Abstract
Aims: We report on the development of a real-time assessment protocol that allows researchers to assess change in BrAC, alcohol responses, behaviors, and contexts over the course of a drinking event. Method: We designed a web application that uses timed text messages (adjusted based on consumption pattern) containing links to our website to obtain real-time participant reports; camera and location features were also incorporated into the protocol. We used a transdermal alcohol sensor device along with software we designed to convert transdermal data into estimated BrAC. Thirty-two college students completed a laboratory session followed by a 2-week field trial. Results: Results for the web application indicated we were able to create an effective tool for obtaining repeated measures real-time drinking data. Participants were willing to monitor their drinking behavior with the web application, and this did not appear to strongly affect drinking behavior during, or 6 weeks following, the field trial. Results for the transdermal device highlighted the willingness of participants to wear the device despite some discomfort, but technical difficulties resulted in limited valid data. Conclusion: The development of this protocol makes it possible to capture detailed assessment of change over the course of naturalistic drinking episodes.
INTRODUCTION
Real-time assessment (a.k.a. ecological momentary assessment, experience sampling) is a procedure that measures individuals in their natural environment as events occur (see Stone et al., 2007; Shiffman et al., 2008). Research using this methodology has examined naturalistic smoking (Shiffman et al., 1996), alcohol (Collins et al., 1998; Kuntsche and Labhart, 2012; see Shiffman, 2009) and eating (Steiger et al., 1999) behaviors that cannot be tested in the laboratory. Previous research has established the validity of using real-time assessment to assess drinking behavior in social drinkers for several weeks at a time (Samo et al., 1989; Sobell et al., 1989; Collins et al., 1998; Hufford et al., 2002) and indicates that monitoring drinking does not substantially affect behavior (Collins et al., 1998; Muraven et al., 2005; Neville et al., 2013).
Although numerous real-time data collection studies have been conducted to capture naturalistic behaviors (see Feldman Barrett and Barrett, 2001; Bolger et al., 2003; Christensen et al., 2003; Hufford and Shiffman, 2003), few studies have repeatedly assessed within-person changes over the course of an event (Kuntsche and Labhart, 2012; Piasecki et al., 2011) and no studies have repeatedly captured objective and subjective drinking measures over the entire duration of a drinking episode or on all drinking days (see also Weaver et al., 2013). In the current study, we sought to design a real-time data collection protocol that would enable us to gain a better understanding of how naturalistic drinking behaviors, including quantity of alcohol consumption, rate of ingestion and subjective responses to alcohol, differ across individuals with and without a variant aldehyde dehydrogenase ALDH2*2 allele (see Luczak et al., 2006, 2014). This methodology is also appropriate for measuring change over the course of a drinking episode for research topics such as decisions to drive, engaging in risky sexual behavior or drinking motives. Clinically, this methodology would be useful for clients and clinicians to monitor relapse (contexts that trigger lapses, reasons for stopping a drinking episode once it begins) or harm reduction behaviors (e.g. limiting binge drinking, not driving while intoxicated). In addition, other substances, eating patterns or social situations could also be assessed using the web application portion of this methodology (without transdermal alcohol sensors) to capture change over the course of other types of events.
The protocol we developed repeatedly assesses objective and subjective measures of alcohol use throughout the course of a drinking episode. We used a transdermal alcohol sensor (TAS) device and a software program we designed to convert these transdermal data into breath alcohol concentration (BrAC) estimates as our objective measures of alcohol consumption. We designed a web application that uses a series of timed text messages (adjusted based on consumption pattern) that contain links to our website to have participants respond in real-time to questions about their naturalistic drinking behaviors, reactions and contexts; photo and global positioning satellite (GPS) features of the smart phone were incorporated into the protocol to further document consumption and context. We first describe the development of the protocol and then report on its feasibility (i.e. reliability of each component of the protocol, validity of TAS data, participant compliance and reactivity) in a college student sample.
MATERIALS AND METHODS
Development of the data collection system
Web application
The Drinking Study web application consists of a timed text messaging system, a website with three sets of web pages for the user, and a data tracking and storage system for the administrator (see Appendix). We chose to use a web-based application (rather than an application specific to one type of phone) to add flexibility to the system, with the end-goal to make this system usable by research participants from their own smart phone regardless of phone brand or service provider (see Kuntsche and Labhart, 2013b, 2014). The application included the following features: (a) flexibility of question administration over the course of the drinking session (e.g. questions specific to the start of a drinking session, with answers to some of these questions integrated into subsequent round questions; ability to vary time intervals between data collection rounds; variable response formats and modified response choices in later rounds to reduce participant burden), (b) notices sent to research staff when participants start drinking, (c) online storage of data on a secure server immediately accessible to research staff and (d) an administrative interface that allows research staff to modify study settings and questions and to manage data collection.
