Abstract
Objective:
This report evaluated the efficacy of three brushes and one biofilm disclosing agent in complete denture cleansing. Methods: Twenty-seven wearers of maxillary dentures were distributed into three groups and received different brushes: Oral B40, conventional toothbrush (Oral B); Denture, denture-specific brush (Condor); Johnson & Johnson, denture-specific brush (Johnson & Johnson). The 60-day experimental period was divided into two techniques: I - brushing (brush associated with a paste - Dentu Creme, Dentco) three times a day; II - brushing and daily application of 1% neutral red on the denture internal surface. Biofilm quantification was carried out weekly and the areas with dye biofilm were obtained by means of Image Tool 2.02 software.
Results:
Biofilm removal was more effective during Technique II (Wilcoxon test: p=0.01) for the three groups of brushes. When the brushes were compared in Technique I, the Kruskal Wallis test indicated statistical difference between Denture X Johnson & Johnson and Denture X Oral B40, in which the Denture was more efficient. For Technique II, there was no statistical difference between brushes (p>0.05).
Conclusion:
The disclosed application promoted more efficacy on biofilm removal, regardless of the brush used. Denture (Condor) was more efficient than the other brushes during Technique I.
Keywords: Complete denture, Biofilm, Cleansers, Disclosing agent, Brushes
Abstract
Este trabalho avaliou três escovas e um evidenciador de biofilme na higiene de próteses totais. Vinte e sete usuários de próteses totais superiores foram distribuídos em três grupos que receberam diferentes escovas: Oral B40 para dentes naturais (Oral B); Denture para dentadura (Condor) e Johnson & Johnson para dentadura (Johnson & Johnson). O período experimental de 60 dias foi dividido em 2 Técnicas: I - utilização das escovas associadas a um dentifrício (Dentu-Creme, Dentco) três vezes ao dia; II – escovação e aplicação diária do evidenciador Vermelho neutro a 1% na superfície interna da prótese total. A quantificação do biofilme foi realizada semanalmente e as áreas com biofilme corado foram medidas com o auxílio do software Image Tool 2.02. A remoção do biofilme foi mais efetiva durante a Técnica II (teste de Wilcoxon: p=0.01) nos três grupos. Comparando as escovas, na Técnica I o teste de Kruskal Wallis indicou diferença significante (p=0.05) entre Denture X Johnson & Johnson e Denture X OralB 40, sendo a Denture a mais eficaz. Na Técnica II, as escovas não apresentaram diferença estatisticamente significante (p>0,05). A aplicação do evidenciador promoveu maior eficácia na remoção do biofilme, independentemente da escova utilizada. Na comparação das escovas na Técnica I, a escova Denture foi a mais eficiente.
INTRODUCTION
Although many reports evaluate the efficacy of denture cleansers, surveys point out that removal of denture biofilm by denture wearers is precarious1–3,5,6,23. Deficient patient orientation, inadequate divulgation of specific denture cleanser materials, intrinsic characteristics of prosthetic appliances and deficient manual dexterity of elderly people have been indicated as causes of poor denture hygiene14,20,22,26. Biofilm removal can be obtained by means of mechanical methods (brushing and ultrasonic devices) associated with chemical methods (alkaline peroxide and hypochlorite, acids, enzymes and disinfectants); among them, brushing is the most common method applied for routine denture biofilm control1,2,17,19,35.
Knowledge on materials and methods for quantification of denture biofilm is an important factor to be considered. While biofilm quantification on natural teeth is significantly studied and published in the literature, its quantification on complete dentures is poorly known, due to the small number of papers published on this matter and because the procedure is not routinely employed by dentists16,24,27,28.
With respect to natural teeth, efficient hygiene control in complete dentures can be obtained by an orientation program, correct use of materials and methods available for denture cleansing and by utilization of a biofilm disclosing agent, allowing quantification and localization of biofilm on dentures, which could allow its removal more effectively5,8,12,16,24,26,28,31,35 .
The aim of this study was to evaluate the efficacy of a biofilm disclosing agent associated with three brushes on hygiene control and maintenance in complete dentures.
