Abstract
Objective
The aim of this study was to investigate the role of peroxiredoxin 1 (Prdx1) in the invasiveness of pancreatic ductal adenocarcinoma (PDAC) cells.
Methods
Immunohistochemistry was used to determine overexpression of Prdx1 in human PDAC tissues. Immunoprecipitation and immunocytochemistry were used to determine the interaction and intracellular distribution of Prdx1 and a member of the mitogen-activated protein kinase (MAPK) family protein, p38 MAPK, in PDAC cells. Finally, immunocytochemistry and Matrigel invasion assay were used to examine the effects of Prdx1 and p38 MAPK on the formation of cell protrusions and PDAC cell invasion.
Results
Prdx1 is overexpressed in human PDAC tissues. Peroxiredoxin 1 interacts with active forms of p38 MAPK, and complexes of Prdx1 and phosphorylated p38 MAPK localize at the leading edges of migrating PDAC cells. Suppression of Prdx1 decreases active p38 MAPK localized in cell protrusions and inhibits the invasiveness of PDAC cells. Consequently, suppression of Prdx1 inhibits membrane ruffling and protrusions. The p38 MAPK inhibitor SB203580 also decreases the formation of membrane protrusions and inhibits invasiveness.
Conclusions
Prdx1 associates with the formation of membrane protrusions through modulation of the activity of p38 MAPK, which in turn promotes PDAC cell invasion.
Key Words: antioxidant defense enzyme, pancreatic cancer, cell invasion, p38 MAPK, actin-cytoskeleton
Among intracellular antioxidant defense enzymes, peroxiredoxins (Prdxs) have recently been characterized as a group of thiol-containing proteins with efficient antioxidant capacity and have been proven to play a critical role in peroxide detoxification.1,2 All of the Prdx proteins contain a conserved cysteine residue in the amino (N)-terminal region that is the active site of catalysis.3 Six Prdx isoforms have been identified in mammalian tissues, and elevated expression of different Prdx isoforms has been documented in numerous malignancies.4–7
Peroxiredoxin 1 (Prdx1) may be a fine-tuner of cellular H2O2 signaling by regulating the activity of binding partners such as c-Jun NH2-terminal kinase (JNK), c-Abl kinase, and PTEN (phosphatase and tensin homolog).8–11 Furthermore, Prdx1 interacts with apoptosis signal-regulating kinase 1 (ASK1), a member of the mitogen-activated protein kinase kinase kinase (MAPKKK) family that activates both MKK4/MKK7-JNK and MKK3/MKK6-p38 mitogen-activated protein kinase (MAPK) signaling cascades, via the thioredoxin-binding domain of ASK1; the redox-sensitive catalytic activity of Prdx1 is required for the interaction with ASK1.12,13 In addition to reports that Prdxs regulate peroxide-mediated signaling cascades, a large number of Prdxs are associated with diverse cellular functions, such as cell proliferation, differentiation, immune response, growth control, tumor promotion, apoptotic processes, and numerous unidentified functions.14 Peroxiredoxin 1 suppresses radiation-induced JNK signaling and apoptosis in lung cancer cells, but the peroxidase activity of Prdx1 is not essential for inhibiting JNK activation.15 Moreover, Prdx1 has been shown to play a role in radioresistance using human lung cancer xenograft models, and significant growth inhibition, radiosensitization, and reduced metastasis have been observed in lung cancer cells stably transfected with antisense Prdx1.15 In addition to the protective role of Prdx1 as a peroxidase, evidence has indicated that other mechanisms influence diverse cellular processes involved in protecting cells from radiation-induced death, cell growth, and cell metastasis. Peroxiredoxin 1 may therefore serve as a therapeutic target and/or a target for inhibiting malignant tumor progression.
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest cancers because of its ability to extensively invade surrounding tissues and metastasize at an early stage.16 Cell motility is critical for a variety of biological processes in normal and pathological conditions including cellular development, tissue repair, and cancer invasion and metastasis.17 The first step of cell motility is the generation of a membrane protrusion in the direction of movement.18 This process is driven by actin polymerization19 and may also require the addition of new membrane at the protrusion site. In our present study, we sought to evaluate the role of Prdx1 in the control of PDAC cell motility and invasion. In the course of this investigation, we found that Prdx1 was transported and accumulated in membrane protrusions and the leading edges of PDAC cells. Further investigation revealed that Prdx1 contributes to the formation of membrane protrusions by increasing p38 MAPK phosphorylation and results in increased invasive properties of PDAC cells.
MATERIALS AND METHODS
Antibodies
Anti-Prdx1 antibody (ab59538) was purchased from Abcam (Cambridge, Mass). Anti-ASK1 (3762), anti-p38 MAPK (9212), anti–phospho-p38 MAPK (9216), anti-JNK (9252), anti–phospho-JNK (9255), and anti–MAPK-activated protein kinase 2 (MAPKAP2) (3042) antibodies were obtained from Cell Signaling (Grand Island, NY). Anti–phospho-ASK1 antibody (3031R) was purchased from Bioss (Woburn, Mass). Anti–phospho-MAPKAPK2 antibody (05-1028) was purchased from Merck (Whitehouse Station, NJ).