The three sets of web pages included the following:
-
The Real-Time web pages were used to record data during drinking episodes. Immediately prior to drinking, participants logged into the website to begin recording a session. In the first round, participants were asked a series of questions including expectations and motives for the drinking episode (Cooper, 1994); these questions only appeared this one time, but some of these responses (e.g. primary reason for drinking) were integrated into questions in subsequent rounds.
At baseline and in every subsequent round, participants recorded their drinking context (location, who they were with), food and substance consumption (alcohol, caffeine, nicotine, other substances), subjective responses to alcohol (e.g. feeling drunk, flushed, sedated; Johnson et al., 1984; Schuckit, 1984; Martin et al., 1993; Holdstock et al., 2000), estimated their blood alcohol concentration (BAC), and wrote any additional comments; at the end of each round they also took a photo (time-stamped and with GPS) of their drink that included their hand for scale, and if they did not currently have a drink they took a photo of their empty hand.
A text message was sent to the phone every 30 min for the first hour and then every 45 min to prompt participants to complete the assessment; a link to the website was provided in the text message or they could login through the icon on the home page of the phone. If a participant did not respond, reminder messages were sent 10 and 20 min after the initial text for that round of data collection. Once a participant had not consumed alcohol for two consecutive rounds (as determined by answering no to the ‘counter variable,’ which asked participants ‘have you had any alcohol since you last logged on?’), the session ended; a text message was then sent to the participant that the session had ended. At any point, participants could manually stop a session by clicking on a ‘sleep’ link on the home page of the website.
On the day after a participant drank, the web application sent a text message at noon with a link to the Day After web pages. Participants reported on the number of drinks they had consumed and amount of time they had drunk the day before, number of hours they slept, any negative effects from drinking (Hurlbut and Sher, 1992; Slutske et al., 2003), and reasons they stopped drinking (Oei et al., 1999). In addition, participants reported on the effects of monitoring on their behavior (see Litt et al., 1998) and their overall enjoyment or displeasure with the assessment procedure.
Six weeks after the field trial, participants were sent an email with a link to log into the Six-Week Follow-Up web pages to assess the effects of participating. Participants completed a 90-day drinking calendar and reported if their drinking behaviors had changed after their participation, and if so how and why.
BrAC estimation from TAS devices
As part of the protocol, participants wore a TAS device, the WrisTAS™ 7 (Giner, Inc.; Newton, MA, USA), to obtain objective alcohol levels. The WrisTAS™ 7 has a transdermal electrochemical sensor cell that continuously measures the local ethanol vapor concentration over the skin surface, with readings taken anywhere from every 10 s up to every 30 min; in the current protocol, readings were taken at 5-min intervals. The device is worn like a wristwatch and is placed on the wrist with a simple Velcro strip (see Appendix Fig. 12 or Leffingwell et al., 2013 for photo of the device).
The relationship between the transdermal alcohol concentration (TAC) data produced by TAS device and the more easily interpreted BrAC varies across individuals and devices (due to skin thickness layers, device anomalies, etc.; see Dumett et al., 2008). Thus, we have developed mathematical models and the BrAC Estimator software for calculating BrAC estimates from TAC data (Luczak et al., 2013; Rosen et al., 2013, 2014). To calibrate the models for an individual wearing a particular TAS device requires an initial laboratory alcohol administration session where both BrAC data (from a breath analyzer) and TAC data (from the particular TAS device) are collected; the parameters determined from this session are used to invert all field trial TAC data into BrAC estimates. Initial results indicate the BrAC estimates produced by our models and software closely match (both in terms of level and timing) BrAC data obtained via breath analyzer (Luczak and Rosen, 2014).
Sample
We ran 32 college students through the protocol between November 2010 and August 2011. Participants were on average 23.1 years old (SD = 2.71); 47% (n = 15) were female and 100% were of Asian heritage (because of our interest in studying the ALDH2 gene; see Goedde et al., 1992). Participants were recruited using advertisements on campus, and a telephone screening followed by an in-person interview ensured they met inclusion criteria: no lifetime personal or first-degree family history of alcohol dependence, no major medical problems, and self-reported weekly drinking (on average) over the past 3 months (Sobell and Sobell, 1992; Bucholz et al., 1994). The study was approved by the UCSD Human Research Protection Program and written informed consent for participation was obtained.
