Abstract
Aims: Alcoholic liver disease (ALD) is linked to binge drinking and cigarette smoking. Heavy chronic ± binge alcohol, or low-level exposures to dietary nitrosamines cause steatohepatitis with insulin resistance and oxidative stress in animal models. This study examines hepatotoxic effects of sub-mutagenic exposures to tobacco-specific nitrosamine (NNK) in relation to ALD. Methods: Long Evans rats were fed liquid diets containing 0 or 26% (caloric) ethanol (EtOH) for 8 weeks. In Weeks 3 through 8, rats were treated with NNK (2 mg/kg) or saline by i.p. injection, 3×/week, and in Weeks 7 and 8, EtOH-fed rats were binge-administered 2 g/kg EtOH 3×/week; controls were given saline. Results: EtOH ± NNK caused steatohepatitis with necrosis, disruption of the hepatic cord architecture, ballooning degeneration, early fibrosis, mitochondrial cytopathy and ER disruption. Severity of lesions was highest in the EtOH+NNK group. EtOH and NNK inhibited insulin/IGF signaling through Akt and activated pro-inflammatory cytokines, while EtOH promoted lipid peroxidation, and NNK increased apoptosis. O6-methyl-Guanine adducts were only detected in NNK-exposed livers. Conclusion: Both alcohol and NNK exposures contribute to ALD pathogenesis, including insulin/IGF resistance and inflammation. The differential effects of EtOH and NNK on adduct formation are critical to ALD progression among alcoholics who smoke.
INTRODUCTION
Alcoholism is a costly public health problem that continues to grow, especially among women and young people (Paula et al., 2010). On a global scale, alcohol abuse is a leading cause of liver-associated morbidity and mortality (Paula et al., 2010; McCullough et al., 2011; Miller et al., 2011). Acute alcohol-related liver injury is usually reversible (Seth et al., 2011), but in some individuals who engage in heavy drinking over long periods of time, steatohepatitis becomes chronic and progressive, ultimately leading to cirrhosis, liver failure, or hepatocellular carcinoma (O'Shea et al., 2010). Mechanistically, chronic alcoholic liver disease (ALD) is linked to injury mediated by complex inter-related pathophysiological processes including: insulin resistance (de la Monte et al., 2008; Pang et al., 2009; Longato et al., 2012), cytotoxicity and lipotoxicity (McVicker et al., 2006; de la Monte et al., 2009a; Cohen and Nagy, 2011; Derdak et al., 2011), inflammation (Ronis et al., 2008; Cohen and Nagy, 2011), oxidative and endoplasmic reticulum (ER) stress (Kaplowitz and Ji, 2006; Pandol et al., 2010; Feldstein and Bailey, 2011; Malhi and Kaufman, 2011; Ramirez et al., 2013), metabolic and mitochondrial dysfunction (Purohit et al., 2009; Ding et al., 2011), decreased DNA synthesis (Sasaki et al., 1994; Banerjee et al., 1998; Pang et al., 2009), and increased cell death (Derdak et al., 2011) (see Supplementary Table S1 for list of abbreviations). ALD progression is marked by activation of pro-fibrogenic pathways (Cohen and Nagy, 2011), setting the stage for cirrhosis and finally liver failure (Vidali et al., 2008; Purohit et al., 2009). Improved understanding of factors contributing to the switch from acute to chronic progressive ALD is needed to identify individuals at risk and refine diagnostic and therapeutic approaches.
Emerging evidence suggests roles for two additional factors in the pathogenesis of ALD: binge drinking and tobacco abuse. First, acute alcoholic hepatitis typically develops in chronic heavy alcohol drinkers who also binge (Seth et al., 2011). Recent publications demonstrated that better models of ALD could be generated in rodents by binge administering alcohol to chronic ethanol-fed mice (Bertola et al., 2013a,b). In addition, a very high percentage of heavy drinkers (up to 80%) also abuse tobacco products, typically by smoking cigarettes (Romberger and Grant, 2004). Moreover, even among non-smokers, secondhand smoke poses significant health risks due to inadvertent exposures to tobacco nitrosamines and their metabolites (Upadhyaya et al., 2009; Thomas et al., 2011). The overwhelming interest in studying adverse effects of alcohol-tobacco dual exposures has focused on carcinogenesis (Johnson et al., 1996; de Boer et al., 1997; Tramacere et al., 2010; Duell, 2012), including hepatocarcinogenesis (Kuper et al., 2000; Seitz and Stickel, 2006; Ha et al., 2012; Purohit et al., 2013), and the roles of the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolites (Go et al., 2005; Duell, 2012).
The concept that NNK and its metabolites could also contribute to ALD pathogenesis and progression stemmed from previous work showing that limited sub-mutagenic doses of other nitrosamines, i.e. streptozotocin (Bolzan and Bianchi, 2002; Koulmanda et al., 2003; Wang et al., 2011) or N-nitrosodiethylamine (NDEA) (de la Monte et al., 2009b; Tong et al., 2009), cause steatohepatitis with hepatic insulin resistance, increased DNA damage, lipid peroxidation, mitochondrial dysfunction, ER stress, and impaired signaling through PI3K-Akt (de la Monte et al., 2009b; Tong et al., 2009). Moreover, although tobacco smoke contains other toxins/carcinogens, e.g. acetaldehyde and acrolein, which generate covalent adducts (Salaspuro, 2011), there are no published reports demonstrating their roles in the pathogenesis of hepatic insulin resistance. The present study tests the hypothesis that chronic exposure to NNK causes steatohepatitis and exacerbates the effects of chronic+binge ethanol exposures on the histological, biochemical and molecular features of experimental ALD.
METHODS
Experimental model
Long Evans male rats were pair-fed for 8 weeks with isocaloric liquid diets containing 0 or 6% (v/v) ethanol. From Weeks 3 through 8, rats in each group were administered intraperitoneal (i.p.) injections (100 µl) of NNK (2 mg/kg) or saline on Mondays, Wednesdays and Fridays, and in Weeks 7 and 8, ethanol-fed rats were binged with 2 g/kg ethanol (100 µl, i.p.) on Tuesdays, Thursdays and Saturdays while controls were treated with saline. At the end of the experiment, blood alcohol levels (tail vein) were measured at 7 AM (basal) and 30 min after binging. Serum was obtained to measure electrolytes, lipids, liver function enzymes, and standard proteins (Marshfield Labs, Marshfield, WI, USA), or endotoxin immunoreactivity (Pierce LAL Chromogenic Endotoxin Quantitation Kit, Thermo Scientific, San Jose, CA, USA).
Liver collection, processing and storage
Portions of fresh liver were fixed in formalin or Karnovsky's, or snap frozen and stored at −80°C. Fixed tissue was divided for paraffin embedding and cryostat sectioning. Adjacent paraffin sections were stained with Hematoxylin and Eosin (H&E), Sirius Red (fibrosis) (Baumgardner et al., 2008). Cryostat sections were stained with Oil-Red O (ORO; lipids) to visualize hepatic steatosis (Caldwell et al., 2010). Karnovsky's fixed tissue was post-fixed in osmium tetroxide and embedded in Spurrs epoxy resin. Ultra-thin (50–60 nm) sections were stained and processed for electron microscopy.
Hepatic lipid and collagen assays
Cholesterol and triglycerides were measured in liver lipid extracts (Setshedi et al., 2011) using the Analox Instruments GM7 Analyzer. Hepatic collagen was measured using the Sirius Red/Fast Green Collagen Staining Kit (Chondrex, Inc., Richmond, VA, USA).
Enzyme-linked immunosorbent assays
Competitive enzyme-linked immunosorbent assays (ELISAs) measured hepatic Keratin-18 (USCN Life Science, Inc., Hubei, PRC), protein carbonyl, 4-hydroxy-2-nonenal (HNE), and isoprostane 8-iso-PGF2α (Cell Biolabs, Inc., San Diego, CA, USA). Luminex bead-based multiplex ELISAs proteins and phosphoproteins through insulin receptor (IR), IGF-1 receptor (IGF-1R), IRS-1, Akt, ribosomal protein S6 kinase (p70S6K) and glycogen synthase kinase 3β (GSK-3β). Hepatic cytokines were measured using a Rat Cytokine 23-Plex bead-based immunoassay (Bio-Rad, Hercules, CA, USA).
O6-methyl-Guanine detection
DNA isolated from rat lung and liver (Lao et al., 2006; Zhang et al., 2009) was subjected to acid hydrolysis (Upadhyaya et al., 2009) and quantified by high-performance liquid chromatography (HPLC). O6-methyl-Gua was detected and quantified by liquid chromatography (LC)-tandem Mass Spectrometry (MS/MS) by capillary LC/electron spray (ESI)-MS/MS using a triple quadrupole mass spectrometer. The ESI source was operated in the positive ion mode. Adducts were quantified with SRM at m/z 166.09 → 149.09 for O6-methyl-Gua and at the corresponding transition m/z 169.11 → 152.12 for [CD3] O6-methyl-Gua. DNA levels were calculated from the guanine content determined by HPLC-UV spectrometric analysis as previously described (Lao et al., 2006). (See Supplementary Detailed Methods).
Statistics
All assays were performed with 8 or 10 samples per group. Data depicted in box plots reflect group medians (horizontal bar), 95% confidence interval limits (upper and lower box limits) and range (whiskers). Data were analyzed using GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA, USA), and inter-group comparisons were made by repeated measures analysis of variance (ANOVA) with post hoc multiple comparison test.