Cell Culture
The human PDAC cell line S2-013, a subline of SUIT-2, was obtained from Dr T. Iwamura (Miyazaki Medical College, Miyazaki, Japan).20 The human PDAC cell line PANC-1 was purchased from the American Type Culture Collection (Manassas, Va). All cells were grown in Dulbecco modified Eagle medium (Gibco-BRL, Carlsbad, Calif) supplemented with 10% heat-inactivated fetal calf serum (FCS) at 37°C in a humidified atmosphere saturated with 5% CO2.
Prdx1 Plasmids
Reverse transcriptase–polymerase chain reaction was used to amplify the entire coding sequence of the Prdx1 cDNA. The resultant polymerase chain reaction product was subsequently inserted into a separate pCMV6-Entry vector (OriGene Technologies, Rockville, Md) bearing a C-terminal myc-DDK-tag (Prdx1WT). The mutant form Prdx1C52A/C173A was generated by site-specific mutagenesis (Genescript, Piscataway, NJ). X-tremeGENE HP DNA Transfection Reagent (Roche, Penzberg, Germany) was used to transiently transfect target cells with resultant Prdx1 plasmids.
Treatment of Cells
To inhibit the activity of p38 MAPK, plated S2-013 cells were treated for 1 hour with 10 μM of a p38 MAPK inhibitor (SB203580; Cell Signaling); to inhibit peroxidase activity, S2-013 cells were treated for 1 hour with 20 mM mercaptosuccinate (Sigma-Aldrich, St Louis, Mo). To assess the peroxidase activity of Prdx1, S2-013 cells, which had been transfected with Prdx1-small interfering RNA (siRNA), were transfected for 48 hours with Prdx1WT or Prdx1C52A/C173A, and then the cells were cultured on fibronectin for 5 hours. Cell lysates were subjected to Western blots.
Immunohistochemical Staining
Paraffin-embedded human PDAC tissue sections were obtained from specimens that had been resected during surgery at the Department of Surgery, Kochi University. In conformity with the principles of the Declaration of Helsinki, informed consent was obtained from each patient. Tissue sections from normal pancreas were purchased from Biochain (Hayward, Calif). The sections were deparaffinized and autoclaved at 108°C for 15 minutes. After endogenous peroxidase activity was quenched by incubation for 30 minutes in 0.33% hydrogen peroxide diluted in methanol, the sections were incubated with fetal bovine serum for blocking. Sections were then incubated with anti-Prdx1 antibody at room temperature for 1 hour and washed with phosphate-buffered saline. Immunodetection was performed with peroxidase-labeled anti–rabbit immunoglobulin (Dako Cytomation, Carpinteria, Calif). Finally, the reactants were developed with 3,3′-diaminobenzidine (Dako Cytomation), and the sections were counterstained with hematoxylin.
Confocal Immunofluorescence Microscopy
Coverslips were coated with 10 μg/mL fibronectin (Sigma-Aldrich) for 1 hour at room temperature. S2-013 cells were seeded on fibronectin-coated glass coverslips and incubated for 5 hours; cells were then fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, covered with blocking solution (3% bovine serum albumin/phosphate-buffered saline), and then incubated with the primary antibody for 1 hour. Alexa488-, Alexa594-, or Alexa647-conjugated secondary antibody (Molecular Probes, Carlsbad, Calif) was used with or without rhodamine-conjugated phalloidin (Cytoskeleton, Denver, Colo). In some experiments, a commercial antibody-labeling technology (Zenon; Life Technologies, Carlsbad, Calif) was used according to the manufacturer’s instructions to conjugate green or red fluorophores to primary antibodies. Each specimen was visualized using a Zeiss LSM 510 META microscope (Carl Zeiss, Gottingen, Germany).
Mice and Orthotopic Implantation of Tumor Cells
Pathogen-free female athymic nude mice (BALB/cSlc-nu/nu, 6 weeks of age) were purchased from Japan SLC, Inc (Shizuoka, Japan). Mice were treated in accordance with the Institutional Animal Care and Use Committee guidelines of Kochi University. S2-013 cells (8.0 × 105) were surgically and orthotopically implanted into the pancreas of each mouse. Each mouse was killed 42 days after the respective implantation; immunohistochemical staining was performed with anti-Prdx1 antibody.
