Abstract
Background and Purpose
For rehabilitation strategies to be effective, training should be based on principles of motor learning, such as feedback-error learning, that facilitate adaptive processes in the nervous system by inducing errors and recalibration of sensory and motor systems. This case report suggests that locomotor resistance training can enhance somatosensory and corticospinal excitability and modulate resting-state brain functional connectivity in a patient with motor-incomplete spinal cord injury (SCI).
Case Description
The short-term cortical plasticity of a 31-year-old man who had sustained an incomplete SCI 9.5 years previously was explored in response to body-weight–supported treadmill training with velocity-dependent resistance applied with a robotic gait orthosis. The following neurophysiological and neuroimaging measures were recorded before and after training. Sensory evoked potentials were elicited by electrical stimulation of the tibial nerve and recorded from the somatosensory cortex. Motor evoked potentials were generated with transcranial magnetic stimulation applied over the tibialis anterior muscle representation in the primary motor cortex. Resting-state functional magnetic resonance imaging was performed to evaluate short-term changes in patterns of brain activity associated with locomotor training.
Outcomes
Somatosensory excitability and corticospinal excitability were observed to increase after locomotor resistance training. Motor evoked potentials increased (particularly at higher stimulation intensities), and seed-based resting-state functional magnetic resonance imaging analyses revealed increased functional connectivity strength in the motor cortex associated with the less affected side after training.
Discussion
The observations suggest evidence of short-term cortical plasticity in 3 complementary neurophysiological measures after one session of locomotor resistance training. Future investigation in a sample of people with incomplete SCI will enhance the understanding of potential neural mechanisms underlying the behavioral response to locomotor resistance training.
Gait retraining strategies after neurological injury have been focused on body-weight–supported treadmill training (BWSTT) approaches, in which repetitive movement of the legs through the gait cycle is enabled by the treadmill and assisted by therapists or robotic devices.1,2 These approaches support motor learning theory underpinnings, such as task-specific practice, by providing locomotor-related sensory cues through feedback pathways to facilitate the production of appropriate muscle activation patterns.3,4 Loading through the legs during stance provides sensory input facilitating the production of extensor muscle activity,4–6 and hip flexor muscle stretch at late stance facilitates the initiation of the swing phase.7 However, guiding the limbs through the “correct” gait movements may not adequately challenge the motor control system for neurological adaptation to enable better functional ambulation.8
Feedback-error learning is based on evidence that sensory and motor neural networks can adapt in response to performance errors during training. Afferent feedback pathways provide information to the central nervous system (CNS) for error detection and adaptation of motor programs.9 Short-term locomotor adaptations have been observed after repeated exposure to an altered movement environment; as a result, persistent changes in afferent input are thought to be mediated by feedback-error learning.9 Body-weight–supported treadmill training can be combined with feedback-error learning by applying resistance to lower limb flexion movements. The addition of resistance will enhance the activation of length- and load-sensitive receptors in the flexor muscles.10 Similar to the effect of load on extensor motoneurons, this approach may produce sustained excitatory drive to flexor motoneurons through afferent feedback pathways.
Short-term locomotor adaptations to robot-applied resistance produced high-stepping or longer-stride aftereffects in people with motor-incomplete spinal cord injury (SCI).11–13 After 3 months of training, these aftereffects were found to be transferred to overground stepping examined immediately after a training session.14 Other indexes of overground ambulatory capacity (eg, 10-Meter Walk Test) and more complex walking tasks (eg, obstacle crossing and stair climbing) also improved after training.14
Feedback-error learning through training with resistive-force fields has been proposed to lead to adaptation of functionally specific sensory and motor areas of the brain. Real-time changes in motor cortex excitability in response to different task demands (eg, locomotor resistance or assistance) have revealed the adaptive nature of muscle-specific pathways.15,16 Motor learning after upper limb resistance training also involves adaptations in somatosensory function, as evidenced by changes in the amplitude of the response to a sensory evoked potential (SEP).17 These findings of adapted pathways are complemented by evidence of more specific changes in sensory and motor cortical regions associated with motor learning. Parietal, frontal, and cerebellar networks showed increased functional connectivity during resting-state functional magnetic resonance imaging (fMRI) after 1 hour of upper limb resistance training. The connectivity strength of these neuronal networks was found to be related to improved behavioral measures of motor learning.18 Thus, neurophysiological and resting-state fMRI measures can be used to determine how locomotor resistance training leads to adaptation of sensory and motor networks through feedback-error learning.
This case report explored whether locomotor resistance training can enhance somatosensory and corticospinal excitability and resting-state functional connectivity as indexes of short-term adaptations after feedback-error learning in a patient with motor-incomplete SCI.