RESULTS
Chronic plus binge ethanol plus tobacco nitrosamine (NNK) exposure model
Over the course of the study, weight gain was similar among the four groups (Supplementary Fig. S1A), and although the final mean body weight of chronic+binge ethanol exposed rats was somewhat reduced relative to the other groups, the differences did not reach statistical significance. Blood alcohol concentrations were measured at 7 AM (basal) and 30 min after the i.p. binge administration of ethanol (2 g/kg) or saline (vehicle). Representative results are shown in Supplementary Fig. S1C and E. As expected, baseline blood alcohol levels were significantly higher in chronic ethanol-fed relative to control rats. However, dual exposures to ethanol and NNK resulted in lower basal blood alcohol levels compared with ethanol feeding alone (Supplementary Fig. S1C). Binge administration of ethanol further increased the blood alcohol concentrations by 2- to 4-fold, but with somewhat lower mean levels in the ethanol+NNK relative to ethanol+vehicle treated rats (Supplementary Fig. S1E). Mean fasting blood glucose levels were higher in all experimental groups relative to control, but the differences only reached significance for rats treated with NNK ± ethanol (Supplementary Fig. S1F).
Serum chemistry assays were used to measure electrolytes, protein content, and indices of hepatic and renal function (Supplementary Table S2). Results were analyzed by ANOVA with post hoc Dunnett (relative to control) and Fisher LSD (multiple comparisons) tests. Most of the differences from control were driven by ethanol exposure, with or without NNK treatments. The only exceptions occurred with respect to ALT and LDH which were significantly reduced by NNK without chronic+binge ethanol feeding. The post hoc Fisher test further demonstrated significant differences between NNK and ethanol treatments and ethanol versus ethanol+NNK or NNK versus ethanol+NNK exposures. The most striking effects of NNK versus control were to reduce serum ALT and LDH levels. Ethanol alone increased serum potassium and the anion gap, and reduced serum chloride, bicarbonate, phosphorous, albumin, creatinine, ALT, Alkaline phosphatase, and LDH. The significant effects of ethanol+NNK relative to control were to increase serum sodium, globulin, urea/creatinine ratio, and anion gap, and reduce chloride, bicarbonate, phosphorous, albumin, albumin/globulin ration, ALT, and alkaline phosphatase. It is noteworthy that the maximum effects of ethanol on ALT and LDH may not have been measured due to the short interval (30 min) in which blood was sampled after binge ethanol administration. The levels of endotoxin immunoreactivity in serum were uniformly low and not significantly different among the groups (data not shown).
Ethanol and NNK-associated liver pathology
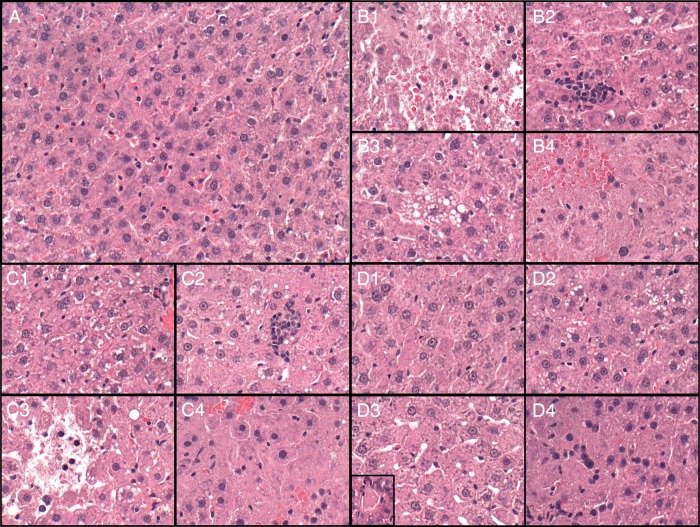
Although the mean liver weights were similar among the groups (Supplementary Fig. S1B), the calculated liver/body weight ratios were higher in the ethanol and ethanol+NNK groups (Supplementary Fig. S1D). Macroscopic examination of the fresh livers revealed the expected reddish-brown parenchyma control specimens, and swelling with parenchymal pallor following ethanol, NNK or both exposures (Supplementary Fig. S2). Histological sections stained with H&E revealed the expected well-delineated chord-like architecture with uniform populations of hepatocytes in control livers (Fig. 1A). NNK and/or ethanol exposures resulted in loss of the regular hepatic chord-like architecture (Fig. 1B–D), foci of necrosis (Fig. 1B1 and C3), lymphomononuclear cell inflammation (Fig. 1B2 and C2), steatosis (Fig. 1B3, C2 and D2), hepatocellular apoptosis (Fig. 1C4 and D3-inset), pyknosis (Fig. 1B4, C4 and D4) and ballooning degeneration (Fig. 1D3) of hepatocytes.
Fig. 1.
Ethanol and NNK effects on liver histology. Long Evans rats fed with isocaloric liquid diets containing 0 or 26% ethanol for 9 weeks were treated with NNK (2 mg/kg) or vehicle (3×/week) during the last 6 weeks, and binged with ethanol (2 g/kg) or saline (3×/week) in the last 2 weeks. Formalin-fixed paraffin-embedded sections of liver were stained with H&E. (A) Control+vehicle liver with normal chord-like architecture, uniform hepatocytes, and no inflammation or necrosis. (B) Control+NNK liver with (B1) necrosis (pale area with shrunken nuclei), (B2) lobular inflammation, (B3) steatosis-vacuoles in center, and (B4) apoptosis with reduced nuclear density, nuclear pleomorphism (pyknotic nuclei, variability in size, homogenization of cytoplasm), and loss of the chord-like architecture. (C) Ethanol diet+vehicle liver with (C1) loss of hepatic chord architecture, nuclear fading or loss, and microvesicular steatosis (vacuoles), (C2) lobular inflammation and microsteatosis-(small vacuoles), (C3) focal necrosis, and (C4) marked distortion of hepatic architecture with apoptosis, reduced nuclear density, and loss of the chord-like architecture. (D) Ethanol diet+NNK treated liver with (D1) drop-out of nuclei, (D2) microsteatosis, (D3, D4) apoptosis, marked distortion of hepatic architecture, and ballooning degeneration (D3-inset). Original magnification-all ×200.
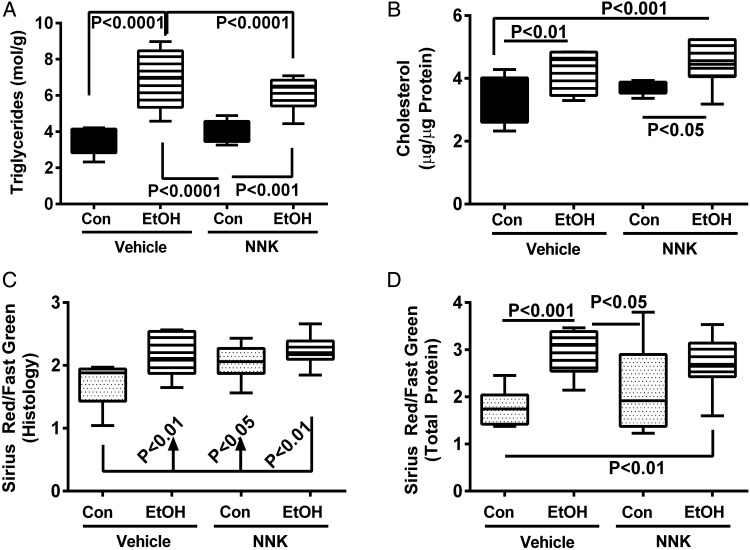
In control livers, ORO staining for lipid was modest and mainly distributed in Zone 3 (Supplementary Fig. S3A). NNK treatment resulted in mild but diffusely increased ORO staining throughout the hepatic lobules (Supplementary Fig. S3B). Chronic+binge ethanol, with or without NNK treatment resulted in strikingly increased pan-lobular ORO staining throughout the liver (Supplementary Fig. S3C and D). Correspondingly, hepatic triglyceride (Fig. 2A) and cholesterol (Fig. 2B) levels were significantly elevated by the ethanol ± NNK exposures relative to control or NNK-only livers. NNK alone slightly increased hepatic triglyceride and cholesterol content relative to control, but the differences were not statistically significant.
Fig. 2.
Quantitative assessments of hepatic lipid and collagen content. (A) Triglycerides and (B) cholesterol were measured in liver lipid extracts using the Analox 7 protocol and apparatus with results normalized to sample weight (triglycerides) or protein content (cholesterol). The Sirius Red/Fast Green assay was used to quantify collagen content in (C) histological sections or (D) aqueous tissue homogenates (see Supplementary Methods). Inter-group comparisons were made by two-way ANOVA. Results of post hoc Tukey multiple comparison tests are shown in the panels.
Sirius Red staining was used to detect collagen deposition as an indicator of early fibrosis. Control livers had minimal or no detectable inter-hepatocyte or sinusoidal fibrosis (Supplementary Fig. S4A). NNK treatment mildly increased collagen fibril abundance, manifested by short Sirius red-stained strands along sinusoids and between hepatocytes (Supplementary Fig. S4B). More prominent fibrosis was observed with chronic+binge ethanol exposures (Supplementary Fig. S4C), and superimposed treatment with NNK further increased collagen fibril abundance (Supplementary Fig. S4D). Fibrosis was quantified by measuring relative levels of collagen in histological sections (Fig. 2C) and aqueous liver homogenates (Fig. 2D) using a commercial Sirius Red/Fast Green assay. Both methods revealed significant ethanol-associated increases in hepatic collagen (fibrosis). In addition, in the NNK-exposed livers, significantly elevated levels of collagen were detected in histological sections but not in homogenates suggesting that the histological section assay may be more sensitive.