Immunostaining Wound-Healing Assay
A plastic pipette tip was used to cut cross-shaped wounds through a confluent cell monolayer; cells were then allowed to polarize and migrate into the wounds. After 4 hours, cells were immunostained with a primary antibody and then incubated with a fluorophore-conjugated secondary antibody as described above. Each specimen was examined using a Zeiss LSM 510 META microscope (Carl Zeiss).
siRNA Treatment
A single mixture with 4 different siRNA oligonucleotides targeting Prdx1 was purchased from Qiagen (FlexiTube GeneSolution GS5052; Valencia, Calif), and a single mixture with 4 different scrambled negative control siRNA oligonucleotides was obtained from Santa Cruz (37007; Santa Cruz, Calif). To examine the effect of the siRNAs on Prdx1 expression, S2-013 cells that expressed PRDX1 were plated in 6-well plates. After 20 hours, the cells were transfected with 80 pmol of siRNA in siRNA transfection reagent (Qiagen) following the manufacturer’s instructions. After a 48-hour incubation, the cells were used for transwell motility and Matrigel invasion assays.
Transwell Motility Assay
Cells (3.0 × 104) were plated in the upper chamber of BD BioCoat Control Culture Inserts (24-well plates, 8-μm pore size; Becton Dickinson, San Jose, Calif). Serum-free culture medium was added to each upper chamber, and medium containing 5% fetal calf serum was added to the bottom chamber. Cells were incubated on the membranes for 12 hours. After a 12-hour incubation, 3 independent visual fields were examined via microscopic observation to count the number of cells that had moved to the bottom chamber.
Matrigel Invasion Assay
A 2-chamber invasion assay was used to assess cell invasion (24-well plates, 8-μm-pore-size membrane coated with a layer of Matrigel extracellular matrix proteins; Becton Dickinson). Cells (4.0 × 104) suspended in serum-free medium were seeded into the upper chamber and allowed to invade toward a 5% fetal calf serum chemoattractant in the lower chamber. After a 20-hour incubation, 3 independent visual fields were examined via microscopic observation to count the number of cells that had moved to the bottom chamber.
Immunoprecipitation
S2-013 cells were incubated on fibronectin for 5 hours and lysed in lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM MgCl2, 0.5% NP-40, protease inhibitor cocktail tablets [Roche], and phosphatase inhibitor cocktail [Nacalai, Kyoto, Japan]). Lysates were immunoprecipitated with Dynabeads Protein G (Dynal, Oslo, Norway) and with anti-Prdx1 antibody or with normal rabbit immunoglobulin G for 2 hours at 4°C. Beads were pelleted on a magnetic rack (Dynal). To examine the interaction of Prdx1 with ASK1, p38 MAPK, and c-JNK, immune complexes were analyzed on Western blots.
Statistical Analysis
GraphPad Prism version 6.0 software (GraphPad Software, Inc, La Jolla, Calif) was used for all statistical analyses. Statistical significance was determined using a 2-tailed Student t test and SDs. For all analyses, P < 0.05 was considered significant.
RESULTS
Overexpression of Prdx1 in PDAC Tissues
Immunohistochemical analysis using a polyclonal antibody against Prdx1 showed strong signals in the cytoplasm in all of the human PDAC tissue sections from 5 patients (Fig. 1A). Although Prdx1 is known to localize primarily in the cytoplasm,10 it is noteworthy that cytosolic Prdx1 accumulated at the cell membranes of PDAC cells (Fig. 1B). No staining was observed in normal pancreatic epithelia (Fig. 1C).
FIGURE 1.
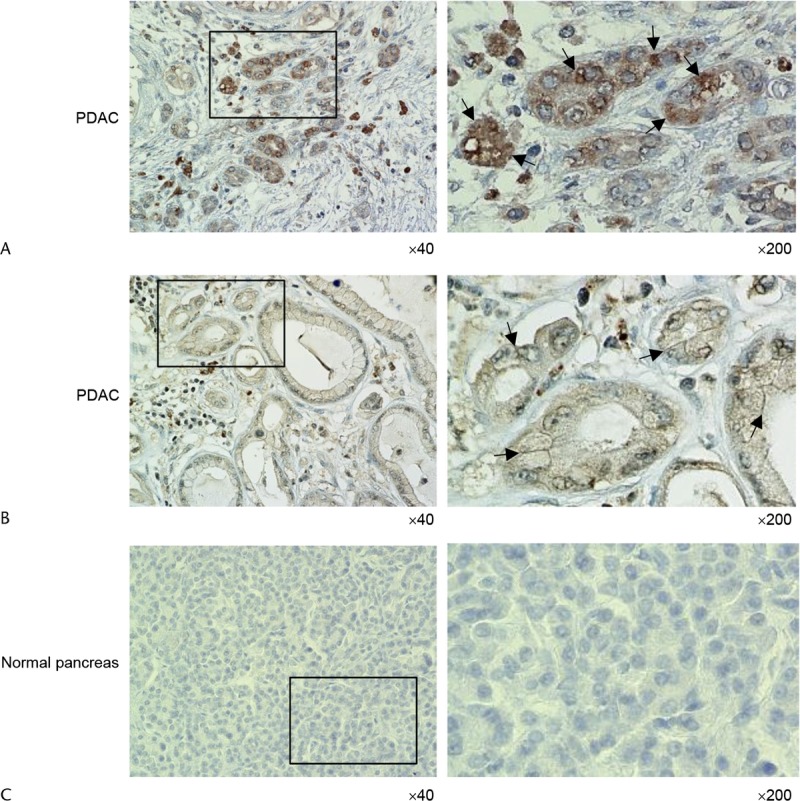
Overexpression of Prdx1 in human PDAC tissues. A, Immunohistochemical staining of PDAC tissues using anti-Prdx1 antibody. Peroxiredoxin 1 staining was primarily present in the cytoplasm of tumor cells. Arrows, Prdx1 localized in the cytoplasm of the cell bodies. The box depicts the position of the section enlarged (original magnifications ×40 [left panel] and ×200 [right panel]). B, Immunohistochemical staining of PDAC tissues using anti-Prdx1 antibody. Focal membrane staining of Prdx1 was observed in tumor cells. Arrows, Prdx1 localized at the cell membranes. The box depicts the position of the section enlarged (original magnifications ×40 [left panel] and ×200 [right panel]). C, Immunohistochemical staining of normal pancreas tissues using anti-Prdx1 antibody. No staining was observed in normal pancreatic epithelia. The box depicts the position of the section enlarged (original magnifications ×40 [left panel] and ×200 [right panel]).