Patient History and Review of Systems
The patient was a 31-year-old man (height=170 cm, weight=60 kg) who had sustained a traumatic motor-incomplete SCI at C5 (as determined with the American Spinal Injury Association Impairment Scale) after a fall 9.5 years earlier. His lower extremity motor scores on this scale were 5 of 25 (right) and 18 of 25 (left), light touch scores were 34 of 56 (right) and 34 of 56 (left), and pin prick scores were 34 of 56 (right) and 36 of 56 (left). The Walking Index for Spinal Cord Injury Scale was scored at 9. He was completely independent with activities of daily living, primarily using a power wheelchair for indoor and outdoor mobility, but was able to stand and walk indoors for short distances with a walker and a Dictus brace (which provides dorsiflexion assistance; Dictus, Henderson, Nevada) on the right. His self-selected comfortable walking speed was 0.18 m/s, as measured with the 10-Meter Walk Test. He had no significant medical history and was not taking prescription medications. The patient's goals were to improve ambulatory capacity and more complex walking skills. He was practicing walking short distances on a daily basis with parallel bars or a wheeled walker.
This patient's limited ambulatory capacity and residual motor function made him an ideal candidate for our approach (ie, application of feedback-error learning through locomotor resistance training). He also had experience with BWSTT on a Lokomat robotic gait orthosis (Hocoma AG, Volketswil, Switzerland) 2 months before our testing. This experience demonstrated his ability to physically tolerate locomotor training. The patient performed a session of unassisted BWSTT, and we quantitatively measured his lower limb flexor muscle strength to further determine the appropriateness of our approach. The patient provided written informed consent.
Examination
We measured the patient's maximum voluntary contraction for bilateral hip and knee flexor muscles using isometric strength testing with the Lokomat L-Force feature.19 Three trials were performed to calculate an average maximum voluntary contraction per joint. Positive force values were recorded for all joints (left hip=15.7 N, left knee=20.8 N, right hip=7.9 N, right knee=1.3 N). The patient also performed 20 minutes of unassisted BWSTT at 1.1 km/h with 10 kg of body-weight support so that we could record his baseline hip and knee kinematic trajectories during treadmill walking. These data were subsequently used to calculate the amount of resistance applied by the Lokomat (see below). The patient's score of 5 for the rate of perceived exertion after the session was equivalent to “hard” on the Borg CR-10 Scale. This examination indicated that the patient had sufficient lower limb strength and was able to physically tolerate 20 minutes of unassisted BWSTT, confirming his capability to undergo our approach.
Clinical Impression
Given the patient's goal of improving functional ambulation and the data from the examination, we considered him to be an appropriate candidate for the application of feedback-error learning through locomotor resistance training. We evaluated changes in sensory and motor neural pathways in response to feedback-error learning using neurophysiological (SEPs and motor evoked potentials [MEPs]) and fMRI measures. If locomotor resistance training is a successful approach for feedback-error learning, then SEPs and MEPs should show the facilitation of neural pathways and increased resting-state functional connectivity should be observed in sensory and motor cortical areas.
Intervention
Locomotor Training
We explored the effect of a single bout of locomotor resistance training on neurophysiological outcomes. Because of the time demands of conducting our evaluations, we obtained SEP and fMRI recordings before and after session 1 and MEP recordings before and after session 2. Each session involved 30 minutes of BWSTT with the Lokomat, and the sessions were scheduled 25 days apart.
Body-weight–supported treadmill training was implemented with customized software control of the Lokomat robotic gait trainer. The drives were programmed to apply a velocity-dependent moment against both hip and knee sagittal-plane movements throughout the step cycle. The instantaneous torque (M) applied to hip (Mh) and knee (Mk) joints was calculated as follows:
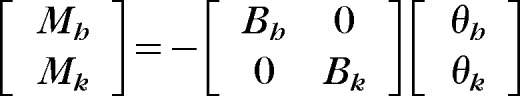 |
where Bh and Bk are the corresponding viscous coefficients (N·m·s/radian) and θh and θk are the angular velocities (radians per second) of the hip and knee, respectively.14 Data from the unassisted BWSTT session were used to determine the hip and knee angular velocities during swing from the time between the onset of hip flexion and the onset of extension with a customized MATLAB (The MathWorks Inc, Natick, Massachusetts) program. The average angular velocities of the hip and knee (θh and θk) during swing were used to determine the desired B values. The amount of resistance (Mh and Mk) was defined as 10% of the maximum voluntary contraction. Because the resistance was velocity dependent, its effect would be greatest during the swing phase, leading to error detection via afferent feedback pathways to the CNS and modification of the locomotor commands to enhance flexor muscle activity to overcome the resistance.9,10
Locomotor resistance training was conducted at 1.1 km/h, the fastest speed tolerated by the patient, with 10 kg of body weight support. During training, the patient was allowed to use his upper extremities on the handrails for balance and light support, if needed. The patient reported his rate of perceived exertion (Borg CR-10 Scale) after training. We monitored heart rate and blood pressure responses to training.