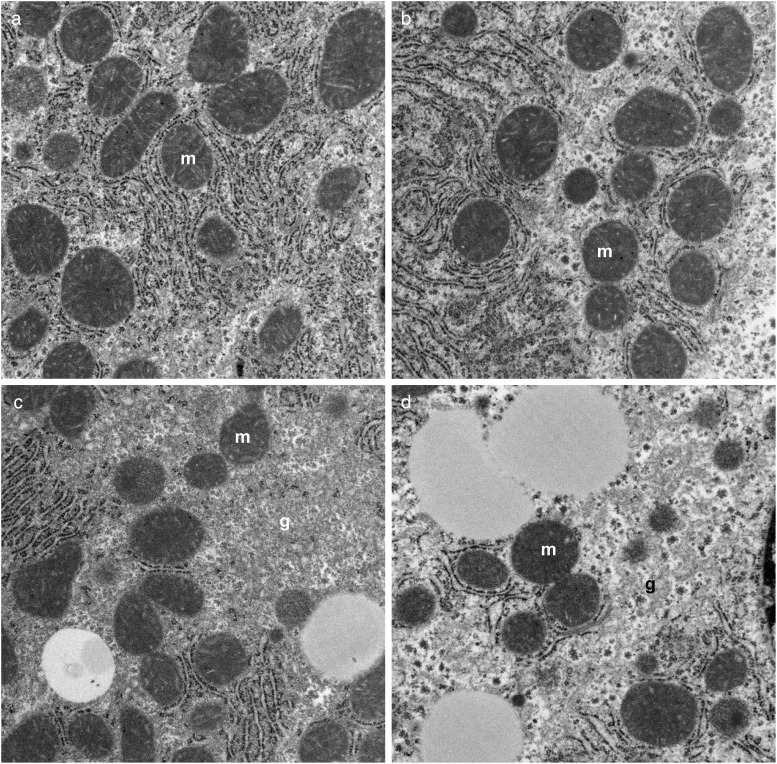
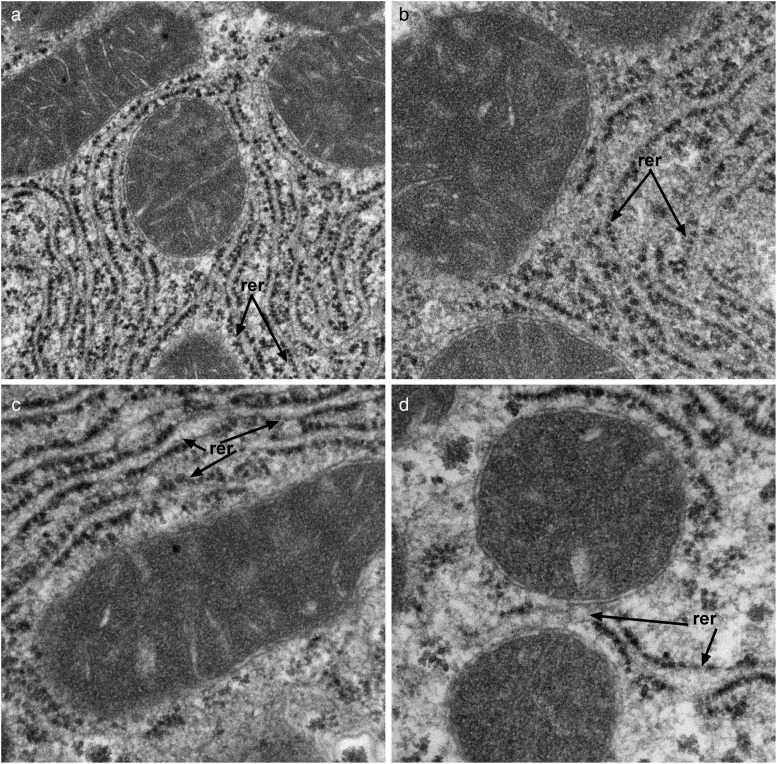
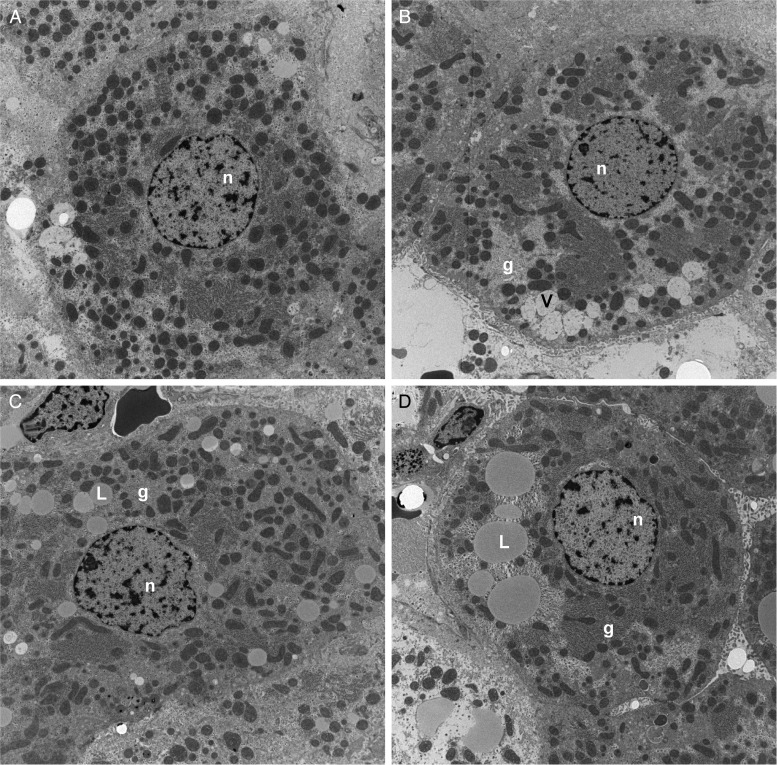
Transmission electron microscopic studies demonstrated that NNK treatment reduced the abundance and altered the distribution of mitochondria, reduced nuclear chromatin, and increased glycogen content and degenerative vacuoles in the cytoplasm relative to control (Supplementary Fig. S3A-a and b). Ethanol had similar effects but also increased cytoplasmic lipid vacuoles (Fig. 3A-c). Combined ethanol+NNK exposures caused striking reductions and alterations in mitochondria and markedly increased intra-hepatocyte lipid droplets (Fig. 3A-d). Higher magnification images demonstrated the expected parallel stacks of rough endoplasmic reticulum (RER) in close juxtaposition with mitochondria in control livers (Fig. 3B-a), and reduced physical associations between mitochondria and RER in the NNK, ethanol and ethanol+NNK groups (Fig. 3B-b–d). Moreover, depletion of RER was most conspicuous in the ethanol+NNK group. In the experimental groups, loss of RER integrity with gaps, dilatations and disorganization of structure was further illustrated at high magnification (Fig. 3C-a–d). It is noteworthy that we did not detect NNK- or ethanol-associated alterations in mitochondrial structure.
Fig. 3B.
(b) NNK, (c), and most strikingly (d) ethanol+NNK decreased the intimate associations between mitochondria (m) and RER relative to (a) control. Note reductions in normal parallel stacks of RER in hepatocytes of the treated groups (original magnification, ×18,000).
Fig. 3C.
Disrupted RER (rer; arrows) morphology with irregular loss of ribosomes and dilatations of the normally parallel ER tubules in the (b) NNK, (c) ethanol, and (d) ethanol+NNK exposed hepatocytes relative to control (a) (original magnification, ×44,000).
Fig. 3A.
Transmission electron microscopic studies. (A) Low magnification images showing reductions in mitochondria and nuclear chromatin (n), and increased lipid droplets (L), degenerative vacuoles (V), and glycogen (g) in (b) NNK-, (c) ethanol, and (d) ethanol+NNK treated livers relative to (a) control (original magnification ×3500).
Ethanol and NNK effects on pro-inflammatory mediators
We used a 23-plex ELISA to assess the effects of chronic+binge ethanol, NNK, or both exposures on inflammatory mediators in liver. The results demonstrated broadly increased expression of pro-inflammatory cytokines in livers of all three experimental groups relative to control. The only reductions were noted with respect to G-CSF and RANTES, while VEGF was not significantly affected by the treatments (Supplementary Table S3). NNK, ethanol and NNK+ethanol all significantly increased hepatic levels of GM-CSF, GRO/KC, IFN-γ, IL-10, IL-13, IL-18, IL-1α, IL-2, IL-4, IL-7, MCP-1 and MIP-3a. Ethanol and Ethanol+NNK increased hepatic expression of EPO, IL-17A, M-CSF and TNF-α. Ethanol exposure alone increased hepatic IL-12 and IL-1β, while NNK only exposure increased expression of IL-6 and IL-1β. There were no instances in which the effects of ethanol and NNK appeared to be additive. Instead, the responses were independently driven by ethanol or NNK, but generally more prominently by ethanol, and not further by dual exposures (Supplementary Table S3).
