Abstract
Background/Aims
The cardiorenal syndrome is a complication in patients hospitalized with chronic heart failure (CHF). The β2-microglobulin (b2M) level is an index of decreased glomerular filtration rate (GFR), tissue turnover and inflammation. It is an emerging new predictive marker of cardiovascular events and mortality, but its role as a biomarker of cardiorenal remodeling and failure is still unknown. TIMP1, an endogenous tissue inhibitor of activated matrix metalloproteinases, is a biomarker of heart remodeling and failure. We aimed to evaluate the circulating profile of b2M and TIMP1 in CHF patients, in sedentary controls with no tissue remodeling and in veteran athletes with physiological cardiorenal remodeling and athlete's heart (AH).
Methods
We investigated the plasma levels of b2M and TIMP1 in 24 subjects with CHF without primitive renal disease, in 25 sedentary controls and in 30 veteran marathoners with AH over 50 years.
Results
The b2M and TIMP1 levels were higher in CHF patients, and there was a correlation between them (r = 0.5287, p < 0.0095). The b2M level correlated with the severity of cardiorenal impairment: with proBNP (r = 0.66, p > 0.0007), percent ejection fraction (r = −0.56, p = 0.0162) and GFR (r = 0.83, p < 0.0001). b2M was also correlated with TIMP1 in AH subjects (r = 0.7548, p < 0.0001) but not in controls. This correlation was independent from GFR in both CHF patients and sedentary controls.
Conclusions
In CHF patients, the plasma levels of b2M and TIMP1 were linked together and correlated with the severity of cardiorenal failure. Moreover, a strong correlation between b2M and TIMP1 characterized cardiovascular remodeling not only in CHF patients but also in AH subjects. These findings suggest that clinicians should use b2M and TIMP1 as associated biomarkers of cardiorenal remodeling and failure.
Key Words: Cardiorenal failure, β2-Microglobulin, TIMP1
Introduction
Patients with heart failure often show impaired renal function, which is a predictor of poor outcome. Indeed, the chronic cardiorenal syndrome is common and has been reported in 63% of all patients hospitalized with congestive heart failure [1,2]. The cardiorenal syndrome is now recognized as a pathophysiological condition, in which the combined cardiac and renal dysfunction amplifies the progression of failure of the individual organs [3,4]. Atherosclerosis, renal vascular disease, diabetes mellitus and hypertension are significant precursors of both heart failure and renal failure. In clinical practice, specialists and general physicians always look for new plasma indices of cardiorenal remodeling, for a better stratification and management of the cardiorenal syndrome. β2-Microglobulin (b2M) represents a biomarker of remodeling and high tissue turnover, inflammation and decreased glomerular filtration rate (GFR) [5,6,7]. As a consequence of renal failure, the serum concentration of b2M can increase up to about the 60-fold normal value [8]. Oddly, clinical data concerning plasma concentrations of b2M and their relationship with diagnostic and prognostic indices of heart failure, such as the B-type natriuretic peptide (BNP) and its amino terminal cleavage fragment NT-proBNP, are still lacking in cardiorenal remodeling, even if b2M is emerging as new predictive marker of cardiovascular events and mortality [9,10,11].
On the other hand, matrix metalloproteinases are the driving force behind myocardial matrix degradation during cardiac remodeling. Tissue inhibitors of matrix metalloproteinases (TIMP1, 2, 3 and 4) firmly regulate matrix metalloproteinase activity [12], but they also display ‘cytokine-like’ properties that are just beginning to be fully characterized [13]. Plasma TIMP1 levels have been reported to be elevated in chronic pressure-overloaded human hearts and in heart failure, and its expression correlates with the degree of interstitial fibrosis [14]. To our knowledge, there is still a complete lack of clinical data and pathophysiological studies concerning the relationship between plasma concentrations of TIMP1 and b2M in the cardiorenal syndrome. In the present study, we investigated the plasma profile of b2M and its relationship not only with NT-proBNP, but also with TIMP1 in patients with chronic heart failure (CHF), choosing a group of veteran athletes with physiological heart remodeling (athlete's heart, AH) and a group of sedentary subjects with no cardiac remodeling as controls (CTL).