Procedure
Laboratory training session
Participants came to the laboratory at 9:00 AM on their first day for a training session that included an alcohol administration session (see Luczak et al., 2002). Participants had to have a breath analyzer reading of 0.000 units when they came and when they left the laboratory, and females had to test negative for pregnancy.
During the first 90 min of the session, participants were trained on the meanings of the subjective items and anchor points for rating scales, how to proceed through a real-time drinking episode, and were given written information that included instructions for the devices, contact numbers, future appointment times and printouts of all questions they would see on the websites. A TAS device was placed on their wrist after obtaining consent for participation. Participants were given an iPhone 4© (Apple, Inc., Cupertino, CA, USA) to use if they did not have their own. Prior to consuming the alcoholic beverage, participants completed the baseline subjective responses on paper. A trained research assistant used the smart phone to proceed through the first round of the website (using the participant's baseline ratings) while the participant observed. All subsequent assessments were completed by the participant.
At 10:30 AM, participants were given alcohol dosed to reach a BrAC peak of ∼0.050 mg% (Watson et al., 1980, 1981). Throughout the laboratory session, participants continued to receive training on the real-time protocol. At the end of the session, participants were quizzed on what to do during the field trial to ensure they understood all procedures.
Field trial
Participants left the laboratory session wearing the TAS device and continued to monitor their alcohol behavior for 2 weeks using this device and the smart phone. Participants were asked to carry the phone with them at all times and to wear the TAS except when they could not avoid environmental alcohol (e.g. science laboratory) or water (swimming, showering).
Participants returned to the laboratory after 1 week to download TAS data and smart phone photos, discuss any problems and receive additional training if necessary based on their first-week performance. Photos and GPS information were used to help prompt participants, add detail to any unclear open-ended responses, rectify any discrepancies in the data and obtain exact alcohol contents from packaging or the names of establishments to contact regarding mixed drink alcohol contents. At the end of the 2 weeks, participants returned to complete a drinking calendar for the past 2 weeks, provide feedback on their experience and receive payment for their participation. Participants were paid up to $320 total ($100 for the laboratory training day, $100 per week of the field trial and $20 for the 6-week assessment).
Six-week follow-up
Participants completed a final assessment 6 weeks later. Following completion, participants could receive feedback on their BrAC and TAS data from the laboratory session and field trial.
RESULTS
The 32 participants recorded data for 140 field trial drinking episodes with 708 rounds of data. The majority of participants (81%) drank on five or fewer occasions (range of 1–11). We first report on the performance of the web application and TAS device, then on the participant adherence to the protocol and feedback.
Web application
Reliability of website and smart phone connectivity
Malfunctions of the web application were more common early in the study, with 28% of participants reporting problems with the web application. Problems typically consisted of getting too many text reminders to complete the session or the session not ending on time. These malfunctions, however, led to very little loss of data.
Loss of data occurred for several reasons. The biggest loss of data occurred in nine sessions where participants had responded they were no longer drinking and then failed to answer the next round; because of the way the counter variable was initially designed, these sessions closed instead of sending another text message for the next round to begin. Adjusting the logic on the counter variable eliminated this problem. Other problems early in the study included three sessions closing after sending a text message to complete the next round, two sessions where the round of data was not completed but text alerts were not sent to remind the participant, four sessions where timing switched one round early from 30-min rounds to 45-min rounds, three incomplete sessions due to poor internet connectivity, and three rounds of partial data for unclear reasons. As each problem was identified, programmers worked to fix the web application, thus minimizing future loss of data.
Participant feedback
At the end of the field trial, 66% of the 31 participants who provided feedback reported they felt very confident they could use the smart phone properly when they had left the initial training session and 78% felt very confident they could use the website (see Table 1). Participants rated the ease of use of the smart phone on a 0 (very easy to use) to 10 (very difficult to use) scale an average of 0.5 (SD = 1.09).
Table 1.
Confidence in ability to properly use devices following the laboratory training session
| Very (%) | Fairly (%) | Slightly (%) | Not at all (%) | |
|---|---|---|---|---|
| TAS device | 84 | 9 | 6 | 0 |
| Smart phone | 66 | 28 | 6 | 0 |
| Web application | 78 | 19 | 3 | 0 |
n = 31. Data collected at the end of the 2-week field trial.