Ethanol and NNK effects indices of cellular injury and O6-methyl-Gua adducts
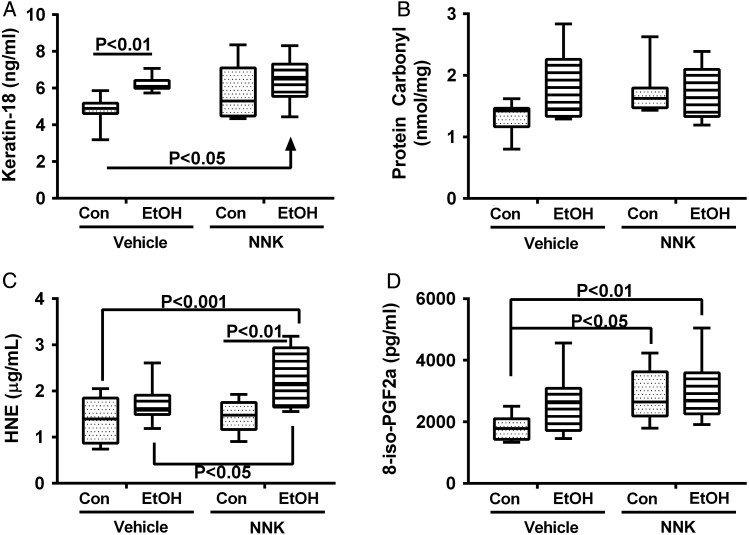
We used competitive ELISAs to measure immunoreactivity to Keratin-18, protein carbonyls, 4-hydroxy-2-nonenal (4-HNE), and 8-epimer of Prostaglandin F2α (8-iso-PGF2α) (Fig. 4). Two-way ANOVA tests demonstrated significant ethanol effects for 4-HNE and 8-iso-PGF2α, NNK effects for all but protein carbonyl and lipid peroxidation adducts, and no significant ethanol × NNK interactive effects (Supplementary Table S4). Post hoc Tukey repeated measures tests demonstrated that Keratin-18 immunoreactivity was increased in both the ethanol+vehicle and ethanol+NNK exposure groups relative to control+vehicle (Fig. 4A), but no significant inter-group differences were obtained with respect to protein carbonyl adduct accumulation (Fig. 4B). Hepatic 8-iso-PGF-2α (Fig. 4D) adducts were significantly elevated by NNK exposures, independent of ethanol. HNE immunoreactivity was highest in the ethanol+NNK exposed livers (Fig. 4C), the only convincing example of an additive effect of NNK and ethanol. Two samples each of lung and liver from control or ethanol+NNK treated rats were analyzed for O6-methyl-Gua adducts. None of the control samples had detectable O6-methyl-Gua, whereas similarly high levels of O6-methyl-Gua were detected in lung (2836 and 1147 fmol/mg DNA) and liver (1434 and 1797 fmol/mg DNA) from the ethanol+NNK group.
Fig. 4.
Ethanol and NNK effects on stress indices in liver. Competitive ELISAs were used to measure (A) cytokeratin 18, (B) protein carbonyl, (C) 4-hydroxy-2-nonenal (HNE) and (D) 8-iso-PGF-2α, with results normalized to sample protein. Inter-group comparisons were made by two-way ANOVA (Supplementary Table S4). Results of post hoc Tukey multiple comparison tests are shown in the panels.
Ethanol and NNK effects on mediators of insulin and IGF-1 signaling in liver
The levels of total and phosphorylated insulin receptor, IGF-1 receptor, IRS-1, Akt, GSK-3β, and p70S6K were measured using 7-Plex bead-based assays. Relative phosphorylation of insulin/IGF-1, IRS-1 Akt, GSK-3β signaling proteins was determined from the calculated ratios of phosphorylated/total proteins. Results of two-way ANOVA tests are shown in Supplementary Table S5, and the corresponding graphs are shown in Figs. 5 and 6 and Supplementary Fig. S5. Ethanol and NNK exposures had significant effects on insulin receptor, IRS-1 and GSK-3β protein levels, whereas only NNK had significant effects on p70S6K protein expression. Ethanol had significant effects on phosphorylated and relative phosphorylated levels of insulin receptor, Akt, GSK-3β, and p70S6K, and significant trends for phosphorylated IRS-1 and relative levels of phosphorylated IGF-1 receptor. In contrast, the effects of NNK on phosphorylated and relative phosphorylated protein levels were less broad and observed with respect to phosphorylated IGF-1 receptor, and relative phosphorylation of the insulin receptor, IGF-1 receptor and Akt. Ethanol × NNK interactive effects were minimal; only trends were measured for phosphorylated and relative phosphorylated levels of IRS-1.
Fig. 5.
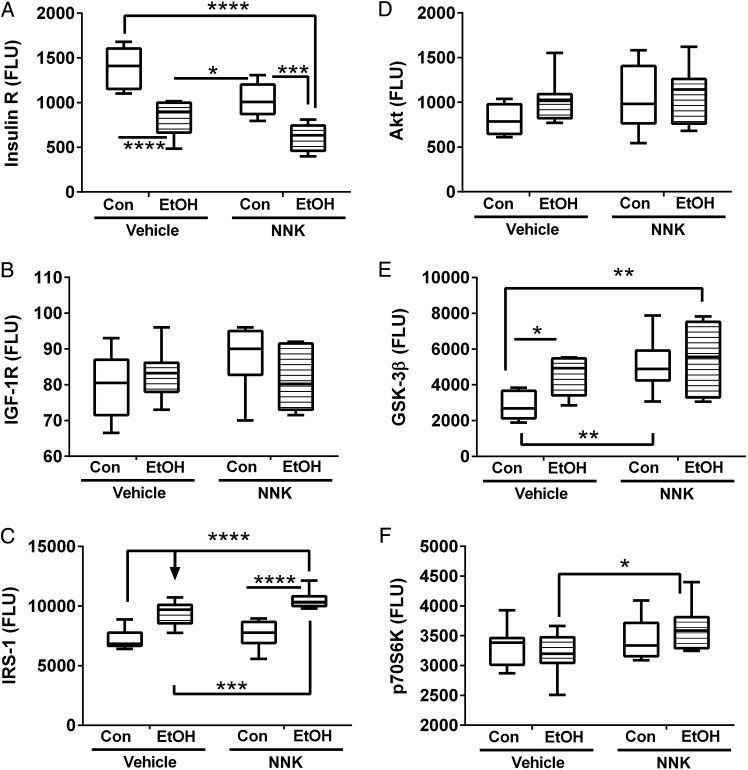
Ethanol and NNK effects on hepatic expression of insulin/IGF-Akt pathways signaling proteins in liver. Liver protein homogenates were used in bead-based multiplex ELISAs to measure immunoreactivity to (A) insulin receptor (Insulin R), (B) IGF-1 receptor (IGF-1R), (C) IRS-1, (D) Akt, (E), GSK-3β, and (F) p70S6K. Data were analyzed by two-way ANOVA (Supplementary Table S5), and results from post hoc Tukey multiple comparison tests are shown in the panels (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Fig. 6.
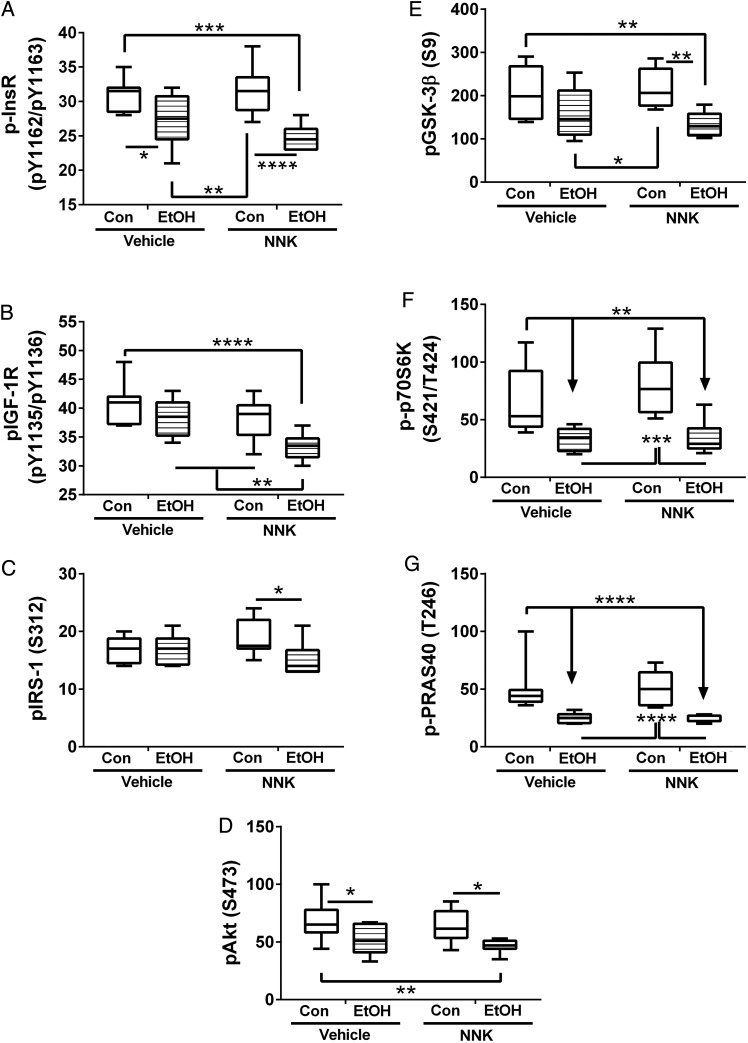
Ethanol and NNK effects on insulin/IGF-Akt pathway activation in liver. Liver protein homogenates were used in bead-based multiplex ELISAs to measure immunoreactivity to (A) pYpY1162/1163-Insulin receptor (p-InsR), (B) pYpY1135/1136-IGF-1R, (C) pS312-IRS-1, (D) pS473 AKT, (E) pS9-GSK-3β, (F) pTpS421/424-p70S6K and (G) pT246-PRAS40. Data were analyzed by two-way ANOVA (Supplementary Table S5), and results from post hoc Tukey multiple comparison tests are shown in the panels (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Insulin receptor expression was progressively reduced in livers exposed to NNK, followed by ethanol, and then ethanol+NNK. Post hoc tests demonstrated these progressive differences to be statistically significant (Fig. 5A). In contrast, IGF-1 receptor (Fig. 5B) and Akt (Fig. 5D) expression did not significantly differ among the groups. IRS-1 immunoreactivity was similar in vehicle and NNK treated control livers, but significantly elevated by ethanol exposure, irrespective of NNK treatment (Fig. 5C). GSK-3β immunoreactivity was significantly elevated in all experimental groups relative to control (Fig. 5E). p70S6K immunoreactivity was similar among the four groups, although a small difference was detected between the ethanol and ethanol+NNK groups (Fig. 5F).