MATERIAL AND METHOD
After research approval by the Institutional Review Board of Ribeirão Preto Dental School - University of São Paulo (no. 2000-136358.0), 27 healthy men and women wearing complete denture (maxillary and mandibular complete dentures) were selected, with approximate mean ages of 50 years, and with no motor deficiency according to the Discipline of Complete Denture of FORP-USP. Patients' maxillary complete dentures were constructed with heat- cured acrylic resin, acrylic teeth, none of them with any break or repair, wearing time ranging from 1 to 3 years and score of biofilm degree of at least 1 (Additive Index 2). Patients were included in the research after being verbally informed, when they read and signed the Consent Term.
Patients were divided into three groups: Toothbrush (Oral B40 - Service Industry and Commerce Ltd., São Paulo, São Paulo, Brazil); Denture brush (Johnson & Johnson - Johnson & Johnson, São Paulo, Brazil); and Denture brush (Denture - Condor S.A., Santa Catarina, Brazil). For all groups, a specific denture paste (Dentu-Creme, Dentco, Inc. Jersey City, USA) was used as an auxiliary brushing agent.
Two hygiene techniques were indicated for all groups; technique I: the subjects were instructed to brush their dentures three times a day, rinsing their mouth with water after brushing and keeping the denture immersed in water overnight; technique II: the instruction was to brush the denture 3 times a day associated with the use of a disclosing agent (1% neutral red) to the last brushing of the day; in this technique, patients were also instructed to rinse their mouth with water after brushing and to keep the denture immersed in water overnight.
Patients received verbal information and practical demonstration of both techniques. After the patients had received instructions and hygiene materials, they were assessed by the investigators, who performed disclosure of the internal surface of maxillary dentures with 1% neutral red solution. The investigator brushed the disclosed dentures until complete removal of the disclosed biofilm (biofilm-free). At first, technique I was employed by all patients for 3 weeks; after this period, the investigator accomplished complete denture biofilm removal by brushing (biofilm-free) and then technique II was employed by all patients for further 3 weeks.
During each technique, the patients attended weekly returns when the maxillary denture was removed, rinsed with running water (5 seconds) and air-dried (10 seconds). Afterwards, the disclosing agent (1% neutral red) was applied on the internal surface with a cotton swab and the denture was rinsed and dried again. The disclosed surfaces were photographed (digital camera, Coolpix, Nikon, Melville, N.Y., USA) with standardized film-object distance and exposure time. The camera was fixed on a stand (CS-4 Copy Stand Testrite, Newark, NJ, USA) at 90° to the internal denture surface. After photographing, dentures were brushed with Denture brush, in order to perform full biofilm removal, and then returned to the patients.
The photographs were transferred to a computer. Biofilm quantification was performed at the end of each hygiene technique by a computerized method. By this method, the total internal surface of the denture and biofilm-covered areas were measured using the Image Tool software (Windows, version 2.02, UTHSC, San Antonio). The percentages of biofilm coverage areas were calculated as the ratio between disclosed areas and the area of denture's internal surface multiplied by 100. Three measurements were performed (one for each visit) of biofilm degree for each treatment (I and II).
Preliminary normality and homogeneity tests applied to the results showed no normal distribution; therefore, the Wilcoxon test was employed to compare the effectiveness of both techniques for each brush group; the Kruskal Wallis test was employed to compare the effectiveness of brushes. These tests were applied to the average of biofilm percentage for each denture in each treatment (3 measurements).
RESULTS
Table 1 shows the mean of three measurements of biofilm percentage on the internal surface of the evaluated complete dentures and the sum of means.
TABLE 1. Means of disclosed biofilm percentages on the internal surface of the evaluated complete dentures and sum of means.
| Ora B40 | Johnson & Johnson | Denture | ||||
|---|---|---|---|---|---|---|
| Technique I | Technique II | Technique I | Technique II | Technique I | Technique II | |
| Mean % | 5 | 0 | 3 | 0 | 0 | 0 |
| 17 | 7 | 2 | 1 | 2 | 0 | |
| 10 | 3 | 9 | 3 | 3 | 0 | |
| 3 | 0 | 2 | 1 | 3 | 0 | |
| 1 | 0 | 1 | 0 | 1 | 0 | |
| 1 | 0 | 9 | 5 | 0 | 0 | |
| 2 | 0 | 10 | 6 | 19 | 5 | |
| 1 | 0 | 1 | 0 | 0 | 0 | |
| 5 | 1 | 12 | 2 | 4 | 3 | |
| ∑ | 45 | 11 | 49 | 18 | 32 | 8 |
Table 2 shows the statistical significance between techniques I and II (Wilcoxon Test).