Neurophysiological Measures
Sensory evoked potentials were measured before and after the first training session, with 2.5 hours between training and testing. Sensory evoked potentials were derived from square wave pulses (0.5-millisecond duration) delivered through surface electrodes to the tibial nerve in the popliteal fossa (SD9 Stimulator with SIU-V Stimulus Isolation Unit, Grass Products, Natus Neurology Inc, West Warwick, Rhode Island) with the patient seated. Stimuli were delivered at 2 Hz for a total of 300 stimulations for 3 different conditions: sensory, motor, and 150% motor thresholds. Intensity was set to the minimum stimulator output required to produce sensation distal to the stimulation site (sensory threshold), to produce a small muscle twitch (motor threshold), and to produce 150% of the motor threshold. Electroencephalographic data were recorded from the Cz, CPz, AFz, C1 to C4, and CP1 to CP4 electrode sites (64-channel NEURO PRAX MR direct-current electroencephalographic system, neuroConn GmbH, Ilmenau, Germany) in accordance with the international 10–20 system for electrode placement and referenced to the right mastoid. Impedance was kept below 5 kΩ. Electroencephalographic data were amplified (20,000 times), notch filtered at 50 Hz, and digitized (2,000 Hz) before being stored for offline analysis. Sensory evoked potentials were extracted with the EEGLAB toolbox (Institute for Neural Computation, University of California, San Diego, California) for MATLAB (MATLAB V2009, The MathWorks Inc) by averaging baseline corrected epochs time locked to stimulation (within −100 to 200 milliseconds) after rejecting trials with any visually identified artifact. The SEP amplitude was measured as the difference between the negative response at 50 milliseconds and mean baseline activity.
Resting-state fMRI was conducted before and after the first training session, with 1.5 hours between training and testing. A single resting-state fMRI scan (echoplanar imaging sequence, repetition time=2,000 milliseconds, echo time=30 milliseconds, flip angle θ=90°, voxel dimension=3 mm3 with a 1-mm gap, 36 slices, field of view=240 × 240 mm, scan time=8.2 minutes per scan) was recorded with the patient lying supine with eyes fixed on a visual stimulus. The patient was instructed to think of nothing in particular and not to fall asleep. Scanning during rest allowed examination of functional connectivity between the motor cortex and other CNS areas without the influence of a specific task. Resting-state fMRI data processing and analysis were carried out with a data processing assistant for resting-state fMRI (DPARSF v2.3; http://www.restfmri.net/forum/DPARSF),20 a MATLAB plug-in based on statistical parametric mapping (http://www.fil.ion.ucl.ac.uk/spm),21 and a resting-state fMRI data analysis toolkit (http://www.restfmri.net).22 For data processing, the first 10 time points were discarded, and slice timing and head motion correction were performed. Functional data were then coregistered to the T1-weighted anatomical image (repetition time=7.7 milliseconds, echo time=3.5 milliseconds, flip angle θ=8°, voxel dimension=1 mm3, 170 slices, field of view=256 mm, scan time=191 seconds) and resampled to a 3-mm isotropic voxel resolution before normalization into Montreal Neurological Institute (MNI) template space.
The data were smoothed with a 4-mm full width at half maximum Gaussian kernel, and the linear trend of the time courses was removed and temporally band-pass filtered (0.01–0.08 Hz). Nuisance covariates (6 head motion parameters, global signal, white matter signal, and cerebrospinal signal) were regressed from the processed data before functional connectivity analysis. A spherical region-of-interest seed was placed within the right motor cortex (MNI template space coordinates: x=8, y= −42, z=66; radius=6 mm) and left motor cortex (MNI template space coordinates: x=−8, y=−42, z=66; radius=6 mm) associated with the motor representation of the contralateral lower extremity. Functional connectivity was quantified for each seed region of interest as the linear correlation of the time course between the seed region of interest and each voxel within the brain.
Motor evoked potentials were measured immediately before and after the second training session. Motor evoked potentials were elicited while the patient was seated. Surface electromyography (EMG) data were recorded bilaterally from the tibialis anterior muscle. Electromyography recordings were amplified (2,000 times) and band-pass filtered (1–200 Hz) before being digitized (1,000 Hz). Transcranial magnetic stimulation was delivered to the leg motor cortex with a 70-mm double-cone coil (Magstim Super Rapid2, The Magstim Company Ltd, Spring Gardens, Whitland, Carmarthenshire, United Kingdom). The optimum site for eliciting an MEP was determined and marked on the patient's head with a washable marker.
The patient was asked to maintain a background activity of approximately 10% of his maximum voluntary contraction (determined by manual muscle testing by a physical therapist). Background EMG data were measured as the mean of the rectified EMG signal at 50 milliseconds before the transcranial magnetic stimulation pulse to verify that no changes occurred during training. A recruitment curve was generated by applying stimuli in 10% increments of stimulator output in sequential order, starting from below the motor threshold to the maximum MEP amplitude.16 Five stimuli were delivered at each intensity, with a 5-second interstimulus interval and a rest period between intensity levels. The average peak-to-peak MEP amplitude was calculated for each intensity level.