Prdx1 Localizes in Cell Protrusions of Migrating PDAC Cells
When grown in normal culture media, the moderately differentiated PDAC cell line S2-013 exhibited strong intracellular expression of Prdx1 in cell membrane protrusions (Fig. 2A). Notably, when S2-013 cells that were initially suspended adhere to an immobilized fibronectin substrate, nascent membrane protrusions (de novo formation of actin patches at the cell periphery) form, and more mature protrusions then promote cell motility and invasion.21 Therefore, we analyzed the subcellular distribution of Prdx1 in PDAC cells cultured on fibronectin. Spreading of S2-013 cells on fibronectin promoted accumulation of Prdx1 in membrane protrusions in which peripheral actin structures were abundant (Fig. 2B). In addition, an immunostaining wound-healing assay was used to analyze the localization of Prdx1 in polarized migrating S2-013 cells (Fig. 2C); results from this assay showed that Prdx1 was recruited to the leading edges of S2-013 cells during wound healing.
FIGURE 2.
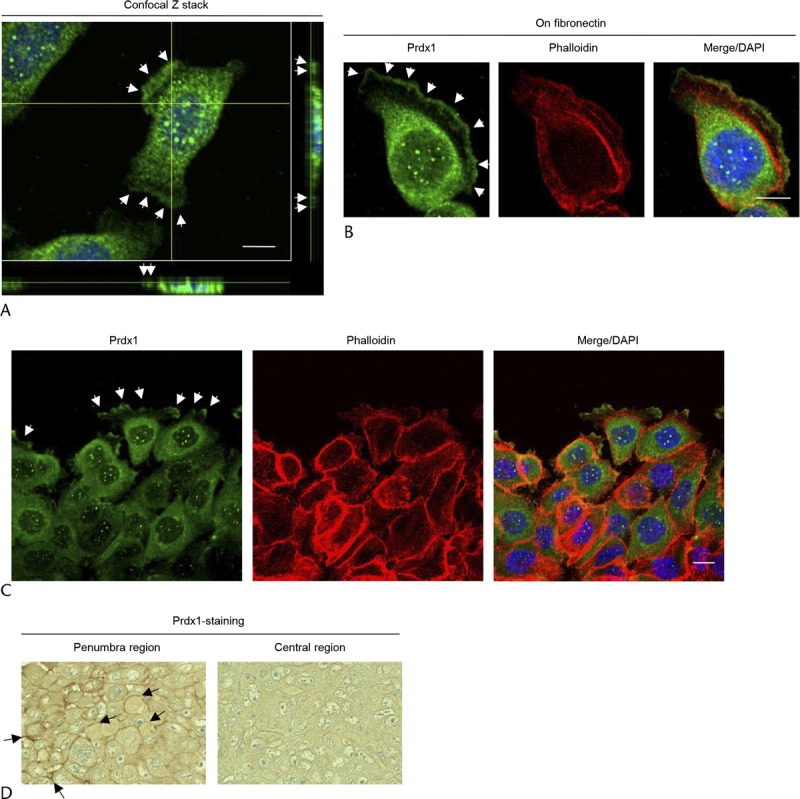
Prdx1 localizes in cell protrusions. A, Confocal Z stack shows the accumulation of Prdx1 (green) in membrane protrusions and nuclear DAPI staining (blue) in S2-013 cells. Arrows, Prdx1 localized in cell protrusions. The lower and light panels in the confocal Z stack show a vertical cross section (yellow lines) through the cells (bar, 10 μm). B, S2-013 cells were incubated on fibronectin and immunocytochemically stained using anti-Prdx1 antibody (green) and phalloidin (red). Actin filaments were labeled by phalloidin. Arrows, Prdx1 localized in cell protrusions. Blue, DAPI staining (bar, 10 μm). C, A confluent S2-013 cell monolayer was wounded. After 4 hours, anti-Prdx1 antibody (green) and phalloidin (red) were used to immunostain the cells. Arrows, Prdx1 at the leading edges of migrating cells. Blue, DAPI staining (bar, 10 μm). D, Immunohistochemical staining with anti-Prdx1 antibody in S2-013 primary pancreatic tumors in mice. Arrows, Prdx1 localized at the cell membranes (representative sections, original magnification ×200).