TAS device
Reliability of the TAS device
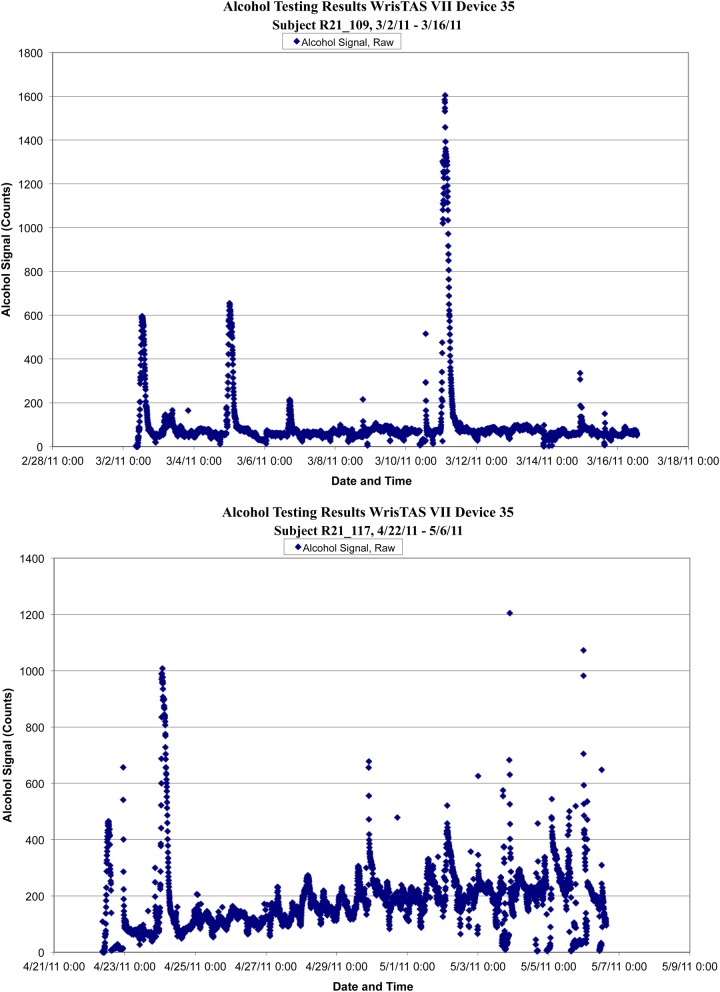
Figure 1 shows TAS data from two participants where the device recorded well (top panel) and poorly (bottom panel). The TAS devices recorded continuously and provided interpretable data over the entire field trial for only 12 (38%) participants. It was originally estimated that we would need to replace the sensor on each device once. However, because of device malfunctions (e.g. low readings attributed to sensor malfunction, flatline readings attributed to wiring malfunction), we returned devices to Giner, Inc. for repair a total of 13 times. Each device was returned at least three times, indicating repeated problems with all four devices. Note that we did not alter the protocol for the participants whose devices failed.
Fig. 1.
TAS data from two participants where the device recorded well throughout the field trial (top panel) and poorly by the end of the field trial (bottom panel).
Validity of TAS data
Within the interpretable TAS data files, we saw a number of spikes in the TAC data during times participants did not report drinking. Some peaks were clearly not drinking sessions as indicated by the rapid increase and decrease in the TAC that were attributed to environmental alcohol (e.g. perfume, hand sanitizer). Other peaks, however, could have been interpreted as drinking episodes based on the shape of the curve. Detailed review of these times with participants led us to note additional situations (e.g. alcohol rubbed on the skin for a flu shot, using markers for several hours) that could be false positives. Because the sensors were not reliable in their recording, it was not possible to determine if some of these spikes were true drinking episodes.
Participant feedback
Feedback was obtained from 31 (97%) participants at the end of the field trial (see Table 1). Most (84%) reported they were very confident they could use the TAS device properly when they left the initial session. On a scale from 0 (very easy to use) to 10 (very difficult to use), participants rated the TAS device an average of 1.2 (SD = 1.80). When asked how aversive it was to wear the device on the 0 (not at all aversive) to 10 (very aversive) scale, the mean rating was 5.2 (SD = 2.31), with one quarter of participants noting skin irritation or rash from the device.