TABLE 2. Statistical difference (Wilcoxon test) between techniques I and II.
| Brushes | Z calculated | Significance Technique I X Technique II |
|---|---|---|
| Oral B40 | 2.70 | 1% (p<0.01) |
| Johnson & Johnson | 2.70 | 1% (p<0.01) |
| Denture | 2.20 | 5% (p<0.05) |
Table 3 shows the results of Kruskal Wallis test applied to the means (%) shown in Table I; this test performs comparisons between brush groups with both techniques.
TABLE 3. Statistical significance, among brushes, for each technique (Kruskal Wallis test).
| Brushes | Difference between μ | Significance | ||
|---|---|---|---|---|
| Technique I | Technique II | Technique I | Technique II | |
| OralB40 X Johnson & Johnson | 2.90 | 1.90 | Ns (p>0.05) | Ns |
| OralB40 X Denture | 4.60 | 3.40 | 5% (p=0.05) | Ns |
| Johnson & Johnson X Denture | 7.40 | 5.30 | 5% (p=0.05) | Ns |
(Ns): non-significant
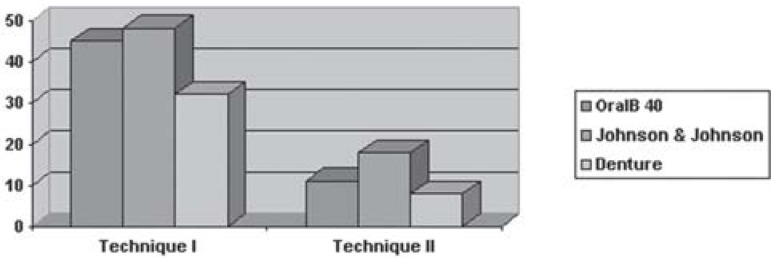
Figure 1 shows the comparison between the sum of means obtained for three brushes in each technique, and compares the techniques with each other
FIGURE 1. Comparison of the sum of means (%) for 3 brushes in each technique and comparison of techniques.
DISCUSSION
The relationship between denture biofilm, mucosal inflammation and Atrophic Chronic Candidiasis has been discussed along the years; there is confirmation that cleansing can help to control or solve the inflammatory conditions of the mucosa by reducing the degree of denture biofilm. Nevertheless, several studies call attention to the precarious oral health of complete denture wearers1,5,11,16,17,19,23,34. The toothbrush is the most important element in any hygiene program and in complete denture hygiene control; brushing is the most common and routine method employed in association with paste as an auxiliary agent.
Some studies have shown that toothbrushes (conventional brushes) can cause wear on the denture materials and do not provide adequate hygiene, since their design is inappropriate to reach the entire denture surfaces (internal and external)7,9,32,33. Such materials are customarily found in other countries, but in the Brazilian market there is only a limited number of specific denture products; as a result, denture wearers make use of conventional brushes to clean their dentures.
Studies regarding natural teeth hygiene emphasize the importance of incentive programs on the control of biofilm formation and many of these indicate home use of disclosing agents as additional means to brushing14,16,25. In the reviews, there are many disclosing agents for natural teeth. However, few solutions are indicated for complete dentures. Complete denture studies indicate the use of these solutions only to quantitatively and qualitatively evaluate the denture biofilm2,3,4,12,17,22,24,28 as a mean to evaluate hygiene products.
Therefore, it is necessary compare the effectiveness of brushes that are available in the Brazilian market, looking for their viable association with alternative methods, as well as using a disclosing agent to make biofilm removal easier and more effective.
Oral B toothbrush (conventional) has established good acceptance and regularity in the Brazilian market along the years. It is composed of 41 soft bristles positioned perpendicularly at the same side of brush's head, which has an oval design. This configuration allows easier and better adaptation to the denture internal surface. The toothbrush has a long anatomic handle that provides a firm grasp.