In the primary pancreatic tumors from nude mice injected with S2-013 cells, Prdx1 enriched in the cytoplasm and near cell membranes was observed in the penumbra of tumors (Fig. 2D). In the central region of tumors derived from S2-013 cells, cytoplasmic and membrane-localized Prdx1 was decreased compared with that in the penumbra (Fig. 2D). Notably, the overexpressed Prdx1 at cell membranes in the penumbra of tumors was similar to the intracellular distribution of Prdx1 localized in the membrane protrusions of fibronectin-stimulated S2-013 cells and in human PDAC tissues. Based on these results, we hypothesized that Prdx1 localized in membrane protrusions may be important to cell motility and invasion of PDAC cells.
Prdx1 Knockdown Reduces Cell Motility and Invasion
Transwell motility and Matrigel invasion assays and siRNA-mediated knockdown were used to examine the effect of Prdx1 on cell motility and invasiveness of S2-013 cells, in which Prdx1 was highly expressed, and in Prdx1 null PANC-1 cells, which are poorly differentiated PDAC cells. Based on Western blot data, Prdx1 expression was markedly lower 72 hours after transfection of Prdx1-siRNA in S2-013 cells than in control cells (Fig. 3A). In transwell motility assays, the motility of S2-013 cells was significantly lower in Prdx1-RNA interference (RNAi)–expressing cells than in control cells; however, no significant differences were observed in PANC-1 cells in which Prdx1 was not expressed (Fig. 3B). In 2-chamber invasion assays, the invasiveness of S2-013 was significantly lower in Prdx1-RNAi–expressing cells than in controls; however, no significant differences were observed in PANC-1 cells (Fig. 3C). Suppression of Prdx1 did not affect cell growth of S2-013 in an in vitro MTT assay (data not shown). These results indicated that Prdx1 increases cell motility and invasiveness of PDAC cells.
FIGURE 3.
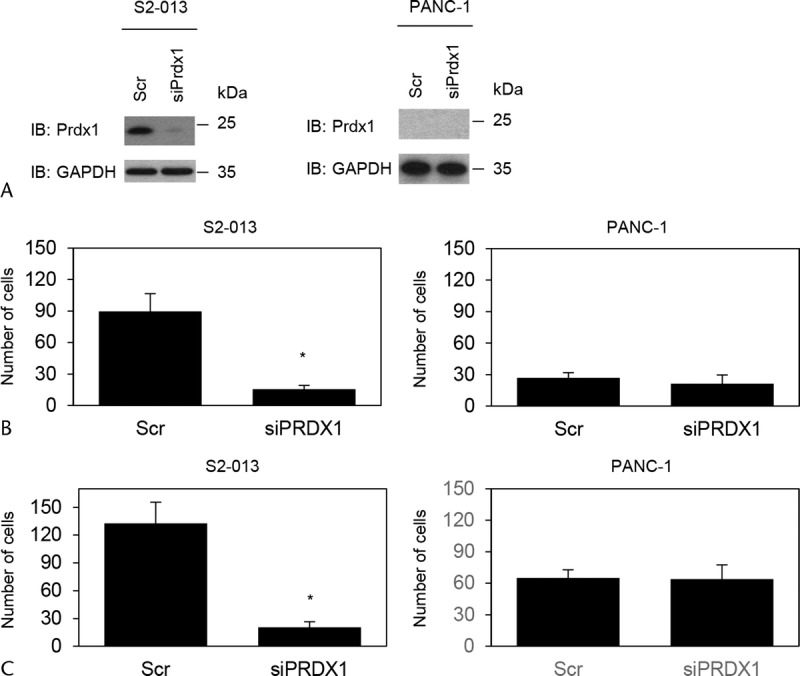
Prdx1 promotes cell motility and invasion of PDAC cells. A, A single mixture with 4 different siRNA oligonucleotides targeting Prdx1 (siPrdx1) or negative scrambled control (Scr) was transiently transfected into S2-013 and PANC-1 cells. Western blots probed with anti-Prdx1 antibody show siPrdx1 cells and control Scr cells. B, Transwell motility assay of control and Prdx1 RNAi cells of S2-013 or PANC-1. Migrating cells in 4 fields per group were scored. Data were derived from 3 independent experiments. Columns, Mean; bars, SD. *P < 0.004 compared with scrambled control (Student t test). C, Control and Prdx1 RNAi cells of S2-013 or PANC-1 were seeded into Matrigel invasion chambers. Invading cells in 4 fields per group were counted. Data were derived from 3 independent experiments. Columns, Mean; bars, SD. *P < 0.003 compared with scrambled control (Student t test).