Participant compliance
Equipment
No devices were lost by participants. The back of one phone was cracked but this did not affect its functioning. One participant damaged the TAS device by showering with it and reported this to staff immediately.
Recording drinking episodes
Although the lack of reliability of the TAS devices made it impossible to determine if participants were omitting recording drinking episodes, participants were unaware of the recording problems so the device at least served as a deterrent for such omissions. Two participants received pre-approval not to record their drinking during special events (wedding due to the TAS device showing, music festival due to interference with the event). Four participants did not record an episode in real time (two forgot the phone, one felt uncomfortable recording with colleagues present, one had no reception); however, all of these episodes were recorded retrospectively on the same day and the day after data were completed. In the feedback session, no additional participants reported having omitted recording a drinking session.
Recording during drinking episodes
Table 2 shows summary information for recording during the drinking episodes. From our data there was some difficulty interpreting the onset of a drinking episode (participants initially reported what they were going to drink instead of what they had already consumed). However, 99 (69%) of the 140 sessions clearly began prior to or immediately at the start of drinking. Once sessions began, only 10% of the drinking episodes had a missing round of data, representing 2% of the total 706 rounds of data, and only 9% of the completed rounds needed a reminder to be sent.
Table 2.
Participant adherence to real-time protocol
| Missed rounds (of those sent text messages) | |
| 0 | 91% |
| 1 | 9% |
| 2+ | 1% |
| Reminder texts | |
| 1 reminder (10 min late) | 7% |
| 2 reminders (10 min late) | 1% |
| Session end reason | |
| Counter variable (2 rounds of not drinking) | 71% |
| Sleep link (end before 2 rounds of not drinking) | 16% |
| Going to bed | 13% |
| Other reason | 3% |
| Had stopped drinking when used sleep link | 15% |
| Website/phone reception problem | 11% |
| Unclear reason | 2% |
n = 140 drinking episodes.
Ending drinking episodes
The majority (71%) of sessions were ended by the counter variable after the participant had not been drinking for two rounds of data collection, although 11% of these in fact ended a round early because the participant answered the counter variable question incorrectly; 16% of the sessions were ended with the sleep link to stop the session early, with participants reporting in 83% of these cases they were in fact going to bed (alternative reasons included swimming, entering a movie theater) and 91% of these participants had already stopped drinking; 11% of the sessions were ended after a website or connectivity problem; and 2% ended due to unclear reasons.
Day after data
All 140 drinking episodes have day after data except for two sessions from one participant (who also did not consistently wear the device). If a participant drank the day before they returned the devices and was scheduled to come in prior to noon, they completed the day after questions on paper and research staff later entered the data into the website.
Six-week follow-up data
We obtained 6-week follow-up data from 26 (81%) participants. However, the website did not work for automatically recontacting participants so all participants were emailed by research staff. We did not obtain data from four participants who had moved and from the two participants who did not consistently wear the TAS device.
TAS device
No participants ended the study early, but two (6%) did not consistently wear the TAS device during the field trial (as determined by the temperature sensor). No participants reported in the feedback session that they tried to trick the TAS device (e.g. having another person wear the device, covering the membrane while drinking).
Reactivity to monitoring
Table 3 shows reactivity to recording drinking. On the day after drinking, participants indicated that monitoring did not affect the amount of alcohol they consumed for the majority (75%) of drinking episodes. Participants reported that recording their behavior was a neutral experience for 38% of the sessions, was fun/interesting for 42% of sessions and was unappealing for 24% of sessions. Note that two drinking sessions (one during a wedding, one with colleagues) were not recorded in real-time due to concerns with social acceptability.
Table 3.
Reactivity to study participation on drinking behavior
| Day after each drinking episode (n = 138 episodes) | |
| 1. If had not been monitoring my drinking, I would have drunk: | |
| Less | 8% |
| Same | 75% |
| More | 17% |
| 2. I found monitoring my drinking to be: | |
| Very fun/interesting | 4% |
| A little-fairly fun/interesting | 36% |
| Neutral Experience | 38% |
| Slightly-fairly unappealing | 21% |
| Very unappealing | 3% |
| Six-week follow-up (n = 26 participants) | |
| Due to my participation, I have been drinking: | |
| 1. Frequency | |
| Less frequently | 25% |
| Same frequency | 71% |
| More frequently | 4% |
| 2. Quantity | |
| Lower quantity | 25% |
| Same quantity | 62% |
| Higher quantity | 13% |
At the 6-week follow-up, 63% of those assessed reported that their study participation had not affected their subsequent alcohol consumption. Six (25%) participants reported drinking less because of their participation, but five of these subjects listed open-ended reasons that seemed unrelated to their participation (e.g. getting in shape, busy with school). Only one participant reported drinking more often after the study, and two participants reported drinking higher average quantities.