Outcome
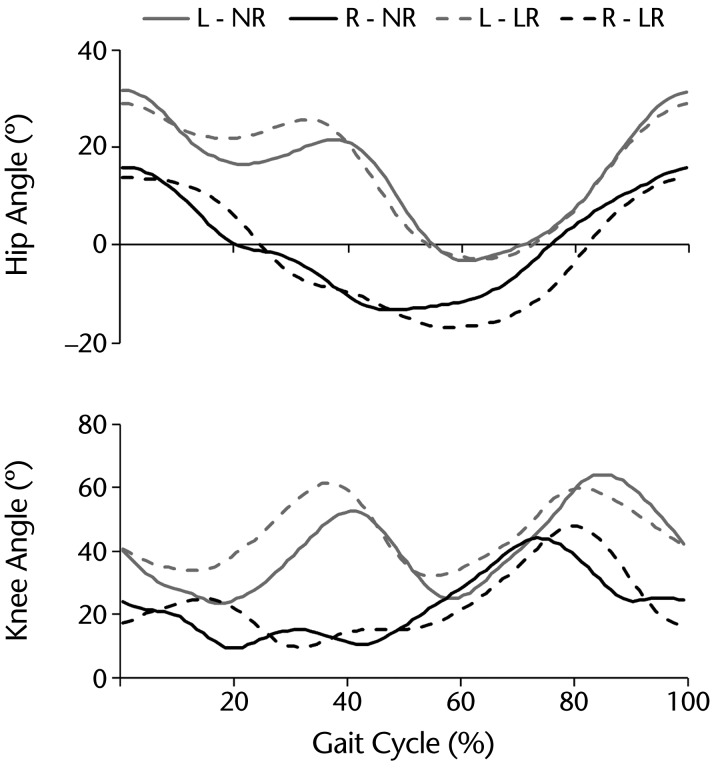
The patient was able to physically tolerate locomotor training with resistance. His rating of perceived exertion after both training sessions was 7 (“very hard”). Average resting heart rate and blood pressure were 76 bpm and 130/70 mm Hg, respectively. Locomotor training increased heart rate and blood pressure to 92 bpm and 147/83 mm Hg, respectively, in the last 5 minutes. Heart rate and blood pressure returned to 82 bpm and 124/80 mm Hg, respectively, at 5 minutes after training. Figure 1 shows the average hip and knee joint angles during the gait cycle with and without resistance (also see the video below).
Figure 1.
Graphic representation of the resistance added during training. Hip and knee joint angles during the gait cycle for the left (L; gray lines) and right (R; black lines) sides are plotted to show nonresistance (NR; solid lines) and Lokomat resistance (LR; dashed lines) walking. Average joint angle curves were calculated from 25 steps and normalized to the percentage of the gait cycle time (0% [heel-strike]–100% [subsequent ipsilateral heel-strike]). Upward deflections represent flexion.
Session 1 Results
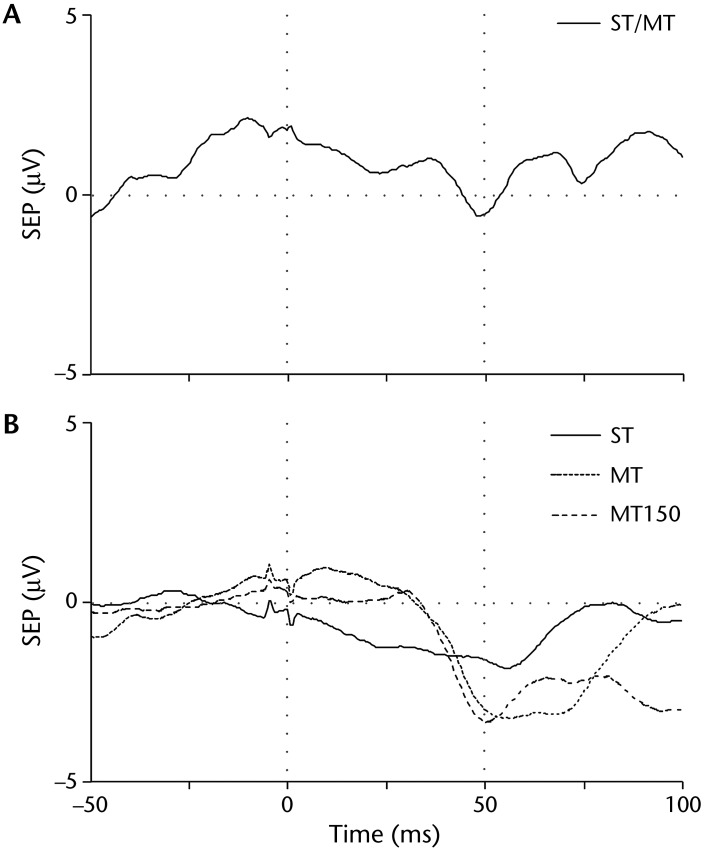
Before training, the sensory threshold for the right leg was at the maximum stimulator output. At this intensity, a palpable muscle twitch distal to the stimulator indicated that the motor threshold had also been reached; therefore, no further stimulation intensities could be tested. We observed a negative response at Cz approximately 50 milliseconds after stimulation (Fig. 2A). During the posttraining assessment, SEPs for both sensory and motor thresholds were reduced in the right leg, and we could test stimulation intensities at up to 150% motor threshold. Sensory evoked potentials showed changes at Cz, with visualization of a negative response at 50 milliseconds for each condition. The amplitude of the negative response at 50 milliseconds was larger for the SEP motor and 150% motor thresholds than for the SEP sensory threshold (Fig. 2B). The patient did not show any observable changes in SEPs for the sensory and motor thresholds for the left leg.