Subjects and Methods
Population Studied
We considered 3 groups of subjects: 24 CHF patients without primitive renal disease, 25 sedentary healthy CTL and 30 veteran marathoners with AH. The CHF patients were recruited from the University Medical Units of Azienda Ospedaliero-Universitaria Pisana (AOUP), Italy; all of them had had a steady clinical history for the preceding 3 months. Of the CHF patients, none was classified as NYHA class I, 10 as NYHA class II, 9 as NYHA class III and 5 as NYHA class IV (New York Heart Association functional classification system). Plasma samples were obtained during a regular periodic control. The veteran marathoners with AH were recruited from athletes who come voluntarily once a year to the Sport Unit of AOUP for a cardiological examination; all of them were eligible for competitive sport, with no electrocardiographic or echocardiographic signs of hypertrophic cardiomyopathy, diastolic or systolic dysfunction or arrhythmias. They had been training and competing continuously for at least 5 years and had a left ventricular (LV) mass >108 g/m2[15]. The marathoners had a training level of 60 km a week, for about 5 h, spread across 3 days. Every 15 days, they ran a half marathon. Almost once a year, they participated in a competitive marathon. Plasma samples were obtained 48 h after their most recent training session. None had been under any continuous medical treatment for the preceding 5 years. Sedentary CTL were recruited through primary care physicians.
Each subject in the 3 groups provided a medical history and underwent clinical examination: 12-lead electrocardiogram, 2D color Doppler echocardiography and abdominal echography. Chest radiography was performed in smokers and those with a professional risk or a family history of lung cancer.
All data were recorded in confidential clinical files. Fasting blood samples, obtained on the same day as the examinations, were used for the analyses of TIMP1 concentrations, b2M, NT-proBNP, C-reactive protein (CRP) and renal function indices. GFRs were estimated using the MDRD formula [16]. Subjects with evidence of neoplastic disease, acute or chronic inflammatory disease, renal and/or hepatic dysfunction or hemostatic disorder were excluded from the study.
Our study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The research protocol was reviewed and approved by the AOUP's Ethical Committee. All participants gave their written informed consent on the study procedure after the purposes had been explained to them.
Plasma TIMP1, b2M and NT-proBNP Measurements
TIMP1 concentrations were measured in heparinized plasma. Each sample was immediately separated, stored at −70°C in multiple aliquots and thawed once (at the time of testing). TIMP1 was measured by a commercial ELISA kit (Biotrak; Amersham Bioscience). b2M was assayed using an automatic analyzer (AxSym; Abbott). NT-proBNP was assayed using Elecsys (Roche).
Echocardiography
Examinations were carried out by two trained echocardiographers, using a Sonos 5500 system with a 4-MHz transducer. A biplanar evaluation of the ejection fractions (EF) was carried out, and monodimensional measurements of the left diastolic and systolic diameters and the septal and parietal thicknesses were performed at the chordal level, just beneath the mitral valve leaflets, following the criteria of the American Society of Echocardiography [17]. The average value based on 3 consecutive measures was calculated (min.-max. variation: <5%). The LV mass was calculated according to the formula of Devereux et al. [18] and indexed on the basis of individually calculated body surface areas. For AH and CTL, echocardiographic data were obtained in a separate examination, subsequent to the visit when blood samples were taken.
Statistical Analysis
The statistical analysis was carried out using the statistical packages Instat 3 and Prism 5.0 (GraphPad Software Inc.). Comparisons of the various parameters between the groups were carried out using the Kruskall-Wallis test, since the data were not normally distributed in the 3 groups. The significance level was set at p < 0.05. Where significance was demonstrated, Mann-Whitney's test was performed between the groups. Correlations between the parameters under examination were determined by Spearman's r analysis. Multiple regression analysis was performed.
Results
Clinical and Serological Characteristics
A summary of the characteristics of the patients, athletes and CTL in the study is shown in table 1. The various data are reported as medians. The same table contains the statistical results of the various comparison tests. Levels of b2M, TIMP1 and NT-proBNP were not significantly different between the healthy CTL and marathoners with AH; the same was observed for the estimated GFR and CRP. The latter was significantly lower in the marathoners with AH than in CHF patients, while there was no significant difference with CTL. In all 3 groups, the median CRP levels were lower than the reference value adopted to suggest an inflammatory disease.
Table 1.