DISCUSSION
Our results for the web application indicate we were able to create a relatively effective tool for the collection and storage of repeated measures data from real-time drinking episodes. To our knowledge, the protocol we designed is the first to repeatedly assess real-time drinking (consumption, context, subjective responses to alcohol) based on the course of the drinking episode—the assessment time points correspond with actual drinking behaviors, items and response choices are adjusted based on the timing of the assessment point within the episode and on participant responses from earlier in the episode, and the program follows participants over the entire duration of a drinking episode at regular (and adjustable) time intervals, including into the descending limb of blood alcohol curve once drinking has stopped. Participants were willing to monitor their behavior over the course of drinking and retrospectively the day after, and monitoring did not appear to strongly affect drinking behavior. Participants also reported the recording during drinking episodes on a smart phone was a relatively easy procedure, although it is not known how this protocol would be viewed by non-college students, a more diverse ethnic sample, or problem drinkers. The compliance and feasibility of the protocol should also continue to improve as more people have their own smart phones and connectivity improves for all service providers. Such high levels of compliance and low levels of reactivity are consistent with prior studies examining alcohol consumption with smart phones (e.g. Kuntsche and Labhart, 2012, 2013a).
Results for the TAS device demonstrate the current technical limitations of the WrisTAS, but the willingness of most participants to wear the device despite some discomfort. Our failure rate with the version 7 WrisTAS is consistent with reports using earlier versions of WrisTAS device (Greenfield et al., 2005; Marques and McKnight, 2009). Other TAS devices (i.e. SCRAM©, Alcohol Monitoring Systems, Inc., Denver, CO, USA) have shown improved reliability with newer models (Marques and McKnight, 2009; Barnett et al., 2014), although the ankle bracelet also has some drawbacks for participant comfort and convenience (see Leffingwell et al., 2013, for comparisons of these two devices). As the reliability of these devices continues to improve, the use of our BrAC Estimator software (which can generate BrAC estimates from TAS data from either device) will help increase their utility to alcohol researchers and clinicians. Even without the transdermal data, however, our real-time data collection protocol produces a comprehensive drinking diary along with time-stamped photos documenting alcohol consumption at 30–45 min intervals over the entire course of the drinking episode that can be used to generate estimates of BrAC. We have previously shown that such a detailed drinking diary data can produce estimates of BrAC of similar accuracy as TAC data modeled into BrAC using our software (see Rosen et al., 2014). Including multiple measures of alcohol consumption in our protocol reduces the reliance on a single measure to obtain valid alcohol consumption data.
Several drawbacks to the protocol are also worth noting. Our data collection protocol was intensive for both participants and research staff. This was due in part to the calibration session required for estimation of BrAC from the TAS devices. We are currently developing models that use detailed drinking diary data to calibrate the TAS device instead of a laboratory drinking session (Coste et al., 2013). The laboratory session, however, provides the opportunity to establish rapport and to train participants both while sober and while under the influence of alcohol, so it would need to be determined how a shorter training session would affect participant compliance and competence. To obtain high quality data requires a detailed training protocol, monitoring of participants during field trial, good communication between participants and researchers, and flexibility and ingenuity of computer programming staff. We also found that the most problematic data improved our protocol the most, and issues with the programming were largely addressed within the first few participants. The TAS device problems, however, persisted throughout the entire study. Thus, we ended with limited numbers of participants who had valid data from both the web application and TAS device in this initial testing of the protocol.
Our protocol would benefit from several refinements. For example, we were not able to automatically link the real-time data collection time points to the TAS data or photos as we had planned. To have this process automated would greatly reduce researcher burden for integrating all components of the real-time data. Additional future goals for the web application include (a) greater flexibility of item content over the course of drinking episodes based on responses to earlier items, changes in context and/or duration of the episode, (b) verifying the compatibility of the web application for use with a variety of smart phones and service providers and (c) ongoing development of the reliability and stability of the web application to make it usable by researchers without requiring any assistance from computer programmers.