Ethanol feeding significantly inhibited phosphorylation of the insulin receptor (Fig. 6A), Akt (Fig. 6D), GSK-3β (Fig. 6E), p70S6K (Fig. 6F), and PRAS40 (Fig. 6G), and combined ethanol+NNK exposures either modestly or significantly reduced phosphorylated levels of insulin receptor (Fig. 6A), IGF-1 receptor (Fig. 6B), IRS-1 (Fig. 6C), and Akt (Fig. 6D) relative to ethanol treatment alone. In contrast, NNK exposure alone had no significant effect on protein phosphorylation relative to control.
Relative levels of insulin receptor phosphorylation were significantly higher in ethanol treated relative to control livers (Supplementary Fig. S5A). However, this effect was due to the greater magnitude in which insulin receptor expression was inhibited compared with the degree of phosphorylation. In contrast, ethanol exposure significantly reduced relative phosphorylation levels of IRS-1 (Supplementary Fig. S5C), Akt (Supplementary Fig. S5D), GSK-3β (Supplementary Fig. S5E), and p70S6K (Supplementary Fig. S5F), and NNK (with or without ethanol) reduced relative phosphorylation of the IGF-1 receptor (Supplementary Fig. S5B), Akt (Supplementary Fig. S5D), and GSK-3β (Supplementary Fig. S5E).
DISCUSSION
Chronic plus binge ethanol and tobacco nitrosamine (NNK) related liver disease
The chronic plus binge ethanol exposure model was used to mimic the human clinical scenario that leads to alcoholic hepatitis. A second goal was to examine NNK tobacco nitrosamine effects on liver injury, steatohepatitis and hepatic insulin resistance, in light of the facts that: (a) a very high percentage of heavy drinkers also smoke (Romberger and Grant, 2004); (b) the causes of progressive alcoholic hepatitis are more complex than alcohol consumption per se; (c) previous studies showed that chronic exposure to sub-mutagenic levels of other nitrosamines causes steatohepatitis (de la Monte et al., 2009b); and (d) NNK causes oxidative injury and DNA damage in liver due to formation of O6-methyl-Gua, pyridyloxobutyl-, and pyridylhydroxybutyl-DNA adducts (Lao et al., 2007; Upadhyaya et al., 2009). Our hypothesis is that the tobacco nitrosamines specifically mediate liver disease that overlaps with and exacerbates the adverse effects of alcohol. The rationale for studying effects of NNK rather than tobacco smoke is that smoking could confound the results by causing pulmonary disease (Gan et al., 2011; McCaskill et al., 2011).
In our ethanol, NNK and ethanol+NNK models, we detected various alterations in serum chemistry. Reduced serum cholesterol and albumin are common findings in heavy drinkers with liver disease, and alcohol-associated increases in anion gap reflect metabolic acidosis. On the other hand, the reduced or unchanged levels of ALT, LDH, AST and alkaline phosphatase were unexpected and discordant with conventional wisdom since acute hepatocellular injury usually causes high liver enzyme levels in serum. Moreover, the data conflict with previous observations in chronic ethanol (Denucci et al., 2010) and chronic+binge ethanol (Miller et al., 2011; Bertola et al., 2013a,b) exposure models. To account for these discrepant results, we considered two potential explanations: (a) impaired hepatocellular function with deterioration of hepatic machinery paradoxically suppressed serum enzyme levels (Kanno et al., 2000); and (b) the assay results may have been compromised due to minute (non-visible) contamination with free hemoglobin. The authors favor the latter since we presented ample evidence of on-going hepatocellular injury in all three models of steatohepatitis, and several reports have highlighted problems occurring with automated selected enzyme chemistry assays, i.e. LDH and transaminases, due to hemoglobin contamination, even in very small amounts (Lippi et al., 2006; Cameron et al., 2009; Koseoglu et al., 2011). None of our samples had visibly detectable hemolysis. However, in contrast to our previous studies in which the serum assays were performed immediately, locally and manually, in the present study, the serum chemistry assays were performed by a commercial laboratory using automated equipment.
In humans, ALD is associated with hepatic steatosis, acute inflammation, necrosis, ballooning degeneration of hepatocytes and disorganization of the lobular architecture (Tannapfel et al., 2011; Yerian, 2011). In the present study, livers from all three experimental groups exhibited steatosis, subacute inflammation, hepatocellular apoptosis or necrosis, and disruption of the chord-like architecture, and therefore mimicked many aspects of human ALD. In contrast to the reports by Bertola et al. (2013a), we did not detect neutrophil infiltration in our chronic+binge ethanol exposure models. This difference could be explained by the higher binge ethanol dose (5 g/kg) used compared with the present experiment (2 g/kg).
Although the NNK-induced steatohepatitis was not as severe as with ethanol, it was worse than with other nitrosamine exposures, i.e. NDEA and streptozotocin (Tong et al., 2009, 2010; Jin et al., 2010), and the features over-lapped with ALD. Moreover, NNK's adverse effects on steatohepatitis appeared to be additive with ethanol, particularly with regard to the severity of fibrosis (Sirius red staining), which marks ALD progression, and ultrastructural pathology manifested by mitochondrial depletion and RER disruption. These findings suggest that chronic exposure to submutagenic nitrosamines present in tobacco smoke can cause steatohepatitis, worsen alcoholic liver disease and possibly enhance ALD progression. Mechanistically, NNK-induced steatohepatitis is likely mediated by NNK metabolism (Atalla et al., 2000) and DNA adduct formation (Lao et al., 2006; Upadhyaya et al., 2008; Stepanov and Hecht, 2009) in liver. Correspondingly, we detected O6-methyl-Gua in the NNK-exposed livers. It is not clear why blood alcohol concentrations were lower in the ethanol+NNK relative to ethanol-only exposures. We speculate that competition for cytochrome P450 enzymes may play a role. For example CYP2E1 is induced by alcohol, generating oxidative radicals that cause cell injury (Lieber, 2004; Bardag-Gorce et al., 2006), and also activating NNK (Yue et al., 2009) which can alter various enzyme activities.
Chronic plus binge alcohol and NNK effects on inflammatory and stress mediators of ALD
The multiplex ELISA results demonstrated broad activation of pro-inflammatory cytokines in all experimental groups. This result contrasts with the relatively modest levels of cytokine activation observed previously in chronic ethanol exposure models (Setshedi et al., 2011; Ramirez et al., 2013). However, the increased levels of TNF-α and IL-1β with ethanol exposures are consistent with previous reports in other studies of ALD (Ronis et al., 2005, 2010; Singh et al., 2007; Bertola et al., 2013b). However, the independent effects of NNK with respect to IL-6 and IL-1β activation are novel. Given their separate effects on inflammation and cytokine activation, the lack of additive ethanol+NNK exposure effects was somewhat surprising since co-administration of ethanol and nitrosamines was previously shown to sharply increase O6-methyl-Gua DNA adduct formation (Chhabra et al., 2000). Nonetheless, the key point is that both ethanol and tobacco nitrosamine exposures cause chronic pro-inflammatory states in liver, and thereby contribute to on-going hepatocellular injury and cell death. Since inflammation worsens insulin resistance, oxidative stress, and dysregulated lipid metabolism, chronic, broad activation of pro-inflammatory mediators due to ethanol and/or low-level tobacco nitrosamine exposures are likely to contribute to ALD pathogenesis and progression.
Besides the activation of pro-inflammatory mediators, NNK and alcohol cause oxidative stress, DNA damage and adduct formation (Bhagwat et al., 1998; Upadhyaya et al., 2008; Pang et al., 2009; Stepanov and Hecht, 2009; Setshedi et al., 2011; Ramirez et al., 2013). Some of these effects are mediated through regulation of cytochrome P450 (CYP) genes and enzymes. For example, nicotine stimulates expression of the CYP2A6 and CYP1A1 which promote oxidative stress due to metabolism of nicotine to NNK and cotinine (Ande et al., 2012). CYP2A6, CYP2A13, CYP2B1 and CYP1A2 promote NNK activation via alpha-hydroxylation (Guo et al., 1991; Smith et al., 1996; Chiang et al., 2011). Ethanol induces CYP2E1 (Lieber, 2004; Bardag-Gorce et al., 2006) and CYP2B1/2 (Yue et al., 2009), which enhance NNK activation (Guo et al., 1991; Smith et al., 1995; Ardies et al., 1996). Activated NNK methylates and pyridyloxobutylates DNA (Peterson et al., 1993); NNK methylation of DNA is marked by the formation of O6-methyl-Gua adducts (Hecht et al., 1993; Chiang et al., 2011). In addition, NNK and other tobacco-specific nitrosamines can form adducts with proteins, such as globin (Carmella and Hecht, 1987; Hecht et al., 1991, 1994; Upadhyaya et al., 2009).