Clinical biochemistry and echocardiographic parameters of the 3 study groups
| Variable | AH (n = 30) | CHF (n = 24) | CTL (n = 25) | Statistical analysis, p values |
|||
|---|---|---|---|---|---|---|---|
| Kruskall-Wallis | Mann-Whitney |
||||||
| AH vs. CHF | CHF vs. CTL | AH vs. CTL | |||||
| Age, years | 59 (54–66) | 70 (66–76) | 56 (49–62) | ||||
| Male/female | 28/2 | 16/8 | 16/9 | ||||
| b2M, ng/ml | 1,096.0 (934.5–1,229.0) | 2,167.0 (1,498–2,703) | 1,131.0 (1,050–1,329) | <0.0001 | <0.0001 | <0.0001 | n.s. |
| TIMP1, ng/ml | 232.1 (187.2–274.7) | 278.8 (214.6–348.1) | 220.8 (192.5–280.5) | 0.0407 | 0.0243 | 0.0319 | n.s. |
| NT-proBNP, pg/ml | 32.0 (21.5–44.5) | 616.0 (135–1,421) | 33.0 (21.5–80.0) | <0.0001 | <0.0001 | <0.0001 | n.s. |
| GFR, ml/min/1.73 m2 | 106.5 (96.0–116.5) | 77.5 (58.7–93.5) | 109.0 (98.0–116.5) | <0.0001 | <0.0001 | <0.0001 | n.s. |
| CRP, mg/dl | 0.095 (0.04–0.2175) | 0.26 (0.1–0.66) | 0.11 (0.04–0.55) | 0.0067 | 0.0010 | n.s. | n.s. |
| LV mass index, g/m2 | 121.0 (105.0–135.5) | 141.7 (119.9–183.5) | 83.3 (78.2–102.0) | <0.0001 | 0.0252 | <0.0001 | <0.0001 |
| Parietal thickness, cm | 1.07 (1.0–1.16) | 1.1 (1.0–1.2) | 1.0 (0.9–1.0) | 0.0026 | n.s. | 0.0004 | 0.0081 |
| Septal thickness, cm | 1.21 (1.13–1.27) | 1.275 (1.163–1.300) | 1.0 (1.0–1.1) | <0.0001 | n.s. | <0.0001 | <0.0001 |
| LVEDD, cm | 5.3 (5.0–5.55) | 5.4 (5.025–5.775) | 5.0 (4.7–5.0) | 0.0003 | n.s. | 0.0002 | 0.0013 |
| EF% | 62 (58–63) | 39 (30–49) | 65 (65–66) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
For all descriptive values, medians with 25th–75th percentiles in parentheses are reported. n.s. = Not significant; LVEDD = LV telediastolic diameter.
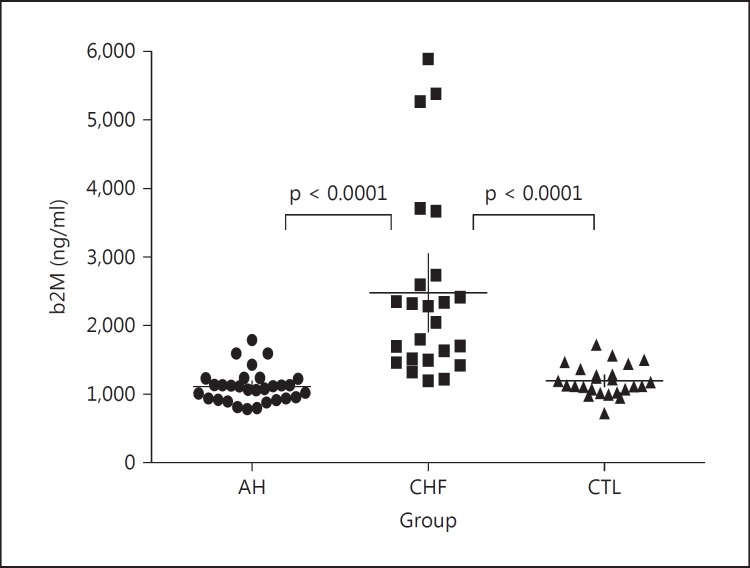
The comparison of data from the CHF patients with those obtained from marathoners with AH and CTL showed important differences: plasma TIMP1 and NT-proBNP levels were higher in the CHF patients than in the athletes and CTL (fig. 1a, b). Also, the estimated GFR was lower in the CHF patients than in athletes and CTL (p < 0.0001), while there was no significant difference between these 2 groups (fig. 1d). The CRP values showed differences (fig. 1), with the median value in the CHF patients being higher than that of the athletes (p = 0.001). As shown in figure 2, plasma b2M was significantly increased in the CHF patients compared with both the veteran marathoners (p < 0.0001) and CTL (p < 0.0001).