The Johnson & Johnson brush was designed by Paranhos, et al.30 (2000) to evaluate a specific dentifrice on the control of Atrophic Chronic Candidiasis in denture wearers. It has 26 tufts with 16-mm long bristles positioned at the same side of the brush's head. The longer length makes the bristles become extra-soft and provides a better range to reach the denture areas. The handle is long with no anatomic feature.
The denture brush has a long handle, the head is elongated and has two groups of soft bristles positioned at each side of the head. One of them is more compact with a wedge-like form and is positioned on the extremity of the head. This bristle displays an anterior angulation and is designed to perform brushing on the internal surface of denture. The other group has a larger number of bristles, positioned perpendicular to the head covering its full extension and is designed to perform brushing on the denture's external surface.
With regard to the biofilm quantification method on complete dentures, disclosure is the most employed method in the literature and is customarily associated with the score attribution method (Index). Nevertheless, some components of this methodology allow variations in results when utilized by different examiners.
In this study, the biofilm quantification methodology was based on utilization of disclosed surfaces and a computerized method (Image Toll 2.02) to quantify biofilm. The Image Tool 2.02 program was employed to obtain final results, since this method supplies numerical data that avoid percentage calculations of the biofilm coverage area. Assessment was performed on the internal denture surface, since it is the area most often used to evaluate cleanser efficacy.
Lovato, et al.21 (2000), studying clinical manipulation of a disclosing agent, highlighted the fact that this agent must have the ability to disclose biofilm, be easy to remove from the denture, and not stain the denture after removal. These authors indicated 1% neutral red, 1% Sodium Fluorescein, Replak and 1% Monosulphate Proflavim as disclosing agent.
The 60-day experimental period was divided between Technique I (brushing) and Technique II (brushing and disclose home application). Before starting Technique I, the denture was disclosed and the biofilm was fully removed by brushing (Denture-Brush-Condor; Dentu-Crème, Dentco) by the investigator. At this moment, the biofilm was photographed, because it was not the purpose of this study to evaluate the improvement of denture hygiene related to the patients' hygiene habits, but rather to evaluate the efficacy of techniques I and II used in this study in association with different brushes.
All 3 brush groups started the experiment with technique I, which was employed for 3 weeks. After the end of Technique I, the patients were instructed to return to their habitual hygiene for 15 days so that the investigator could repeat disclosing and brushing of the maxillary complete dentures, to provide full biofilm removal (biofilm-free) before initiation of Technique II (3 weeks). This 15-day interval was important to reduce the influence from one technique on the other.
Throughout the entire experimental period, the patients returned weekly for biofilm quantification, since 7 days is an appropriate period for new biofilm deposition and to allow for good patient control. The 1% Neutral Red was selected due to its good affinity with biofilm, removal efficacy from denture surface and because it does not damage the denture components (teeth and base).
Regarding the efficacy of brushes during Technique II, no significant difference was found among the three groups. These results indicate that it is not necessary to use a specific brush when an auxiliary agent for biofilm identification was employed prior to brushing. By using disclosing, all brushes proved to be efficient. This can be explained by the fact that disclosure promoted biofilm visualization by the patient 5,18,20,25. In agreement with Kipot, et al.18 (1984), it is essential that patients be instructed, trained and motivated to continue adequate oral hygiene.
The increase in life expectancy of the Brazilian population further increases the needs of edentate patients. This study aimed at contributing with maintenance of patients' health, since there is direct association between denture biofilm control and associated pathologies as Chronic Atrophic Candidiasis.
CONCLUSION
Home use of a disclosing agent improved biofilm control in dentures for all 3 groups of brushes. Denture brush was more effective than the others in Technique I, while there was no difference among brushes in Technique II. Therefore, without the use of a disclosing biofilm agent, a specific brush for denture hygiene should be indicated. On the other hand, any type of brush can be used if associated with home use of a disclosing agent.
ACKNOWLEDGMENTS
The authors would like to thank PhD Geraldo Maia Campos for his assistance in developing the statistics; and FAPESP, for its financial assistance in this work (00/05088-3).