Beside causing permanent macromolecular injury including DNA damage (Becher et al., 1993; Wang et al., 2012b), NNK adducts can alter gene expression (Devereux et al., 1993) by interacting with or methylation of DNA promoter regions (Cloutier et al., 1999; Pulling et al., 2001, 2004; Vuillemenot et al., 2006; Kimura et al., 2012; Wang et al., 2012a). In addition, CYP2E1, together with alcohol dehydrogenase and catalase, oxidizes alcohol to acetaldehyde, which is toxic and leads to adduct formation on proteins, nucleic acids and lipids (Setshedi et al., 2011), unless efficiently metabolized to acetate and water by aldehyde dehydrogenase 2 (Yu et al., 2010). Complete metabolism of alcohol in the presence of NNK may be limited since NNK causes Hepatotoxic effects of ethanol and NNK can be manifested by cellular injury and death, mitochondrial dysfunction, disruption of the cytoskeleton, and lipid and protein adduct accumulation.
Cytokeratin 18 marks hepatocellular injury with pre-Mallory inclusions in ALD (Savolainen et al., 1994), and can serve as a non-invasive serum biomarker for hepatocellular injury and death following toxin exposure (Cave et al., 2011), NAFLD (Smith and Adams, 2011) and heavy alcohol misuse (Gonzalez-Quintela et al., 2011; Lavallard et al., 2011). Carbonylation is a marker of protein oxidation that reflects stress and is elevated in steatohepatitis (Videla et al., 2004). HNE is a dominant aldehydic product of lipid peroxidation of membrane polyunsaturated fatty acids that exerts cytotoxic effects, including via post-translational modification and impairment of ALDH function (Song et al., 2011). In addition, human heavy drinkers with ALD were found to have elevated serum levels of anti-HNE, suggesting host immune mediated injury contributes to liver disease (Mottaran et al., 2002). Isoprostane 8-iso-PGF2α is formed by free radical-catalyzed peroxidation of arachidonic acid independent of cyclo-oxygenase and therefore marks lipid peroxidation (Morrow et al., 1992, 1994; Sodergren et al., 2000; Yin et al., 2007). Isoprostane levels along with upregulated mRNA expression of lipoxygenases have been observed in human livers with ALD or NAFLD (Raszeja-Wyszomirska et al., 2012). Nitrosylation can impair function of alcohol metabolizing enzymes, including aldehyde dehydrogenase, and thereby exacerbate the toxic effects of ethanol due to acetaldehyde buildup (Moon et al., 2007). Altered protein and lipid structures have been recognized as important features of ALD for decades (Takahashi et al., 1991; Rouach et al., 1997). Our studies showed that ethanol and NNK each increased lipid adducts, although the indices of lipid peroxidation differed in that 8-iso-PGF-2α was driven more by NNK exposures, while HNE was increased by ethanol, with or without NNK co-exposures. Although hepatic protein carbonylation was not significantly increased by ethanol or NNK, the intra-group variances were high. Future studies will be directed toward examining adduct formation on specific rather than global proteins, as the consequences may be relevant to the observed impairments in liver function.
Chronic plus binge alcohol and NNK effects on hepatic insulin/IGF-1 signaling mediators
Previous studies demonstrated significant impairments in insulin signaling in both experimental models and humans with ALD (Onishi et al., 2003; Ronis et al., 2007; de la Monte et al., 2008; Denucci et al., 2010; Setshedi et al., 2011; Longato et al., 2012; Ramirez et al., 2013). Insulin resistance was linked to three key pathogenic factors: steatosis with dysregulated lipid metabolism; inflammation, and oxidative and ER stress (de la Monte et al., 2009a; Longato et al., 2012; Ramirez et al., 2013). However, since impairments in insulin signaling occur even after short durations of ethanol exposure, they could be regarded as the initiating event, although once established, ALD treatment would likely have to be multi-pronged to interrupt the cascade of progressive degeneration (de la Monte et al., 2009a; de la Monte, 2012). In our consideration of the potential role of tobacco nitrosamines as mediators or co-factors in ALD, we evaluated effects of NNK on hepatic insulin/IGF-1 signaling mechanisms.
We measured total and phosphorylated proteins involved in signaling through Akt since Akt has a key role in regulating cell growth, survival and metabolism, including lipid homeostasis in liver (Roberts et al., 2000; Li et al., 2005; Michl and Downward, 2005; Leavens and Birnbaum, 2011). The studies showed that both ethanol and NNK impaired expression of the insulin receptor and that the effects were additive. Reduced insulin receptor expression could contribute to impairments in insulin signaling in the liver by limiting functional activation of downstream pathways. In contrast, ethanol mainly was responsible for inhibiting phosphorylation of the insulin receptor, and phosphorylation of the insulin receptor, Akt, and GSK-3β, but in some instances, e.g. IRS-1, NNK co-exposures further impaired phosphorylation of signaling molecules. However, both ethanol and NNK exposures contributed significantly to the relative reductions in phosphorylation of the IGF-1 receptor (utilized for cellular proliferation and regeneration), Akt and GSK-3β. Decreased total and relative levels of GSK-3β phosphorylation and increased levels of GSK-3β protein occurred with ethanol or NNK, indicating that both contributed to increased GSK-3β activation. Importantly, GSK-3β activation opposes the positive effects of PI3K-Akt signaling and stimulation of growth, metabolism, and cell survival, and its inhibition of oxidative stress, inflammation, and cell death (apoptosis) (Pap and Cooper, 1998; Pearl and Barford, 2002; McCubrey et al., 2014).
Conclusions
These studies provide new evidence that chronic, low-dose (sub-mutagenic) NNK tobacco nitrosamine exposures cause liver injury with molecular, biochemical and histopathological features that overlap with the effects of alcohol, and suggest that they can contribute to the pathogenesis of ALD. Although both ethanol and NNK caused hepatic inflammation, oxidative stress, steatosis and impairments in insulin/IGF-1 signaling through Akt, the characteristics and severities of these effects were distinctive. The most prominent distinguishing effects of ethanol were to inhibit insulin signaling at multiple levels of the cascade, cause severe hepatic steatosis and increase lipid peroxidation, whereas the main effects of NNK were to impair mitochondrial function and increase oxidative injury, GSK-3β activation and nitrotyrosine adducts. Future studies will determine the degree to which the observed effects of NNK are mediated by O6-methyl-Gua adducts with attendant increased DNA damage (genomic and mitochondrial), inhibition of alcohol metabolizing enzymes with increased acetaldehyde accumulation, and increased ER stress with impairment of the ubiquitin-proteasome pathway.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Alcohol and Alcoholism online.
Funding
This was supported by AA-11431, AA-12908, and a Diversity Supplement to AA-11431 from the National Institute of Alcohol Abuse and Alcoholism, T32 DK-60415 from NIDDK, and CA-81301 from the National Cancer Institute.
Conflict of interest statement
None declared.