The creation of a flexible and easily modifiable repeated measures real-time data collection web application coupled with objective alcohol data collection has the potential to be a powerful assessment tool. This study indicates our protocol is feasible for participants in its current form, and the reliability of the data collection should only continue to improve as the technology for the devices advances. The development of such a real-time data collection system allows for additional research questions to be addressed in the future by our laboratory and other alcohol researchers studying naturalistic change over the course of drinking episodes.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Alcohol and Alcoholism online.
Funding
This work was supported by National Institutes of Health grants R01AA11257 and R21AA17711 and a grant from the Alcoholic Beverage Medical Research Foundation. These funding sources had no other role other than financial support.
Conflict of interest statement
None declared.
Acknowledgements
We thank Steven Q. Evans and Tim Taplin for their development of the web application.
REFERENCES
- Barnett N, Mead EB, Glynn TB. Predictor of detection of alcohol use episodes using a transdermal alcohol sensor. Exp Clin Psychopharmacol. 2014;22:86–95. doi: 10.1037/a0034821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E. Diary methods: capturing life as it is lived. Annu Rev Psychol. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, et al. A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Christensen TC, Feldman Barrett L, Bliss-Moreau E, et al. A practical guide to experience-sampling procedures. J Happiness Stud. 2003;4:53–78. [Google Scholar]
- Collins RL, Morsheimer ET, Shiffman S, et al. Ecological momentary assessment in a behavioral drinking moderation training program. Exp Clin Psychopharmacol. 1998;6:306–15. doi: 10.1037//1064-1297.6.3.306. [DOI] [PubMed] [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: development and validation of a four-factor-model. Psychol Assess. 1994;6:117–28. [Google Scholar]
- Coste K, Rosen IG, Luczak SE. Using drinking diary data to calibrate software for estimating BAC from field-collected transdermal alcohol sensor data. Alcohol Clin Exp Res. 2013;37:132A. [Google Scholar]
- Dumett M, Rosen IG, Sabat J, et al. Deconvolving an estimate of breath measured blood alcohol concentration from biosensor collected transdermal ethanol data. Appl Math Comput. 2008;196:724–43. doi: 10.1016/j.amc.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman Barrett L, Barrett DJ. An introduction of computerized experience sampling in psychology. Soc Sci Comput Rev. 2001;19:175–85. [Google Scholar]
- Goedde HW, Agarwal DP, Fritze G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. 1992;88:344–6. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- Greenfield TK, Tujague J, Bond J, et al. A pilot study of self-report alcohol consumption diary vs. transdermal alcohol sensor accuracy. Alcohol Clin Exp Res. 2005;29:103A–30A. [Google Scholar]
- Holdstock L, King AC, De Wit H. Subjective and objective responses to ethanol in moderate/heavy drinkers and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–94. [PubMed] [Google Scholar]
- Hufford MR, Shiffman S. Assessment methods for patient-reported outcomes. Dis Manag Health Out. 2003;11:77–86. [Google Scholar]
- Hufford MR, Stone AA, Shiffman S, et al. Paper vs. electronic diaries: compliance and subject evaluations. Appl Clin Trials. 2002:38–43. http://images.alfresco.advanstar.com/alfresco_images/pharma/2014/08/22/bb34ba55-e950-4531-8c08-aa1f3da6e0e3/article-26823.pdf . [Google Scholar]
- Hurlbut SC, Sher KJ. Assessing alcohol problems in college students. J Am Coll Health. 1992;41:49–58. doi: 10.1080/07448481.1992.10392818. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Nagoshi CT, Schwitters SY, et al. Further investigation of racial/ethnic differences and of familial resemblances in flushing response to alcohol. Behav Genet. 1984;14:171–8. doi: 10.1007/BF01065539. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Labhart F. Investigating the drinking patterns of young people over the course of the evening at weekends. Drug Alcohol Depend. 2012;124:319–24. doi: 10.1016/j.drugalcdep.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Labhart F. ICAT: development of an internet-based data collection method for ecological momentary assessment using personal cell phones. Eur J Psychol Assess. 2013a;29:140–8. doi: 10.1027/1015-5759/a000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Labhart F. Using personal cell phones for ecological momentary assessment. An overview of literature and practical recommendations. Eur Psychol. 2013b;18:3–11. doi: 10.1027/1015-5759/a000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Labhart F. The future is now—using personal cellphones to gather data on substance use and related factors. Addiction. 