Prdx1 Binds to an Active Form of P38 MAPK in Cell Protrusions
We hypothesized that Prdx1 may affect cell motility and invasion through regulating the ASK1-associated signaling cascades. We used anti-Prdx1 to perform immunoprecipitation with extracts from S2-013 cells that had been grown on fibronectin; we then analyzed the coimmunoprecipitation of Prdx1 with phosphorylated ASK1, p38 MAPK, or JNK. Peroxiredoxin 1 immunoprecipitated with phosphorylated p38 MAPK (phospho-p38 MAPK), but not with phospho-ASK1 or phospho-JNK (Fig. 4A). Immunofluorescence indicated that phospho-p38 MAPK was localized in both the cytoplasm and in cell protrusions of fibronectin-stimulated S2-013 cells and that Prdx1 was abundantly colocalized with phospho-p38 MAPK accumulated in cell protrusions (arrows in Fig. 4B). Peroxiredoxin 1 did not colocalize with phospho-ASK1 or phospho-JNK in fibronectin-stimulated S2-013 cells (data not shown). The localization in cell protrusions is characteristic of factors that modulate and are essential for cell migration.
FIGURE 4.
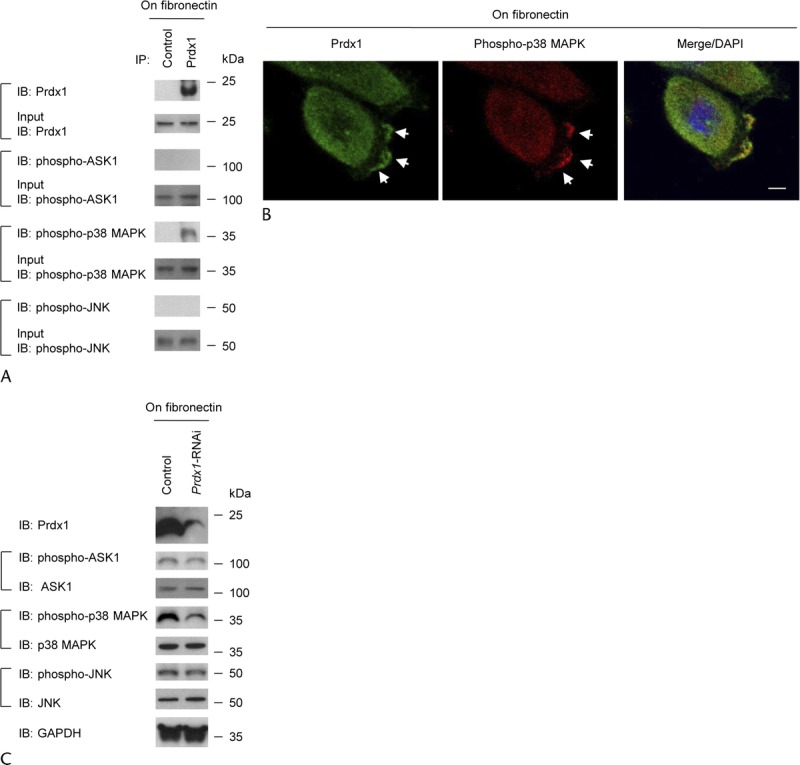
Prdx1 associates with phospho-p38 MAPK in cell protrusions. A, Immunoprecipitation of Prdx1 from S2-013 cells cultured on fibronectin. Immunoprecipitates were examined on Western blots probed with antibodies against phospho-ASK1, phospho-p38 MAPK, and phospho-JNK. Rabbit immunoglobulin G was used as an isotype control. B, Immunocytochemical staining in S2-013 cells cultured on fibronectin; anti-Prdx1 (green) and anti–phospho-p38 MAPK (red) antibodies were used to label endogenous proteins. Arrows, Prdx1 colocalized with phospho-p38 MAPK in cell protrusions. Blue, DAPI staining (bar, 10 μm). C, Western blotting of total cell lysates probing for phospho-ASK1, phospho-p38 MAPK, and phospho-JNK in scrambled control and Prdx1 RNAi S2-013 cells cultured on fibronectin.
We assessed the effect of Prdx1 on the regulation of p38 MAPK activity using Prdx1 RNAi-S2-013 cells. Suppression of Prdx1 resulted in a decrease in active p38 MAPK compared with scrambled siRNA-transfected cells; all cells were incubated on fibronectin (Fig. 4C), suggesting that endogenous Prdx1 is associated with an increase in the activity of p38 MAPK. The amounts of phospho-ASK1 and phospho-JNK were not affected by suppression of Prdx1 (Fig. 4C).