REFERENCES
- Ande A, Earla R, Jin M, et al. An LC-MS/MS method for concurrent determination of nicotine metabolites and the role of CYP2A6 in nicotine metabolite-mediated oxidative stress in SVGA astrocytes. Drug Alcohol Depend. 2012;125:49–59. doi: 10.1016/j.drugalcdep.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardies CM, Smith TJ, Kim S, et al. Induction of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) activation in rat lung microsomes by chronic ethanol consumption and repeated running exercise. Cancer Lett. 1996;103:209–18. doi: 10.1016/0304-3835(96)04216-4. [DOI] [PubMed] [Google Scholar]
- Atalla A, Breyer-Pfaff U, Maser E. Purification and characterization of oxidoreductases-catalyzing carbonyl reduction of the tobacco-specific nitrosamine 4-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK) in human liver cytosol. Xenobiotica. 2000;30:755–69. doi: 10.1080/00498250050119826. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Mohr L, Wands JR, et al. Ethanol inhibition of insulin signaling in hepatocellular carcinoma cells. Alcohol Clin Exp Res. 1998;22:2093–101. [PubMed] [Google Scholar]
- Bardag-Gorce F, French BA, Nan L, et al. CYP2E1 induced by ethanol causes oxidative stress, proteasome inhibition and cytokeratin aggresome (Mallory body-like) formation. Exp Mol Pathol. 2006;81:191–201. doi: 10.1016/j.yexmp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Baumgardner JN, Shankar K, Hennings L, et al. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr. 2008;138:1872–9. doi: 10.1093/jn/138.10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher R, Lag M, Schwarze PE, et al. Chemically induced DNA damage in isolated rabbit lung cells. Mutat Res. 1993;285:303–11. doi: 10.1016/0027-5107(93)90119-z. [DOI] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013a;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013b;58:1814–23. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat SV, Vijayasarathy C, Raza H, et al. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–9. doi: 10.1016/s0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512:121–34. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Caldwell S, Ikura Y, Dias D, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53:719–23. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SJ, Gerhardt G, Engelstad M, et al. Interference in clinical chemistry assays by the hemoglobin-based oxygen carrier, Hemospan. Clin Biochem. 2009;42:221–4. doi: 10.1016/j.clinbiochem.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Hecht SS. Formation of hemoglobin adducts upon treatment of F344 rats with the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N’-nitrosonornicotine. Cancer Res. 1987;47:2626–30. [PubMed] [Google Scholar]
- Cave M, Falkner KC, Henry L, et al. Serum cytokeratin 18 and cytokine elevations suggest a high prevalence of occupational liver disease in highly exposed elastomer/polymer workers. J Occup Environ Med. 2011;53:1128–33. doi: 10.1097/JOM.0b013e31822cfd68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra SK, Anderson LM, Perella C, et al. Coexposure to ethanol with N-nitrosodimethylamine or 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone during lactation of rats: marked increase in O(6)-methylguanine-DNA adducts in maternal mammary gland and in suckling lung and kidney. Toxicol Appl Pharmacol. 2000;169:191–200. doi: 10.1006/taap.2000.9068. [DOI] [PubMed] [Google Scholar]
- Chiang HC, Wang CY, Lee HL, et al. Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced gene mutation—a mammalian cell-based mutagenesis approach. Toxicol Appl Pharmacol. 2011;253:145–52. doi: 10.1016/j.taap.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Cloutier JF, Drouin R, Castonguay A. Treatment of human cells with N-Nitroso(acetoxymethyl)methylamine: distribution patterns of piperidine-sensitive DNA damage at the nucleotide level of resolution are related to the sequence context. Chem Res Toxicol. 1999;12:840–9. doi: 10.1021/tx990025f. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis. 2011;12:3–9. doi: 10.1111/j.1751-2980.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MF, Sanderson RJ, Damhuis RA, et al. The effects of alcohol and smoking upon the age, anatomic sites and stage in the development of cancer of the oral cavity and oropharynx in females in the south west Netherlands. Eur Arch Otorhinolaryngol. 1997;254:177–9. doi: 10.1007/BF00879269. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Alcohol-induced liver and brain degeneration: roles of insulin resistance, toxic ceramides, and endoplasmic reticulum stress. In: Sova Ms., editor. Alcohol, Nutrition, and Health Consequences. New York: Humana Press; 2012. [Google Scholar]
- de la Monte SM, Yeon JE, Tong M, et al. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008;23:e477–86. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, et al. The liver-brain axis of alcohol-mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health. 2009a;6:2055–75. doi: 10.3390/ijerph6072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lawton M, et al. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol Neurodegener. 2009b;4:54. doi: 10.1186/1750-1326-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denucci SM, Tong M, Longato L, et al. Rat strain differences in susceptibility to alcohol-induced chronic liver injury and hepatic insulin resistance. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/312790. doi:10.1155/2010/312790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdak Z, Lang CH, Villegas KA, et al. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. J Hepatol. 2011;54:164–72. doi: 10.1016/j.jhep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux TR, Belinsky SA, Maronpot RR, et al. Comparison of pulmonary O6-methylguanine DNA adduct levels and Ki-ras activation in lung tumors from resistant and susceptible mouse strains. Mol Carcinog. 1993;8:177–85. doi: 10.1002/mc.2940080308. [DOI] [PubMed] [Google Scholar]
- Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 2011;236:546–56. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog. 2012;51:40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Bailey SM. Emerging role of redox dysregulation in alcoholic and nonalcoholic fatty liver disease. Antioxid Redox Signal. 2011;15:421–4. doi: 10.1089/ars.2011.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan G, Hu R, Dai A, et al. The role of endoplasmic reticulum stress in emphysema results from cigarette smoke exposure. Cell Physiol Biochem. 2011;28:725–32. doi: 10.1159/000335766. [DOI] [PubMed] [Google Scholar]
- Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol (Fayetteville, NY) 2005;35:205–11. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Tome S, Fernandez-Merino C, et al. Synergistic effect of alcohol consumption and body mass on serum concentrations of cytokeratin-18. Alcohol Clin Exp Res. 2011;35:2202–8. doi: 10.1111/j.1530-0277.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Smith TJ, Ishizaki H, et al. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by cytochrome P450IIB1 in a reconstituted system. Carcinogenesis. 1991;12:2277–82. doi: 10.1093/carcin/12.12.2277. [DOI] [PubMed] [Google Scholar]
- Ha NB, Ha NB, Ahmed A, et al. Risk factors for hepatocellular carcinoma in patients with chronic liver disease: a case-control study. Cancer Causes Control. 2012;23:455–62. doi: 10.1007/s10552-012-9895-z. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Murphy SE. Hemoglobin adducts as biomarkers of exposure to and metabolic activation of carcinogenic tobacco-specific nitrosamines. Biomed Environ Sci. 1991;4:93–103. [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Foiles PG, et al. Tobacco-specific nitrosamine adducts: studies in laboratory animals and humans. Environ Health Perspect. 1993;99:57–63. doi: 10.1289/ehp.939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Foiles PG, et al. Biomarkers for human uptake and metabolic activation of tobacco-specific nitrosamines. Cancer Res. 1994;54:1912s–17s. [PubMed] [Google Scholar]
- Jin X, Zeng L, He S, et al. Comparison of single high-dose streptozotocin with partial pancreatectomy combined with low-dose streptozotocin for diabetes induction in rhesus monkeys. Exp Biol Med (Maywood) 2010;235:877–85. doi: 10.1258/ebm.2010.009361. [DOI] [PubMed] [Google Scholar]
- Johnson NW, Warnakulasuriy S, Tavassoli M. Hereditary and environmental risk factors; clinical and laboratory risk matters for head and neck, especially oral, cancer and precancer. Eur J Cancer Prev. 1996;5:5–17. [PubMed] [Google Scholar]
- Kanno N, LeSage G, Glaser S, et al. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–61. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21(Suppl 3):S7–9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- Kimura S, Paiz J, Yoneda M, et al. Deficiency of CCAAT/enhancer binding protein family DNA binding prevents malignant conversion of adenoma to carcinoma in NNK-induced lung carcinogenesis in the mouse. Mol Cancer. 2012;11:90. doi: 10.1186/1476-4598-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseoglu M, Hur A, Atay A, et al. Effects of hemolysis interferences on routine biochemistry parameters. Biochem Med. 2011;21:79–85. doi: 10.11613/bm.2011.015. [DOI] [PubMed] [Google Scholar]
- Koulmanda M, Qipo A, Chebrolu S, et al. The effect of low versus high dose of streptozotocin in cynomolgus monkeys (Macaca fascilularis) Am J Transplant. 2003;3:267–72. doi: 10.1034/j.1600-6143.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- Lao Y, Villalta PW, Sturla SJ, et al. Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2006;19:674–82. doi: 10.1021/tx050351x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao Y, Yu N, Kassie F, et al. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N’-nitrosonornicotine. Chem Res Toxicol. 2007;20:246–56. doi: 10.1021/tx060208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallard VJ, Bonnafous S, Patouraux S, et al. Serum markers of hepatocyte death and apoptosis are non invasive biomarkers of severe fibrosis in patients with alcoholic liver disease. PLoS One. 2011;6:e17599. doi: 10.1371/journal.pone.0017599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200–15. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- Li XL, Man K, Ng KT, et al. The influence of phosphatidylinositol 3-kinase/Akt pathway on the ischemic injury during rat liver graft preservation. Am J Transplant. 2005;5:1264–75. doi: 10.1111/j.1600-6143.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol (Fayetteville, NY) 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lippi G, Salvagno GL, Montagnana M, et al. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:311–6. doi: 10.1515/CCLM.2006.054. [DOI] [PubMed] [Google Scholar]
- Longato L, Ripp K, Setshedi M, et al. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid Med Cell Longev. 2012;2012:479348. doi: 10.1155/2012/479348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, et al. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol Clin Exp Res. 2011;35:1106–13. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Bertrand FE, et al. Multifaceted roles of GSK-3 and Wnt/beta-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014;28:15–33. doi: 10.1038/leu.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AJ, O'Shea RS, Dasarathy S. Diagnosis and management of alcoholic liver disease. J Dig Dis. 2011;12:257–62. doi: 10.1111/j.1751-2980.2010.00470.x. [DOI] [PubMed] [Google Scholar]
- McVicker BL, Tuma DJ, Kubik JL, et al. Ethanol-induced apoptosis in polarized hepatic cells possibly through regulation of the Fas pathway. Alcohol Clin Exp Res. 2006;30:1906–15. doi: 10.1111/j.1530-0277.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43:1133–9. doi: 10.1055/s-2005-858638. [DOI] [PubMed] [Google Scholar]