Figure 2.
Average sensory evoked potentials (SEPs) for the sensory threshold (ST), motor threshold (MT), and 150% motor threshold (MT150) before training (A) and after training (B) for the left hemisphere over the Cz electrode (right leg). Before training, the ST and MT were at the maximum stimulator output; therefore, no further conditions were tested. After training, data for all conditions could be collected. The amplitude of the negative response at 50 milliseconds (vertical gray dotted line) was larger for the MT and MT150 than for the ST after training.
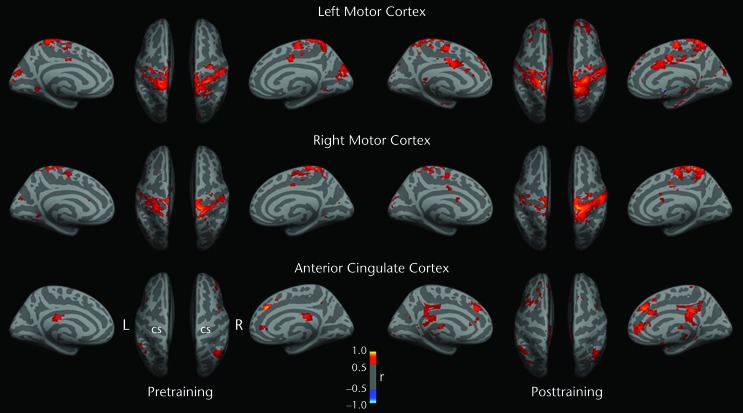
Before training, resting-state fMRI-based correlation analyses showed greater intrahemispheric and interhemispheric homotopic functional connectivities for the left leg representation in the motor cortex than for the right leg representation in the motor cortex. After training, increased local intrahemispheric homotopic functional connectivity strength and decreased interhemispheric homotopic functional connectivity strength were observed for the right motor cortex seed. The functional connectivity topography and strength for the left motor cortex seed were largely unchanged after training (Fig. 3).
Figure 3.
Functional connectivity (FC) results projected onto inflated medial and superior cortical surfaces in standard Montreal Neurological Institute (MNI) template space. At baseline, greater FC strength was observed when the left (L) motor cortex leg representation was seeded than when the right (R) motor cortex leg representation was seeded. After training, increased FC was observed for the leg areas of both primary motor cortexes and multiple cortical regions. When the right motor cortex was seeded, connectivity strength appeared to show increased lateralization after training; this feature was not apparent for the left motor cortex. Warmer colors represent higher positive correlations between seed activity and voxelwise brain resting activity. The right anterior cingulate cortex (MNI coordinates: x=5, y=34, z=28) was used as a control site for changes in nonmotor cortical regions. This region was shown by resting-state fMRI to be functionally connected to nonmotor prefrontal cortical regions related to attentional processing and executive function.32 The spatial pattern of connectivity was largely unchanged after training. CS=central sulcus.
Session 2 Results
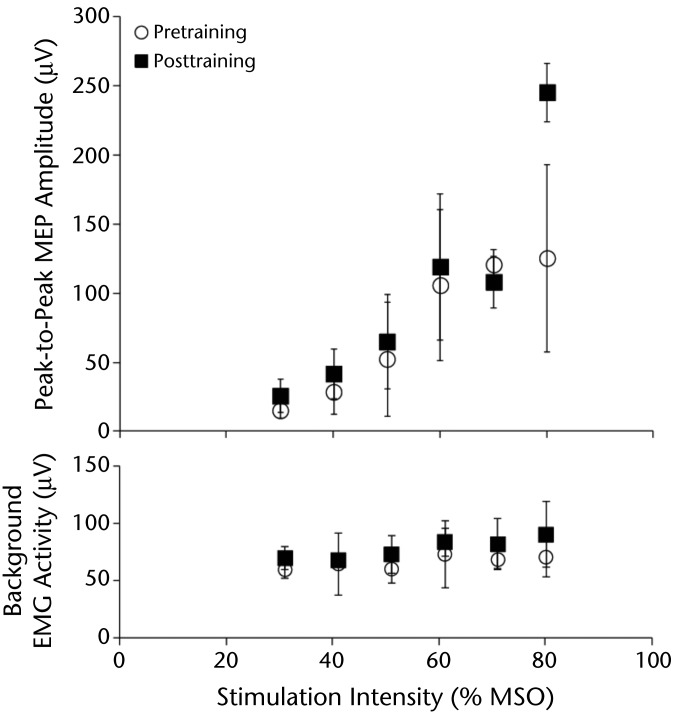
Average peak-to-peak MEP amplitudes increased with higher stimulation intensities for the left leg. Posttraining peak-to-peak MEP amplitudes were larger than pretraining peak-to-peak MEP amplitudes for most stimulation intensities, in particular, the highest intensity (Fig. 4). Before and after training, no MEPs could be elicited from the right leg.