Prdx1 Promotes Cell Invasion via Association With the p38 MAPK Signaling Cascade
The p38 MAPK inhibitor SB203580 acts primarily to block the catalytic activity of p38 MAPK.22 One of the physiological substrates of p38 MAPK is MAPKAP2.23 To determine the selectivity of SB203580, we confirmed that pretreatment of fibronectin-stimulated S2-013 cells with SB203580 decreased the level of phospho-MAPKAP2, but the amounts of phospho-ASK1 and phospho-JNK were not affected, confirming the specificity of SB203580 (Fig. 5A). We determined the effect of SB203580 on the invasiveness of scrambled control and Prdx1-knockdown S2-013 cells. Pretreatment of scrambled control S2-013 cells with SB203580 significantly inhibited cell invasion in a 2-chamber assay, whereas this treatment did not alter the invasiveness of Prdx1-knockdown S2-013 cells (Fig. 5B). Suppression of Prdx1 also resulted in a decrease in active MAPKAP2 via a decrease in active p38 MAPK compared with scrambled siRNA-transfected cells; all S2-013 cells were incubated on fibronectin (Fig. 5C). These results indicated that Prdx1-associated regulation of the p38 MAPK signaling cascade plays a role in modulating PDAC cell invasion.
FIGURE 5.
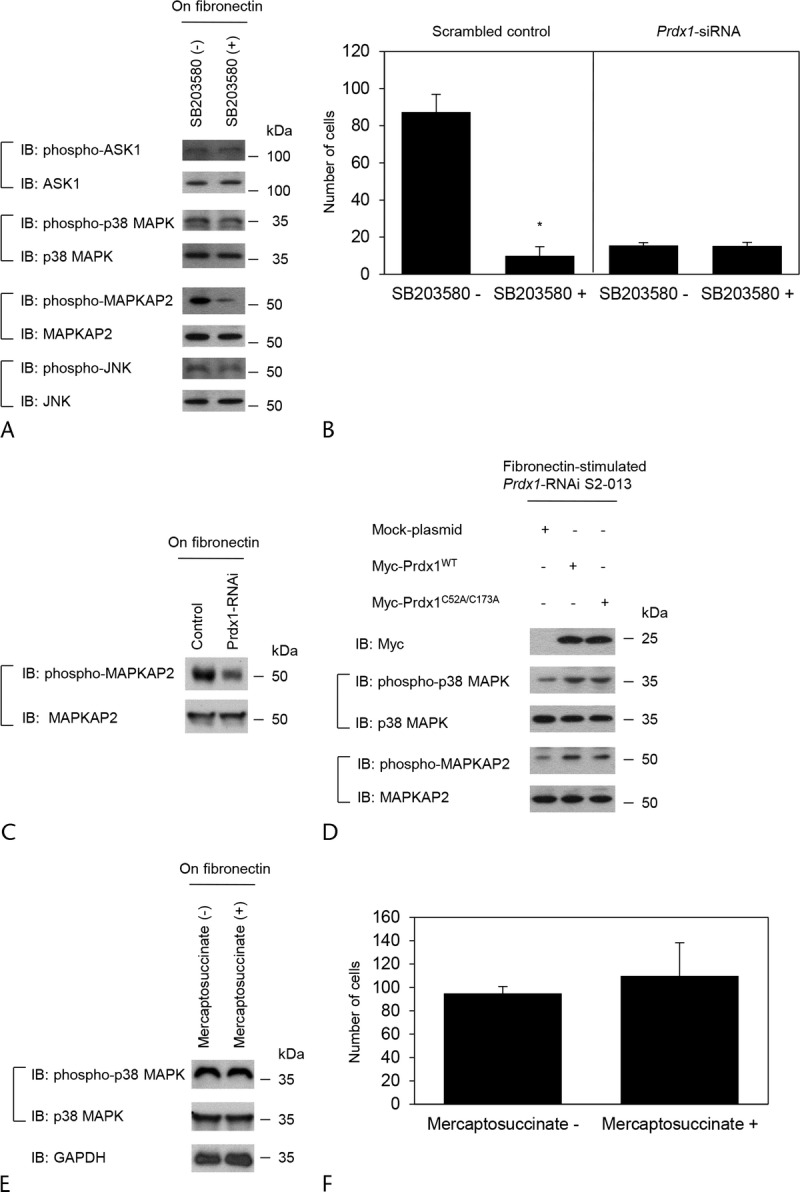
Prdx1 plays a role in increasing the activity of p38 MAPK. A, S2-013 cells were treated with SB203580, and then the cells were incubated on fibronectin. Western blotting of steady-state levels of the cells was performed using antibodies against phospho-ASK1, phospho-p38 MAPK, phospho-MAPKAP2, and phospho-JNK. B, Scrambled control S2-013 cells or Prdx1-knockdown S2-013 cells treated with SB203580 were plated on Matrigel invasion chambers. Invading cells in 4 fields per group were counted. Data were derived from 3 independent experiments. Columns, Mean; bars, SD. *P < 0.001 compared with nontreated cells (Student t test). C, Western blotting of total cell lysates probing for phospho-MAPKAP2 in scrambled control and Prdx1 RNAi S2-013 cells cultured on fibronectin. D, Prdx1-knockdown S2-013 cells were transfected for 48 hours with the indicated combinations of plasmids encoding Myc-Prdx1WT and Myc-Prdx1C52A/C173A. The cells were incubated on fibronectin for 5 hours, and then Western blotting of steady-state levels of the cells was performed using anti-myc, anti–phospho-p38 MAPK and anti–phospho-MAPKAP2 antibodies. E, S2-013 cells were treated with mercaptosuccinate, and then the cells were incubated on fibronectin. Western blotting of steady-state levels of the cells was performed using anti–phospho-p38 MAPK antibody. F, S2-013 cells treated with mercaptosuccinate were plated on Matrigel invasion chambers. Invading cells in 4 fields per group were counted. Data were derived from 3 independent experiments. Columns, Mean; bars, SD.