Fig. 1.
Boxplots of TIMP1 (a), NT-proBNP (b), CRP (c) and GFR (d) distributions and levels in the 3 study groups. All indices of cardiorenal dysfunction are elevated in the CHF patients in comparison with the 2 control groups. CRP shows a significant difference only between the CHF patients and the marathoners with AH. Median values and 25th-75th percentiles are indicated by horizontal and vertical lines, respectively. Significant differences (p value) between the groups are given in each graph.
Fig. 2.
b2M distributions and levels in the 3 groups. In the CHF patients, b2M shows higher values than in the marathoners with AH and CTL. Median values and 25th-75th percentiles are indicated by horizontal and vertical lines, respectively. Significant differences (p value) between the groups are given in each graph.
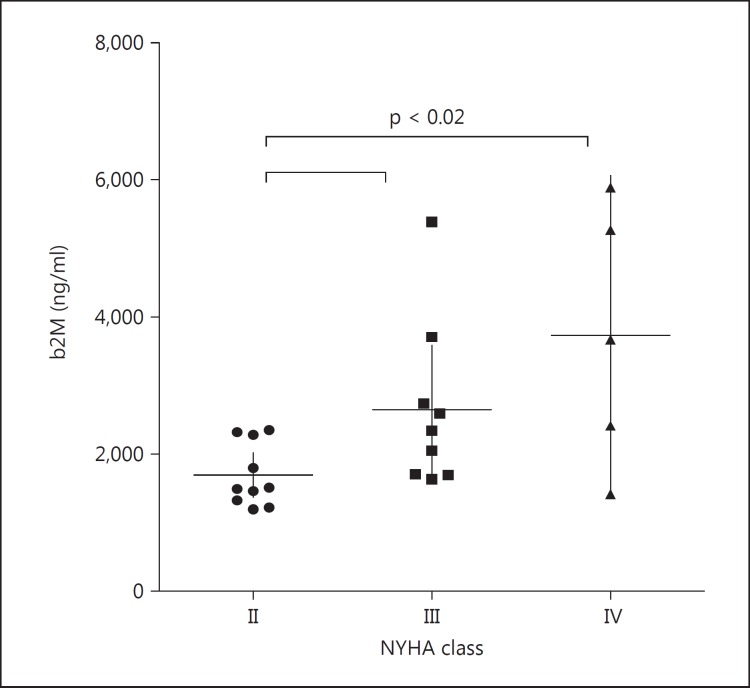
Comparing the b2M values of patients in different NYHA classes, those of patients belonging to higher NYHA classes were significantly higher (p = 0.0188). The comparison test showed that the differences were significant between patients in class II and those in classes III and IV, while no significant differences were found between the values of patients in class III and those of patients in class IV (fig. 3).
Fig. 3.
b2M values in CHF patients of different NYHA classes (from II to IV). Patients in NYHA classes III and IV show higher levels of b2M compared with patients in NYHA class II. Median values and 25th-75th percentiles are indicated by horizontal and vertical lines, respectively. Significant differences (p value) between the groups are given in each graph.
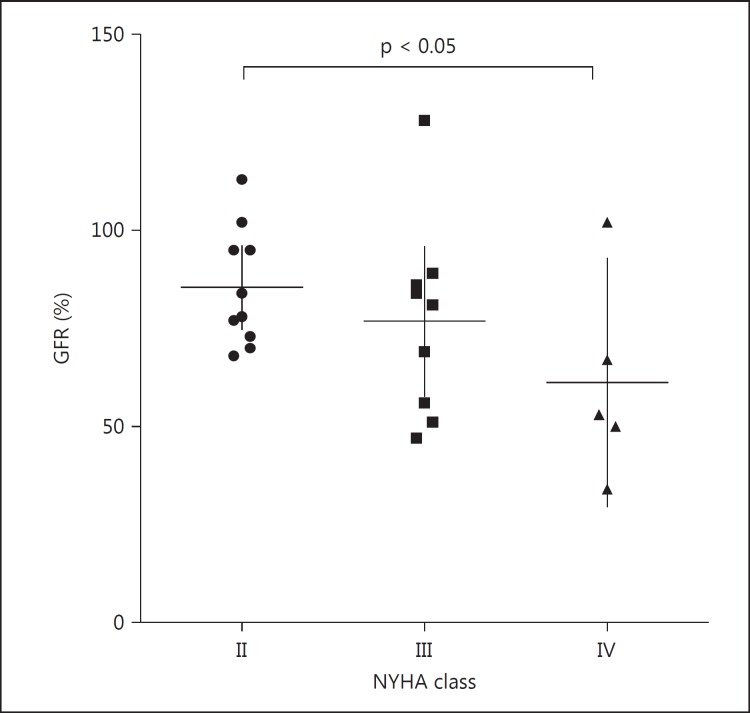
The evaluation of the differences between GFR in the different NYHA classes demonstrated a significant difference between class II and IV (p = 0.0496; fig. 4).