- Miller AM, Horiguchi N, Jeong WI, et al. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787–93. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Abdelmegeed MA, Song BJ. Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS Lett. 2007;581:3967–72. doi: 10.1016/j.febslet.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Kato T, et al. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90:2502–7. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Minton TA, Mukundan CR, et al. Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J Biol Chem. 1994;269:4317–26. [PubMed] [Google Scholar]
- Mottaran E, Stewart SF, Rolla R, et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- Onishi Y, Honda M, Ogihara T, et al. Ethanol feeding induces insulin resistance with enhanced PI 3-kinase activation. Biochem Biophys Res Commun. 2003;303:788–94. doi: 10.1016/s0006-291x(03)00407-8. [DOI] [PubMed] [Google Scholar]
- O'Shea RS, Dasarathy S, McCullough AJ Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- Pandol SJ, Gorelick FS, Gerloff A, et al. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis. 2010;28:776–82. doi: 10.1159/000327212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, de la Monte SM, Longato L, et al. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol. 2009;50:1192–201. doi: 10.1016/j.jhep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–32. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Paula H, Asrani SK, Boetticher NC, et al. Alcoholic liver disease-related mortality in the United States: 1980–2003. Am J Gastroenterol. 2010;105:1782–7. doi: 10.1038/ajg.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH, Barford D. Regulation of protein kinases in insulin, growth factor and Wnt signalling. Curr Opin Struct Biol. 2002;12:761–7. doi: 10.1016/s0959-440x(02)00386-x. [DOI] [PubMed] [Google Scholar]
- Peterson LA, Liu XK, Hecht SS. Pyridyloxobutyl DNA adducts inhibit the repair of O6-methylguanine. Cancer Res. 1993;53:2780–5. [PubMed] [Google Scholar]
- Pulling LC, Klinge DM, Belinsky SA. p16INK4a and beta-catenin alterations in rat liver tumors induced by NNK. Carcinogenesis. 2001;22:461–6. doi: 10.1093/carcin/22.3.461. [DOI] [PubMed] [Google Scholar]
- Pulling LC, Vuillemenot BR, Hutt JA, et al. Aberrant promoter hypermethylation of the death-associated protein kinase gene is early and frequent in murine lung tumors induced by cigarette smoke and tobacco carcinogens. Cancer Res. 2004;64:3844–8. doi: 10.1158/0008-5472.CAN-03-2119. [DOI] [PubMed] [Google Scholar]
- Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Kwon OS, et al. Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci. 2013;92:3–9. doi: 10.1016/j.lfs.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez T, Longato L, Dostalek M, et al. Insulin resistance, ceramide accumulation and endoplasmic reticulum stress in experimental chronic alcohol-induced steatohepatitis. Alcohol Alcohol. 2013;48:39–52. doi: 10.1093/alcalc/ags106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raszeja-Wyszomirska J, Safranow K, Milkiewicz M, et al. Lipidic last breath of life in patients with alcoholic liver disease. Prostaglandins Other Lipid Mediat. 2012;99:51–6. doi: 10.1016/j.prostaglandins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Roberts RA, James NH, Cosulich SC. The role of protein kinase B and mitogen-activated protein kinase in epidermal growth factor and tumor necrosis factor alpha-mediated rat hepatocyte survival and apoptosis. Hepatology. 2000;31:420–7. doi: 10.1002/hep.510310223. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Butura A, Sampey BP, et al. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39:619–30. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronis MJ, Wands JR, Badger TM, et al. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–85. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Butura A, Korourian S, et al. Cytokine and chemokine expression associated with steatohepatitis and hepatocyte proliferation in rats fed ethanol via total enteral nutrition. Exp Biol Med (Maywood) 2008;233:344–55. doi: 10.3181/0707-RM-203. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Korourian S, Blackburn ML, et al. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol (Fayetteville, NY) 2010;44:157–69. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach H, Fataccioli V, Gentil M, et al. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–5. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- Salaspuro M. Acetaldehyde and gastric cancer. J Dig Dis. 2011;12:51–9. doi: 10.1111/j.1751-2980.2011.00480.x. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hayashi N, Ito T, et al. Influence of ethanol on insulin receptor substrate-1-mediated signal transduction during rat liver regeneration. Alcohol Alcohol Suppl. 1994;29:99–106. [PubMed] [Google Scholar]
- Savolainen VT, Lalu K, Penttila A, et al. Cytokeratin inclusions in alcoholic liver disease and their relation to the amount of alcohol intake. Liver. 1994;14:281–7. doi: 10.1111/j.1600-0676.1994.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–60. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- Seth D, Haber PS, Syn WK, et al. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089–105. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res. 2011;35:2139–51. doi: 10.1111/j.1530-0277.2011.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Jiang Y, Benlhabib E, et al. Herbal mixtures consisting of puerarin and either polyenylphosphatidylcholine or curcumin provide comprehensive protection against alcohol-related disorders in P rats receiving free choice water and 15% ethanol in pure water. J Med Food. 2007;10:526–42. doi: 10.1089/jmf.2006.228. [DOI] [PubMed] [Google Scholar]
- Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Stoner GD, Yang CS. Activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human lung microsomes by cytochromes P450, lipoxygenase, and hydroperoxides. Cancer Res. 1995;55:5566–73. [PubMed] [Google Scholar]
- Smith TJ, Guo Z, Guengerich FP, et al. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by human cytochrome P450 1A2 and its inhibition by phenethyl isothiocyanate. Carcinogenesis. 1996;17:809–13. doi: 10.1093/carcin/17.4.809. [DOI] [PubMed] [Google Scholar]
- Sodergren E, Vessby B, Basu S. Radioimmunological measurement of F(2)-isoprostanes after hydrolysis of lipids in tissues. Prostaglandins Leukot Essent Fatty Acids. 2000;63:149–52. doi: 10.1054/plef.2000.0172. [DOI] [PubMed] [Google Scholar]
- Song BJ, Abdelmegeed MA, Yoo SH, et al. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteomics. 2011;74:2691–702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Hecht SS. Mitochondrial DNA adducts in the lung and liver of F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and (S)-4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2009;22:406–14. doi: 10.1021/tx800398x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, French SW, Wong PT. Alterations in hepatic lipids and proteins by chronic ethanol intake: a high-pressure Fourier transform infrared spectroscopic study on alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1991;15:219–23. doi: 10.1111/j.1530-0277.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Tannapfel A, Denk H, Dienes HP, et al. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. 2011;458:511–23. doi: 10.1007/s00428-011-1066-1. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Guo H, Carmella SG, et al. Metabolites of a tobacco-specific lung carcinogen in children exposed to secondhand or thirdhand tobacco smoke in their homes. Cancer Epidemiol Biomarkers Prev. 2011;20:1213–21. doi: 10.1158/1055-9965.EPI-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Neusner A, Longato L, et al. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17:827–44. [PMC free article] [PubMed] [Google Scholar]
- Tong M, Longato L, de la Monte SM. Early limited nitrosamine exposures exacerbate high fat diet-mediated type 2 diabetes and neurodegeneration. BMC Endocr Disord. 2010;10:4. doi: 10.1186/1472-6823-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramacere I, Negri E, Bagnardi V, et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 1: overall results and dose-risk relation. Oral Oncol. 2010;46:497–503. doi: 10.1016/j.oraloncology.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Upadhyaya P, Kalscheuer S, Hochalter JB, et al. Quantitation of pyridylhydroxybutyl-DNA adducts in liver and lung of F-344 rats treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2008;21:1468–76. doi: 10.1021/tx8001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya P, Lindgren BR, Hecht SS. Comparative levels of O6-methylguanine, pyridyloxobutyl-, and pyridylhydroxybutyl-DNA adducts in lung and liver of rats treated chronically with the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Drug Metab Dispos. 2009;37:1147–51. doi: 10.1124/dmd.109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali M, Stewart SF, Albano E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends Mol Med. 2008;14:63–71. doi: 10.1016/j.molmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Videla LA, Rodrigo R, Orellana M, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–8. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- Vuillemenot BR, Hutt JA, Belinsky SA. Gene promoter hypermethylation in mouse lung tumors. Mol Cancer Res. 2006;4:267–73. doi: 10.1158/1541-7786.MCR-05-0218. [DOI] [PubMed] [Google Scholar]
- Wang S, Kamat A, Pergola P, et al. Metabolic factors in the development of hepatic steatosis and altered mitochondrial gene expression in vivo. Metabolism. 2011;60:1090–9. doi: 10.1016/j.metabol.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao SL, Li Y, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone induces retinoic acid receptor beta hypermethylation through DNA methyltransferase 1 accumulation in esophageal squamous epithelial cells. Asian Pac J Cancer Prev. 2012a;13:2207–12. doi: 10.7314/apjcp.2012.13.5.2207. [DOI] [PubMed] [Google Scholar]
- Wang XY, Jensen-Taubman SM, Keefe KM, et al. Achaete-scute complex homolog-1 promotes DNA repair in the lung carcinogenesis through matrix metalloproteinase-7 and O(6)-methylguanine-DNA methyltransferase. PLoS One. 2012b;7:e52832. doi: 10.1371/journal.pone.0052832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerian L. Histopathological evaluation of fatty and alcoholic liver diseases. J Dig Dis. 2011;12:17–24. doi: 10.1111/j.1751-2980.2010.00472.x. [DOI] [PubMed] [Google Scholar]
- Yin H, Gao L, Tai HH, et al. Urinary prostaglandin F2alpha is generated from the isoprostane pathway and not the cyclooxygenase in humans. J Biol Chem. 2007;282:329–36. doi: 10.1074/jbc.M608975200. [DOI] [PubMed] [Google Scholar]
- Yu HS, Oyama T, Isse T, et al. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact. 2010;188:367–75. doi: 10.1016/j.cbi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Yue J, Khokhar J, Miksys S, et al. Differential induction of ethanol-metabolizing CYP2E1 and nicotine-metabolizing CYP2B1/2 in rat liver by chronic nicotine treatment and voluntary ethanol intake. Eur J Pharmacol. 2009;609:88–95. doi: 10.1016/j.ejphar.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang M, Villalta PW, et al. Analysis of pyridyloxobutyl and pyridylhydroxybutyl DNA adducts in extrahepatic tissues of F344 rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2009;22:926–36. doi: 10.1021/tx900015d. [DOI] [PMC free article] [PubMed] [Google Scholar]