF TWO FORMS OF MUSCLE UDPG-ALPHA-GLUCAN TRANSGLUCOSYLASE BY A PHOSPHORYLATION-DEPHOSPHORYLATION REACTION SEQUENCE. Biochemistry. 1963 Jul-Aug;2:669–675. doi: 10.1021/bi00904a009. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Huijing F., Larner J. On the mechanism of action of adenosine 3',5' cyclophosphate. Proc Natl Acad Sci U S A. 1966 Aug;56(2):647–653. doi: 10.1073/pnas.56.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Glycogen synthetase kinase 2 (GSK 2); the identification of a new protein kinase in skeletal muscle. FEBS Lett. 1974 Oct 1;47(1):162–166. doi: 10.1016/0014-5793(74)80450-3. [DOI] [PubMed] [Google Scholar]
- ROSELL-PEREZ M., LARNER J. STUDIES ON UDPG-ALPHA-GLUCAN TRANSGLUCOSYLASE. V. TWO FORMS OF THE ENZYME IN DOG SKELETAL MUSCLE AND THEIR INTERCONVERSION. Biochemistry. 1964 Jan;3:81–88. doi: 10.1021/bi00889a014. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Rapino N. G. Partial purification and characterization of an adenosine 3',5'-monophosphate-dependent protein kinase from rabbit gastric mucosa. Biochim Biophys Acta. 1974 May 20;350(1):201–204. doi: 10.1016/0005-2744(74)90218-6. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Schlender K. K., Larner J. Purification and properties of glycogen synthase I from skeletal muscle: two kinetic forms. Biochim Biophys Acta. 1973 Jan 12;293(1):73–83. doi: 10.1016/0005-2744(73)90377-x. [DOI] [PubMed] [Google Scholar]
- Schlender K. K. Regulation of renal glycogen synthase. Interconversion of two forms in vitro. Biochim Biophys Acta. 1973 Feb 28;297(2):384–398. doi: 10.1016/0304-4165(73)90086-x. [DOI] [PubMed] [Google Scholar]
- Schlender K. K., Wei S. H., Villar-Palasi C. UDP-glucose:glycogen alpha-4-glucosyltransferase I kinase activity of purified muscle protein kinase. Cyclic nucleotide specificity. Biochim Biophys Acta. 1969 Nov 4;191(2):272–278. doi: 10.1016/0005-2744(69)90246-0. [DOI] [PubMed] [Google Scholar]
- Soderling T. R., Hickenbottom J. P., Reimann E. M., Hunkeler F. L., Walsh D. A., Krebs E. G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1970 Dec 10;245(23):6317–6328. [PubMed] [Google Scholar]
- Soderling T. R., Park C. R. Recent advances in glycogen metabolism. Adv Cyclic Nucleotide Res. 1974;4(0):283–333. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. Specificity of activation of glycogen synthase I from skeletal muscle and heart. Biochim Biophys Acta. 1973 Jan 12;293(1):84–93. doi: 10.1016/0005-2744(73)90378-1. [DOI] [PubMed] [Google Scholar]
- Villar-Palasi C., Larner J., Shen L. C. Glycogen metabolism and the mechanism of action of cyclic AMP. Ann N Y Acad Sci. 1971 Dec 30;185:74–84. doi: 10.1111/j.1749-6632.1971.tb45238.x. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D. Protein kinases: aspects of their regulation and diversity. Recent Prog Horm Res. 1973;29:329–359. doi: 10.1016/b978-0-12-571129-6.50012-9. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]