Fig. 4.
GFR in the different NYHA classes. The GFR decreases from class II to class IV. It is significantly lower in class IV vs. class II.
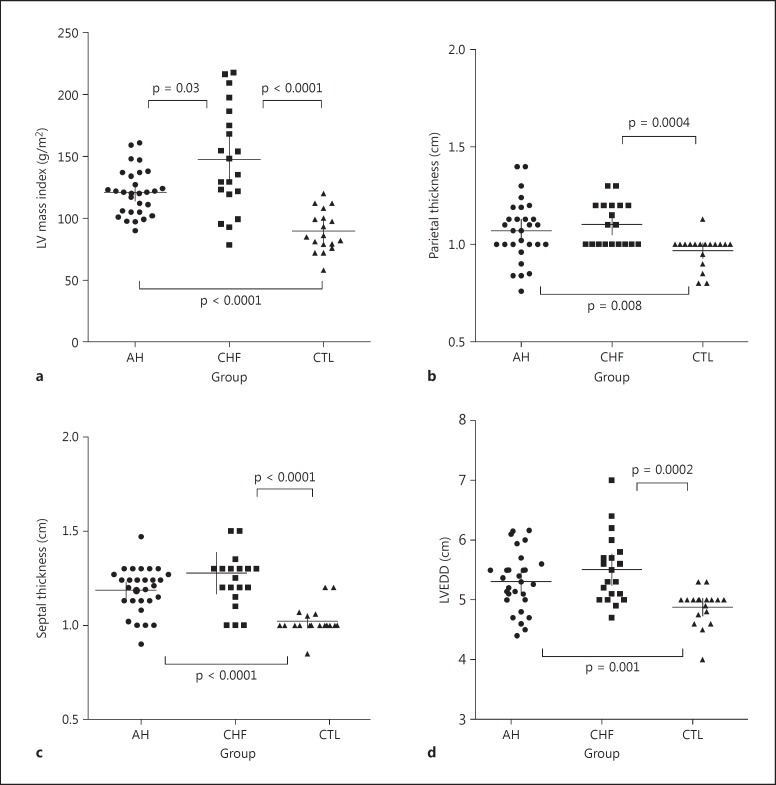
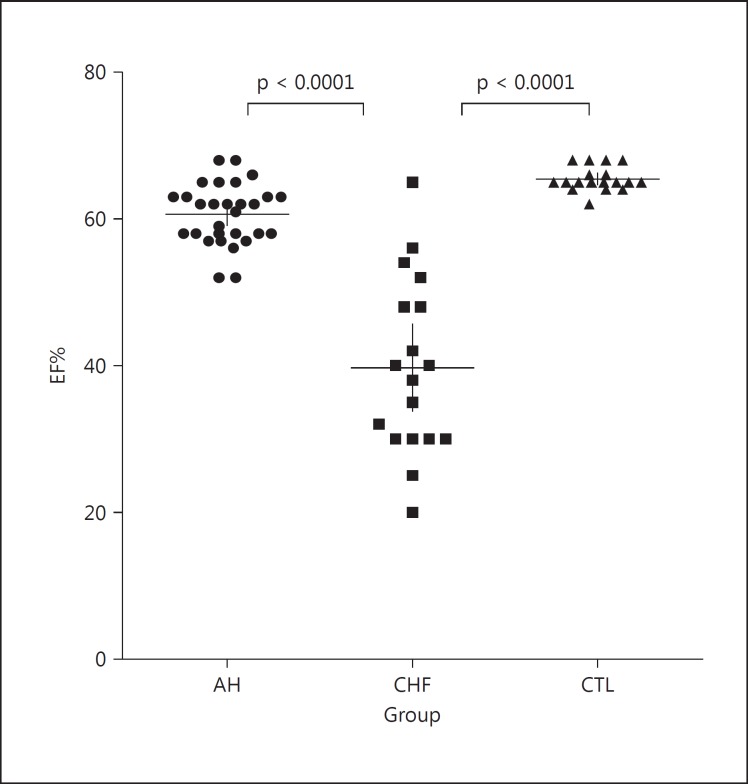
Different results were found in the echocardiographic parameters between the CTL, marathoners with remodeled AH and CHF patients, as expected. All indices of hypertrophic remodeling showed no significant differences between the athletes and CHF patients, since they showed similar values; nevertheless, the LV mass index was slightly lower in the marathoners with AH than in CHF patients, but significantly higher in both groups compared to the values of the CTL group (fig. 5). Differences between the CHF patients and the athletes were mainly limited to ventricular function: the mean value of percent EF was significantly lower in the CHF patients than in the athletes (fig. 6).