REFERENCES
- 1.Abelson DC. Denture plaque and denture cleansers: review of the literature. Gerodontics. 1985;1:202–206. [PubMed] [Google Scholar]
- 2.Ambjornsen E, Valderhaug J, Norheim PW, Floystrand F. Assessment of an additive index for plaque accumulation on complete maxillary dentures. Acta Odontol Scand. 1982;40:203–208. doi: 10.3109/00016358209019813. [DOI] [PubMed] [Google Scholar]
- 3.Andruciolli MCD, Macedo LD, Lara EHG, Panzeri H, Paranhos HFO. Avaliação de pasta higienizadora de próteses totais sobre o biofilme dentadura em pacientes com estomatite de dentadura. Pesqui Odontol Bras. 2002;16:84–84. [Google Scholar]
- 4.Budtz–Jørgensen E. The significance of Candida albicans in denture stomatitis. Scand J Dent Res. 1974;82:151–190. doi: 10.1111/j.1600-0722.1974.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Budtz-JÆRgensen E, Kelstrup J. Enzyme as a denture cleansers. Scand J Dent Res. 1977;58:209–215. doi: 10.1111/j.1600-0722.1977.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 6.Cabargas JM, Cruzat CP, Mierzo HP. Eficacia de limpiador quimico de protesis. Estudo clinico e in vitro. Rev Odontol Chile. 2001;15:1–7. [Google Scholar]
- 7.Cruz PC, Freitas KM, Peracini A, Silva-Lovato CH, Paranhos HFO. Método químico versus método químico-mecânico: comparação clínica na eficácia da remoção de biofilme da Prótese Total. Braz Oral Res. 2005;19:99–99. [Google Scholar]
- 8.Fernandes RAG, Zaniquelli O, Paranhos HFO. Análise dos métodos de contagem de pontos e planímetro na quantificação do biofilme da dentadura – um estudo de validação metodológica. Pesqui Odontol Brás. 2002;16:63–68. doi: 10.1590/s1517-74912002000100011. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez RAG, Zaniquelli O, Ito IY, Silva-Lovato CH, Paranhos HFO. Efetividade de três escovas específicas na remoção do biofilme de próteses totais. Braz Oral Res. 2004;18:190–190. [Google Scholar]
- 10.Gornitsky M, Paradis I, Landaverde G, Malo A-M, Velly AM. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J Can Dent Assoc. 2002;68:39–45. [PubMed] [Google Scholar]
- 11.Hoad-Reddick G, Grant AA, Griffits CS. Investigation into the cleanness of dentures in an elderly population. J Prosthet Dent. 1990;64:48–52. doi: 10.1016/0022-3913(90)90152-3. [DOI] [PubMed] [Google Scholar]
- 12.Hutchins PW, Parker WA. A clinical evaluation of the ability of denture cleaning solutions to remove dental plaque from prosthet devices. N Y State Dent J. 1973;39:363–367. [PubMed] [Google Scholar]
- 13.Iacopino DMD, Willian F, Wathen WF. Oral candidal infection and denture stomatitis: a comprehensive review. J Am Dent Assoc. 1992;123:46–51. doi: 10.14219/jada.archive.1992.0023. [DOI] [PubMed] [Google Scholar]
- 14.Jagger DC, Harrison A. Denture cleansing-the best approach. Br Dent J. 1995;178:413–417. doi: 10.1038/sj.bdj.4808788. [DOI] [PubMed] [Google Scholar]
- 15.Jeganathan S, Payne JÁ, Thean HPY. Denture stomatitis in an elderly edentulous Asian population. J Oral Rehabil. 1997;24:468–472. doi: 10.1046/j.1365-2842.1997.00523.x. [DOI] [PubMed] [Google Scholar]
- 16.Keng SB, Lim M. Denture plaque distribution and effectiveness of a perborate-containing denture cleanser. Quintessence Int. 1996;27:341–345. [PubMed] [Google Scholar]
- 17.Kuc IM, Samaranayake LP, Van Heyst EN. Oral health and microflora in an institucionalised elderly population in Canada. Int Dent J. 1999;49:33–40. doi: 10.1111/j.1875-595x.1999.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 18.Kipioti A, Tsamis A, Mitsis F. Disclosing agents in plaque control. Evaluation of their role during periodontal technique. Clin Prev Dent. 1984;6:9–13. [PubMed] [Google Scholar]