Figure 4.
Average peak-to-peak motor evoked potential (MEP) amplitudes from the left tibialis anterior muscle (less affected side) before (white circle) and after (black square) session 2 of treadmill walking with Lokomat resistance. Motor evoked potentials were higher for most stimulation intensities tested after training, in particular, the highest intensity. Stimulation intensity is expressed as a percentage of maximum stimulator output (% MSO). The mean background rectified electromyography (EMG) activity 50 milliseconds before the stimulus for each stimulation intensity is plotted. Error bars denote standard deviations. We were not able to elicit MEPs for the right limb, which had more severe sensorimotor impairments.
Discussion
This case report demonstrates how feedback-error learning can be implemented with BWSTT in a patient with incomplete SCI to challenge locomotor control and adapt CNS mechanisms. Feedback-error learning was applied through locomotor resistance training in adults who were healthy and people with incomplete SCI12,14,15; however, our case report is the first to describe how this approach supports the neurophysiological underpinnings of motor learning theory after CNS injury. We observed short-term changes in cortical activity after a session of Lokomat training with resistance, suggesting sensorimotor adaptations in response to the activation of afferent feedback pathways. Our findings suggest that exposure to walking in an altered movement environment, such as resistive forces, changes sensory and motor representations of the lower limb to facilitate recalibration to an appropriate motor program.9
Before training, it was difficult to elicit SEPs from the patient, whereas both sensory and motor responses showed larger amplitudes in the leg motor cortex area after training. A possible explanation for this outcome is that increased transmission in spinal feedback pathways from proprioceptive muscle afferents leads to the facilitation of sensorimotor pathways.23 A change in somatosensory function after feedback-error learning seems possible because of direct connections between somatosensory areas and the primary motor cortex,24–26 along with other motor areas in the brain.27 Changes in SEPs on the right side may have reflected the sparing of sensory tracts, even though no MEPs could be produced. Recent evidence showed that motor learning produced changes in sensory function, in terms of a larger SEP magnitude and enhanced sensorimotor connectivity, after resistance training of an upper limb motor task in adults who were healthy.17,18 Importantly, these changes were not observed in patients practicing the motor task without resistance or during passive movements (without motor activation).17,18 The increased SEPs detected in our case report likely were specific to the effect of resistance, in agreement with the theory that resistance drives error feedback along sensory pathways to the CNS.
An important outcome for our patient was the larger MEP amplitudes after resistance training, indicating enhanced excitability of the corticospinal tract to the leg muscles. This outcome was consistent with the roles of the primary motor cortex and corticospinal tract in modulating locomotor adaptations.16 Barthelemy et al15 showed that MEPs in the tibialis anterior muscle could be modulated by locomotor adaptations to forces around the ankle in adults who were able-bodied. The application of a force that assisted ankle dorsiflexion resulted in MEP suppression, whereas a resistive force resulted in MEP facilitation during walking. In addition, locomotor adaptations to walking with the Lokomat (compared with regular treadmill walking) included increased MEP amplitudes elicited in the knee flexor muscles.16 We also observed increased MEPs (recorded while the patient was seated) after only one training session. Such enhanced corticospinal excitability could underlie the retention of motor adaptations that we previously observed after months of Lokomat resistance training.28 Facilitation of the corticospinal pathway from the primary motor cortex provides evidence of the successful application of feedback-error learning.
Although primary motor cortex projections through the corticospinal tract could have been accessed by transcranial magnetic stimulation, resting-state fMRI provided the opportunity to characterize changes in the functional connectivity of the motor cortex with other CNS areas related to motor learning and walking function. Resting-state fMRI activity patterns after training revealed alterations in functional connectivity for the leg motor cortex representations, adjacent cortical regions, and homotopic regions in the contralateral hemisphere, primarily for the right motor cortex. These changes may have been linked to underlying short-term neural modulation, such as improved synaptic efficiency, from exposure to resistive forces during training.