Fig. 5.
Echocardiographic parameters of LV geometry and remodeling in the 3 groups. In CHF patients and marathoners with AH, the LV mass index (a), parietal thickness (b), septal thickness (c) and left ventricular telediastolic diameter (LVEDD; d) are significantly higher than in sedentary CTL. Median values and 25th-75th percentiles are indicated by horizontal and vertical lines, respectively. Significant differences (p value) between the groups are given in each graph.
Fig. 6.
Echocardiographic estimation of LV ejection fraction (EF%) in the 3 groups. Only in the CHF patients, there is a significant LV dysfunction. Median values and 25th-75th percentiles are indicated by horizontal and vertical lines, respectively. Significant differences (p value) between the groups are given in each graph.
Correlations
As in the CHF patients (r = 0.5287, p < 0.0095), the athletes' b2M levels showed a strong positive correlation with the TIMP1 levels (r = 0.7548, p < 0.0001), while this relationship was not seen in the sedentary CTL. In the CHF patients, b2M was also positively correlated with NT-proBNP, and it was negatively correlated with GFR and EF% (table 2). Multiple linear regression analysis was performed: the results indicated that all mentioned parameters were independent of each other both in the CHF patients and marathoners with AH.
Table 2.
Correlations between b2M, TIMP1 and the main parameters of cardiorenal dysfunction (GFR, NT-proBNP and EF%) in the 3 study groups
| GFR | NT-proBNP | EF% | |
|---|---|---|---|
| AH | |||
| b2M | |||
| r | –0.49 | ||
| p | 0.0065 | n.s. | n.s. |
| TIMP1 | |||
| r | –0.3672 | ||
| p | 0.0459 | n.s. | n.s. |
| CHF | |||
| b2M | |||
| r | –0.8327 | 0.6553 | –0.5576 |
| p | <0.0001 | 0.0007 | 0.0162 |
| TIMP1 | n.s. | n.s. | n.s. |
| CTL | |||
| b2M | |||
| r | 0.4117 | ||
| p | n.s. | 0.0409 | n.s. |
| TIMP1 | |||
| r | 0.02 | ||
| p | n.s. | 0.92 | n.s. |
n.s. = Not significant.
Discussion
The main findings of this study are the following: (1) the circulating b2M and TIMP1 levels are higher in CHF patients than in veteran athletes and sedentary CTL; (2) in CHF patients, b2M correlates with NT-proBNP and GFR values and is associated with the severity of the disease, as higher values were found in patients of higher NYHA class, and (3) b2M and TIMP1 are strongly correlated, independent of GFR values, both in CHF patients and in marathoners with physiological remodeling (veteran athletes), but not in sedentary CTL.
A cardiorenal impairment, without primitive renal disease, has been found in more than 60% of all patients hospitalized for CHF, and the pathophysiological cardiorenal connection is currently recognized: the deterioration of renal function is proportional to the severity of heart failure [2]. Moreover, b2M is known to correlate with GFR impairment in renal dysfunction [19]. The increase of b2M in CHF patients, in addition to its association with the severity of heart disease and renal impairment, suggests a new role for b2M as biomarker of heart failure progression and prognosis.