- 19.Kulak-Ozkan Y, Kazazoglu E, Arikan A. Oral higyene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J Oral Rehabil. 2002;29:300–304. doi: 10.1046/j.1365-2842.2002.00816.x. [DOI] [PubMed] [Google Scholar]
- 20.Lang NP, Ostergaard E, Löe H. A fluorescent plaque disclosing agent. J Periodontol Res. 1972;7:59–67. doi: 10.1111/j.1600-0765.1972.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 21.Lovato CH, Paranhos HFO, Ito IY. Aplicação clínica de evidenciadores de biofilme em próteses totais. RPG. 2000;7:311–319. [Google Scholar]
- 22.Mäkila E, Taulio-Korvenm AA. Denture and oral brush for elderly people. Proc Finn Dent Soc. 1988;84:197–200. [PubMed] [Google Scholar]
- 23.Marchini L, Vieira PC, Bossan TP, Montenegro FL, Cunha VP. Self-reported oral hygiene habits among institutionalized elderly and their relationship to the condition of the oral tissues in Taubate, Brazil. Gerodontology. 2006;23:33–37. doi: 10.1111/j.1741-2358.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 24.Moran M, Zuckerberg DA, Monica F. Denture cleanser plaque removal and antibacterial effects. J Dent Res. 1983;62:71–71. [Google Scholar]
- 25.Moskona D, Kaplan I. Oral lesions in elderly denture weares. Clin Prevent Dent. 1992;14:11–14. [PubMed] [Google Scholar]
- 26.Murray ID, Mccabe JF, Storer R. The relationship between the abrasivity and cleaning power of the dentifrice-type denture cleaners. Br Dent J. 1986;161:205–212. doi: 10.1038/sj.bdj.4805932. [DOI] [PubMed] [Google Scholar]
- 27.Nikawa H, Hamada T, Yamashiro H, Kumagai H. A review of in vivo methods to evaluate the efficacy of denture cleansers. Int J Prosthodontics. 1999;12:153–159. [PubMed] [Google Scholar]
- 28.Palenik CJ, Miller CH. In vitro testing of three denture cleaning systems. J Prosthet Dent. 1984;51:751–754. doi: 10.1016/0022-3913(84)90369-x. [DOI] [PubMed] [Google Scholar]
- 29.Paranhos HFO, SILVA CHL. Comparative study of methods for the quantification of biofilm on complete dentures. Braz Oral Res. 2004;18:215–223. doi: 10.1590/s1806-83242004000300007. [DOI] [PubMed] [Google Scholar]
- 30.Paranhos HFO, Lara EHG, Panzeri H, Candido RC, Ito IY. Capacity of denture plaque removal and antimicrobial action of a specific paste formulated for denture cleaning. Braz Dent J. 2000;11:97–104. [PubMed] [Google Scholar]
- 31.Raybin M. Disclosing solutions. Dent Itens Int. 1945;67:235–243. [Google Scholar]
- 32.Richmond R, Macfarlane TV, McCord JF. An evaluation of the surface changes in PMMA biomaterial formulations as a result of toothbrush/dentifrice abrasion. Dent Mater. 2004;20:124–132. doi: 10.1016/s0109-5641(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 33.Salles AES, Macedo LD, Silva-Lovato CH, Paranhos HFO. Avaliação da eficácia de agentes auxiliares higienizadores na remoção do biofilme em próteses totais. Braz Oral Res. 2005;19:191–191. [Google Scholar]
- 34.Sheen SR, Harrison A. Assessment of plaque prevention on dentures using an experimental cleanser. J Prosthet Dent. 2000;84:594–601. doi: 10.1067/mpr.2000.110498. [DOI] [PubMed] [Google Scholar]
- 35.Silva CHL, Paranhos HFO, Ito IY. Evidenciadores de biofilme em prótese total: avaliação clínica e antimicrobiana. Pesqui Odontol Bras. 2002;16:270–275. doi: 10.1590/s1517-74912002000300015. [DOI] [PubMed] [Google Scholar]