2014;109:1052–3. doi: 10.1111/add.12540. [DOI] [PubMed] [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, et al. Transdermal alcohol monitoring: 21st century measurement for an age-old problem. Alcohol Clin Exp Res. 2013;37:16–22. doi: 10.1111/j.1530-0277.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Ecological momentary assessment (EMA) with treated alcoholics: methodological problems and potential solutions. Health Psychol. 1998;17:48–52. doi: 10.1037//0278-6133.17.1.48. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG. Estimating BrAC from transdermal alcohol concentration data using the BrAC estimator software program. Alcohol Clin Exp Res. 2014;38:2243–52. doi: 10.1111/acer.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Elvine-Kreis BJ, Shea SH, et al. Genetic risk for alcoholism relates to level of response to alcohol in Asian American men and women. J Stud Alcohol. 2002;63:74–82. [PubMed] [Google Scholar]
- Luczak SE, Shea SH, Hsueh A, et al. ALDH2 is associated with alcohol-induced blackouts in Asian-American college students. J Stud Alcohol. 2006;67:349–53. doi: 10.15288/jsa.2006.67.349. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG, Weiss J. Determining blood and/or breath alcohol concentration from transdermal alcohol data. Int Fed Automatic Control. 2013:473–8. [Google Scholar]
- Luczak SE, Yarnell LM, Prescott CA, et al. Effects of ALDH2*2 on alcohol consumption and problems over four years of college. J Abnorm Psychol. 2014;123:130–40. doi: 10.1037/a0035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques PR, McKnight AS. Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res. 2009;33:703–11. doi: 10.1111/j.1530-0277.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty R, et al. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–6. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Shiffman S, et al. Daily fluctuations in self-control demands and alcohol intake. Psych Addict Behav. 2005;19:140–7. doi: 10.1037/0893-164X.19.2.140. [DOI] [PubMed] [Google Scholar]
- Neville FG, Williams DJ, Goodall DA, et al. An experimental trial exploring the impact of continuous transdermal alcohol monitoring upon alcohol consumption in a cohort of male students. PLoS One. 2013;8:e67386. doi: 10.1371/journal.pone.0067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei TPS, Sweeton J, Dingle G, et al. Psychometric properties of a Quitting Time for Alcohol Questionnaire: factor structure, reliability and validity. Addict Behav. 1999;24:383–98. doi: 10.1016/s0306-4603(98)00077-x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, et al. The subjective effects of alcohol–tobacco co-use: an ecological momentary assessment investigation. J Abnorm Psychol. 2011;120:557–71. doi: 10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen IG, Luczak SE, Hu W, et al. Discrete-time blind deconvolution for distributed parameter systems with dirichlet boundary input and output with application to a transdermal biosensor data. SIAM Conf Control Appl. 2013:160–7. [Google Scholar]
- Rosen IG, Luczak SE, Weiss J. Blind deconvolution for distributed parameter systems with unbounded input and output and determining blood alcohol concentration from transdermal biosensor data. Appl Math Comput. 2014;231:357–76. doi: 10.1016/j.amc.2013.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samo JA, Tucker JA, Vuchinich RE. Agreement between self-monitoring, recall, and collateral observation measures of alcohol consumption in older adults. Behav Assess. 1989;11:391–409. [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–84. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–97. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, et al. First lapses to smoking: within-subject analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Hunt-Carter EE. Development and initial validation of the Hangover Symptoms Scale: prevalence and correlates of hangover symptoms in college students. Alcohol Clin Exp Res. 2003;27:1442–50. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MC. Alcohol Time-Line Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allens JP, editors. Measuring Alcohol Consumption. New Jersey: Humana Press; 1992. [Google Scholar]
- Sobell MB, Bogardis J, Schuller R, et al. Is self-monitoring of alcohol consumption reactive? Behav Assess. 1989;11:447–58. [Google Scholar]
- Steiger H, Gauvin L, Jabalpurwala S, et al. Hypersensitivity to social interactions in bulimic syndromes: relationship to binge eating. J Consult Clin Psychol. 1999;67:765–75. doi: 10.1037//0022-006x.67.5.765. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Atienza AA, et al. The Science of Real-Time Data Capture: Self-Report in Health Research. New York: Oxford University Press; 2007. [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects. J Stud Alcohol. 1981;42:547–56. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weaver ER, Horyniak DR, Jenkinson R, et al. ‘Let's get Wasted!’ and other apps: characteristics, acceptability, and use of alcohol-related smartphone applications. JMIR Mhealth Uhealth. 2013;1:e9. doi: 10.2196/mhealth.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]