Our data demonstrate that b2M levels simultaneously follow the development of renal and cardiac failure, and this evidence is in agreement with the fact that b2M correlates with the following indices of cardiac disease and failure: increased NT-proBNP and decreased EF%. On the other hand, in athletes with physiological heart remodeling and no evidence of renal or cardiac failure, the b2M levels were comparable to those of sedentary CTL. Interestingly, the multiple regression analysis showed a strong correlation between b2M and TIMP1, independent of GFR, both in CHF patients and veteran marathoners, but not in sedentary CTL.
In summary, b2M and TIMP1 are linked together both in physiological and pathological remodeling; interestingly, even though echocardiographic parameters are indicative of high tissue remodeling both in marathoners with AH and in CHF patients, only CHF patients show elevated values of TIMP1 and b2M. From a pathophysiological point of view, this is one of the most interesting results of our research and could lead to a better understanding of the pathophysiological links between heart and renal remodeling and failure. Indeed, TIMP1 is now recognized as a biomarker of ongoing adverse cardiac remodeling [12], as changes in the plasma levels of TIMP1 have been the focus of several large-scale cardiovascular outcome studies and have been associated with increased mortality [20,21]. Moreover, elevated TIMP1 plasma levels have been associated with major cardiovascular risk factors: the presence of LV hypertrophy, of heart failure in pressure overload hypertrophy and of myocardial fibrosis and dysfunction [22].
Little or nothing is known about TIMP1 in relation to cardiorenal failure progression. On the other hand, b2M is associated with kidney disease and a decreased GFR, while its relationship with cardiac diseases is still little investigated. Only in recent years has b2M been proposed as a marker of cardiovascular mortality risk in patients with carotid atherosclerosis or chronic renal disease. Moreover, in one proteome study, it has been identified as a causative risk biomarker for coronary heart disease in postmenopausal women [23]. Questions arise about the factors determining the strong bidirectional correlation between b2M and TIMP1 in heart remodeling. In our opinion, this tight link could be based on the role which these two proteins can play in high cell turnover processes through the regulation of apoptosis, cell survival, growth, migration, differentiation, angiogenesis, fibrosis, inflammation and overall extracellular matrix remodeling.
b2M is expressed by all nucleated cells. TIMP1 inhibits matrix degradation and is overexpressed in substitutive fibrosis. As a consequence, cardiac and renal cell dysfunction and death could determine an increase in b2M levels; while high concentrations of circulating TIMP1 could reflect an increased deposition of fibrosis, both in the myocardial interstitium and in renal tubulointerstitial tissue. Moreover, a decreased GFR, associated with a significant renal impairment, hinders the clearance of TIMP1 and b2M and thus further contributes to elevate their plasma concentrations, especially in the final stages of cardiorenal disease. Since the cardiorenal syndrome has been underrepresented in clinical studies [24], there is still only little evidence available to guide clinicians in the optimal management and follow-up of patients with both conditions. Instead, when both heart and renal failure occur together, it is important to have biomarkers, just like b2M and TIMP1, able to risk-stratify patients by looking at both their cardiac and renal aspects. Of course, the clinical use of b2M and TIMP1 is not less important, in addition to BNP and other novel biomarkers of cardiorenal remodeling and inflammation, such as GDF 15 [25], in the differentiation between physiological and pathological cardiac remodeling. Finally, the results of our study might be tested for and extended to all cardiorenal syndrome subtypes. They may be of primary importance in providing a new clinical tool in early diagnosis and follow-up.
The current study has some limitations. Our observational research was carried out on a relative small sample of patients and veteran athletes with heart remodeling. In particular, none of our CHF patients exhibited symptoms of early NYHA class I. Additionally, there are limitations to echocardiography for an accurate assessment of LV mass and remodeling indices; however, magnetic resonance imaging was not possible for our subjects, although it would have provided a better estimate of these parameters.
Conclusions and Clinical Perspectives
The design of the study arose from recent evidences stating the role of b2M not only as a biomarker of high tissue turnover and remodeling, inflammation and a decreased GFR, but also as an emerging new predictive marker of cardiovascular events and mortality. We studied b2M profiles in CHF patient of different NYHA classes and compared them with those of sedentary CTL and veteran athletes. For the first time, we showed that b2M plasma levels are increased in CHF and are associated with the severity of the disease. In addition, we highlighted the correlation between b2M and NT-proBNP in CHF and a new strong correlation between b2M and TIMP1 not only in CHF, but also after physiological remodeling (AH). Thus b2M, in addition to NT-proBNP and TIMP1, might provide diagnostic and prognostic complementary information about cardiorenal function and failure in physiological and pathological heart remodeling. Also, in patients with CHF and cardiorenal syndrome, b2M and TIMP1, in addition to NT-proBNP, could be a powerful combination of biomarkers for predicting morbidity and mortality.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the personnel of the 2nd Medical Clinic of the University of Pisa for their valuable assistance. This study was supported by grants from the University of Pisa.