Our findings are similar to those of a previous study showing functional connectivity changes in the cerebellar cortex and parietal and frontal motor areas after exposure to resistive forces during an upper limb motor task.18 Specifically, Vahdat et al18 reported that changes in connectivity between the cerebellar cortex and frontal motor areas (primary motor cortex and supplementary motor area) were dependent on motor learning. Sensorimotor relationships adapted through learning to correct for resistive forces can be retained after training. Our measures of short-term cortical plasticity may reflect the modulation of both sensory and motor pathways in response to locomotor adaptations derived from feedback-error learning. We also observed greater changes for the right motor cortex seed (associated with the less affected side) than for the left motor cortex seed, suggesting that functional connectivity may depend on the integrity of preserved corticospinal tracts (our patient had more severe motor impairments in the right lower limb). It also is interesting to speculate whether the observed increase in local connectivity strength in the right sensorimotor regions may have been related to the observed increases in both MEPs and SEPs after training. It is possible that increasing local connectivity strength could have had a summative effect that contributed to the observed increases in evoked potentials. Future research should examine whether resting-state functional connectivity is correlated with measures of sensorimotor excitability and how these measures may relate to behavioral assessments of sensorimotor impairment in people with SCI.
It has been proposed that adaptation through feedback-error learning depends on both sensory and motor systems. Motor learning can stem from changes in motor commands, sensory feedback, or both. One possibility is that motor learning involves adjustments to motor commands that recalibrate the CNS contribution, resulting in subsequent downstream effects that augment proprioceptive feedback. Another possibility is that feedback-error learning involves recalibration of both sensory and motor systems.18,29 Previous evidence showed that active involvement in the production of movement is required to properly engage feedback-error pathways.29
Because of the nature of a case report, it is not possible to determine whether BWSTT alone (without resistance) would have had the same result as locomotor training with resistance. Because the locomotor resistance paradigm was a relatively novel activity for our patient, it is possible that any type of extended or intense locomotor training—compared with his usual daily routine—would have enhanced sensorimotor pathways. Previous studies showed differences in behavioral and neural indexes of motor adaptation between patients training with resistance and patients training without resistance for a novel upper limb motor task.18,29 Although adaptations to locomotor behavior were reported for this paradigm in adults who were able-bodied and people with CNS injury,12,14,30 future research should focus on understanding neural mechanisms after long-term training and on observations of functional improvements to confirm how feedback-error learning theory is applied through locomotor resistance training.
Although this case report provides important information about how locomotor resistance training supports feedback-error learning, several limitations need to be addressed. Because of time constraints, we did not perform repeated baseline assessments to ensure the stability of the neurophysiological measures before the intervention. Although there is inherent variability in outcomes, we took precautions to reduce variability from the environment and investigators. However, changes in the patient's arousal state may have influenced the outcomes. Future controlled studies with masked assessors and a sham condition are warranted to determine the benefit of locomotor resistance training.
During data collection, resistance may have changed because of reapplication of the electrodes for the SEPs; this possibility may explain the reduced sensory and motor stimulation thresholds after training. We were unable to match M-wave amplitudes between pretraining and posttraining evaluations; therefore, changes in current delivery may have influenced SEP amplitudes. We attempted to control for this potential confound by reestablishing stimulation thresholds before SEP recording at each session. We used manual palpation to determine the appropriate level of background EMG activity while eliciting MEPs. We cannot discount the possibility that when we interpreted changes in MEP amplitudes after training, the slight increase in background EMG activity at 80% maximum stimulator output affected the resultant MEP amplitudes. However, Devanne et al31 previously reported similar maximum MEP amplitudes for background contractions of 10% to 40% of maximum voluntary effort. Furthermore, we might have generated different recruitment curves had we used a random rather than a serial stimulation approach. However, because we used the same approach before and after the intervention, it is unlikely that order affected the results. In addition, the patient used his upper extremity to provide light support during the swing phase, mostly with the more affected side, similar to his compensatory strategies for overground walking. The use of the upper extremity for support may have reduced the load on the extensor muscles during training. However, this possibility likely would not have affected the activation of the flexor muscles and the response to the added resistance.2,10
In summary, we have shown that locomotor resistance training may be a successful approach for implementing feedback-error learning theory in gait rehabilitation strategies after incomplete SCI. The data suggest that changes in resting-state functional connectivity, SEPs, and MEPs can be detected after 1 training session and may be sensitive to the sensorimotor adaptations underlying the feedback-error learning associated with locomotor resistance training. However, the findings described in this case report should be interpreted with caution, given some of the methodological limitations and given that only one patient was treated. Nevertheless, the measures provide a complementary and integrative view of possible neural mechanisms underlying the response to locomotor resistance training, which has been shown to enhance the recovery of functional ambulation after incomplete SCI.14 These findings raise interesting possibilities for the design and evaluation of gait rehabilitation strategies for people with incomplete SCI.