References
- 1.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 2.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome: J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifson AR, Hessol NA, Buchbinder SP, O'Malley PM, Barnhart L, Segal M, Katz MH, Holmberg SD. Serum b2-microglobulin and prediction of progression to AIDS in HIV infection. Lancet. 1992;339:1436–1440. doi: 10.1016/0140-6736(92)92030-j. [DOI] [PubMed] [Google Scholar]
- 6.Walters MT, Stevenson FK, Goswami R, Smith JL, Cawley MI. Comparison of serum and synovial fluid concentrations of b2-microglobulin and C reactive protein in relation to clinical disease activity and synovial inflammation in rheumatoid arthritis. Ann Rheum Dis. 1989;48:905–911. doi: 10.1136/ard.48.11.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floege J, Ehlerding G. Beta-2-microglobulin-associated amyloidosis. Nephron. 1996;72:9–26. doi: 10.1159/000188801. [DOI] [PubMed] [Google Scholar]
- 8.Gejyo F, Homma N, Suzuki Y, Arakawa M. Serum levels of b2-microglobulin as a new form of amyloid protein in patients undergoing long-term hemodialysis. N Engl J Med. 1986;314:585–586. doi: 10.1056/NEJM198602273140920. [DOI] [PubMed] [Google Scholar]
- 9.Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke HD, Vanholden R, Diouf M, Choukroun G, Massy ZA. Plasma beta-2-microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82:1297–1303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 10.Amighi J, Hoke M, Mlekusch W, Schlager O, Exner M, Haumer M, Pernicka E, Koppensteiner R, Minar E, Rumpold H, Schillinger M, Wagner O. Beta-2-microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke. 2011;42:1826–1833. doi: 10.1161/STROKEAHA.110.600312. [DOI] [PubMed] [Google Scholar]
- 11.Astor BC, Shafi T, Hoogeveen RC, Matsushita F, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of ESRD, cardiovascular disease and mortality in the general population. Am J Kidney Dis. 2012;59:653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘Embracing the MMP-independent-side of the family’. J Mol Cell Cardiol. 2010;48:445–453. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Zile MR, Desantis SM, Baicu CF, Stroud RE, Thompson SB, McClure CD, Mehurg SM, Spinale FG. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail. 2011;4:246–256. doi: 10.1161/CIRCHEARTFAILURE.110.958199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelliccia A, Kinoshita N, Pisicchio C, Quattrini F, Dipaolo FM, Ciardo R, Di Giacinto B, Guerra E, De Blasiis E, Casasco M, Culasso F, Maron BJ. Long-term clinical consequences of intense, uninterrupted endurance training in olympic athletes. J Am Coll Cardiol. 2010;5:1619–1625. doi: 10.1016/j.jacc.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP, Weiner RB. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57:1126–1166. doi: 10.1016/j.jacc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 19.Donadio C. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients: diagnostic accuracy of urinary β-trace protein. Am J Physiol Renal Physiol. 2010;299:F1407–F1423. doi: 10.1152/ajprenal.00507.2009. [DOI] [PubMed] [Google Scholar]
- 20.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart. 2006;151:1101.e1–1101.e8. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J. 2004;25:1509–1516. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 23.Prentice RL, Paczesny S, Aragaki A, Amon LM, Chen L, Pitteri SJ, McIntosh M, Wang P, Buson Busald T, Hsia J, Jackson RD, Rossouw JE, Manson JE, Johnson K, Eaton C, Hanash SM. Novel proteins associated with risk for coronary heart disease or stroke among postmenopausal women identified by in-depth plasma proteome profiling. Genome Med. 2010;2:48. doi: 10.1186/gm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377–1384. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 25.Kahli A, Guenancia C, Zeller M, Grosjean S, Stamboul K, Rochette L, Girard C, Vergely C. Growth differentiation factor-15 (GDF-15) levels are associated with cardiac and renal injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. PLoS One. 2014;9:e105759. doi: 10.1371/journal.pone.0105759. [DOI] [PMC free article] [PubMed] [Google Scholar]