Supplementary Material
Footnotes
All procedures were approved by the University of British Columbia Ethics Board.
Dr Borich was supported by the Heart and Stroke Foundation. Ms Peters is supported by the Canadian Institutes for Health Research. Support was provided to Dr Boyd by the Canada Research Chairs and the Michael Smith Foundation for Health Research. Dr Lam was supported by a Canadian Institutes for Health Research New Investigator Award.
References
- 1. Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- 2. Dobkin BH, Harkema S, Requejo P, Edgerton VR. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil. 1995;9:183–190. [PubMed] [Google Scholar]
- 3. Edgerton VR, de Leon RD, Tillakaratne N, et al. Use-dependent plasticity in spinal stepping and standing. Adv Neurol. 1997;72:233–247. [PubMed] [Google Scholar]
- 4. Harkema SJ, Hurley SL, Patel UK, et al. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. [DOI] [PubMed] [Google Scholar]
- 5. Sinkjaer T, Andersen JB, Ladouceur M, et al. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res. 1991;87:679–687. [DOI] [PubMed] [Google Scholar]
- 7. Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. [DOI] [PubMed] [Google Scholar]
- 8. Lewek MD, Cruz TH, Moore JL, et al. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearson KG. Neural adaptation in the generation of rhythmic behavior. Annu Rev Physiol. 2000;62:723–753. [DOI] [PubMed] [Google Scholar]
- 10. Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol. 2006;95:766–773. [DOI] [PubMed] [Google Scholar]
- 11. Lam T, Noonan VK, Eng JJ. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2008;46:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Houldin A, Luttin K, Lam T. Locomotor adaptations and aftereffects to resistance during walking in individuals with spinal cord injury. J Neurophysiol. 2011;106:247–258. [DOI] [PubMed] [Google Scholar]
- 13. Yen SC, Schmit BD, Landry JM, et al. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res. 2012;216:473–482. [DOI] [PubMed] [Google Scholar]
- 14. Lam T, Pauhl K, Krassioukov A, Eng JJ. Using robot-applied resistance to augment body-weight–supported treadmill training in an individual with incomplete spinal cord injury. Phys Ther. 2011;91:143–151. [DOI] [PubMed] [Google Scholar]
- 15. Barthelemy D, Alain S, Grey MJ, et al. Rapid changes in corticospinal excitability during force field adaptation of human walking. Exp Brain Res. 2012;217:99–115. [DOI] [PubMed] [Google Scholar]
- 16. Zabukovec JR, Boyd LA, Linsdell MA, Lam T. Changes in corticospinal excitability following adaptive modification to human walking. Exp Brain Res. 2013;226:557–564. [DOI] [PubMed] [Google Scholar]
- 17. Nasir SM, Darainy M, Ostry DJ. Sensorimotor adaptation changes the neural coding of somatosensory stimuli. J Neurophysiol. 2013;109:2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci. 2012;31:16907–16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolliger M, Banz R, Dietz V, Lünenburger L. Standardized voluntary force measurement in a lower extremity rehabilitation robot. J Neuroeng Rehabil. 2008;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friston KJ, Frith CD, Liddle PF, et al. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. [DOI] [PubMed] [Google Scholar]
- 22. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez M, Brezun JM, Zennou-Azogui Y, et al. Sensorimotor training promotes functional recovery and somatosensory cortical map reactivation following cervical spinal cord injury. Eur J Neurosci. 2009;30:2356–2367. [DOI] [PubMed] [Google Scholar]
- 24. Darian-Smith C, Darian-Smith I, Burman K, Ratcliffe N. Ipsilateral cortical projections to areas 3a, 3b, and 4 in the macaque monkey. J Comp Neurol. 1993;335:200–213. [DOI] [PubMed] [Google Scholar]
- 25. Jones EG, Coulter JD, Hendry SH. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol. 1978;181:291–347. [DOI] [PubMed] [Google Scholar]
- 26. Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J Comp Neurol. 1999;403:431–458. [PubMed] [Google Scholar]
- 28. Lam T, Bigelow A, Krassioukov A, Eng JJ. Treadmill training with Lokomat-applied resistance to enhance ambulation in people with incomplete spinal cord injury. In: Proceedings of the American Physical Therapy Association Combined Sections Meeting Alexandria, VA: American Physical Therapy Association; 2012. [Google Scholar]
- 29. Ostry DJ, Darainy M, Mattar AA, et al. Somatosensory plasticity and motor learning. J Neurosci. 2010;30:5384–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam T, Wirz M, Lunenburger L, Dietz V. Swing phase resistance enhances flexor muscle activity during treadmill locomotion in incomplete spinal cord injury. Neurorehabil Neural Repair. 2008;22:438–446. [DOI] [PubMed] [Google Scholar]
- 31. Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. [DOI] [PubMed] [Google Scholar]
- 32. Margulies DS, Kelly AM, Uddin LQ, et al. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.