The catalytic functions of Prdx1 are critically dependent on residues Cys52 in the N-terminal portion and Cys173 in the C-terminal portion of the protein.13,24 If the peroxidase activity of Prdx1 is necessary for regulating p38 MAPK activity, the catalytic function of the Cys52 and Cys173 residues might be required. We constructed wild-type Prdx1 (Prdx1WT) and a mutant form (Prdx1C52A/C173A) in which Cys52 and Cys173 were altered to alanine residues. After Prdx1 knockdown, S2-013 cells were transiently transfected with Myc-tagged Prdx1WT or Myc-tagged Prdx1C52A/C173A; all cells were incubated on fibronectin. Peroxiredoxin 1C52A/C173A increased phosphorylated p38 MAPK and phosphorylated MAPKAP2 to the same levels as Prdx1WT in Prdx1-knockdown S2-013 cells, compared with Prdx1-knockdown S2-013 cells transfected mock vector (Fig. 5D). These results indicated that the catalytic functions of Prdx1 were not essential for regulating p38 MAPK activity. In addition, S2-013 cells were incubated in the presence or absence of mercaptosuccinate, which inhibits the peroxidase activity; all cells were incubated on fibronectin. This treatment did not alter the activity of p38 MAPK (Fig. 5E). These results indicated that the peroxidase activity was not associated with modulating p38 MAPK activity. Furthermore, pretreatment with mercaptosuccinate did not significantly alter cell invasion of S2-013 cells (Fig. 5F). This suggests that cell invasion did not require the peroxidase activity in PDAC cells.
Prdx1 Induces the Formation of Membrane Protrusions
To determine if Prdx1 and the active form of p38 MAPK localized in cell protrusions could potentially be a novel participant in inducing the protrusions, we next analyzed peripheral actin structures in the membrane ruffles of control and Prdx1 RNAi S2-013 cells cultured on fibronectin. Similar with the results of Figure 4B, Prdx1 was abundantly colocalized with phospho-p38 MAPK accumulated in cell protrusions of scrambled control S2-013 cells (white arrows in Fig. 6A). Phospho-MAPKAP2 was accumulated in cell protrusions of scrambled S2-013 control cells (yellow arrows in Fig. 6A); however, Prdx1 did not colocalize with phospho-MAPKAP2 in cell protrusions of scrambled control cells (Fig. 6A). Suppression of Prdx1 decreased the expression of phospho-p38 MAPK and phospho-MAPKAP2 near the plasma membranes (Fig. 6A). The finding that the expressions of phospho-p38 MAPK and phospho-MAPKAP2 in the cytoplasm of the cell bodies were not altered by knockdown of Prdx1 (Fig. 6A) indicates that suppression of Prdx1 decreased the expression of phospho-p38 MAPK and phospho-MAPKAP2 near the plasma membranes. Furthermore, suppression of Prdx1 decreased the peripheral actin structures (yellow arrowheads in Fig. 6A) compared with control cells (white arrowheads in Fig. 6A). Knockdown of Prdx1 significantly inhibited the formation of fibronectin-mediated membrane protrusions in S2-013 cells (Fig. 6B). To evaluate the ability of p38 MAPK to induce such membrane ruffles, we next examined the actin cytoskeletal structures of S2-013 cells on fibronectin in the absence or presence of SB203580. SB203580 significantly inhibited these surface actin rearrangements and the formation of cell protrusions that were stimulated by seeding on fibronectin (Figs. 6C, D). These results indicate that the function of Prdx1 in increasing p38 MAPK activity via producing complexes of Prdx1 and phospho-p38 MAPK in cell protrusions plays a role in inducing peripheral actin cytoskeletal rearrangements and the formation of protrusions and thereby promotes cell invasion of PDAC cells.
FIGURE 6.
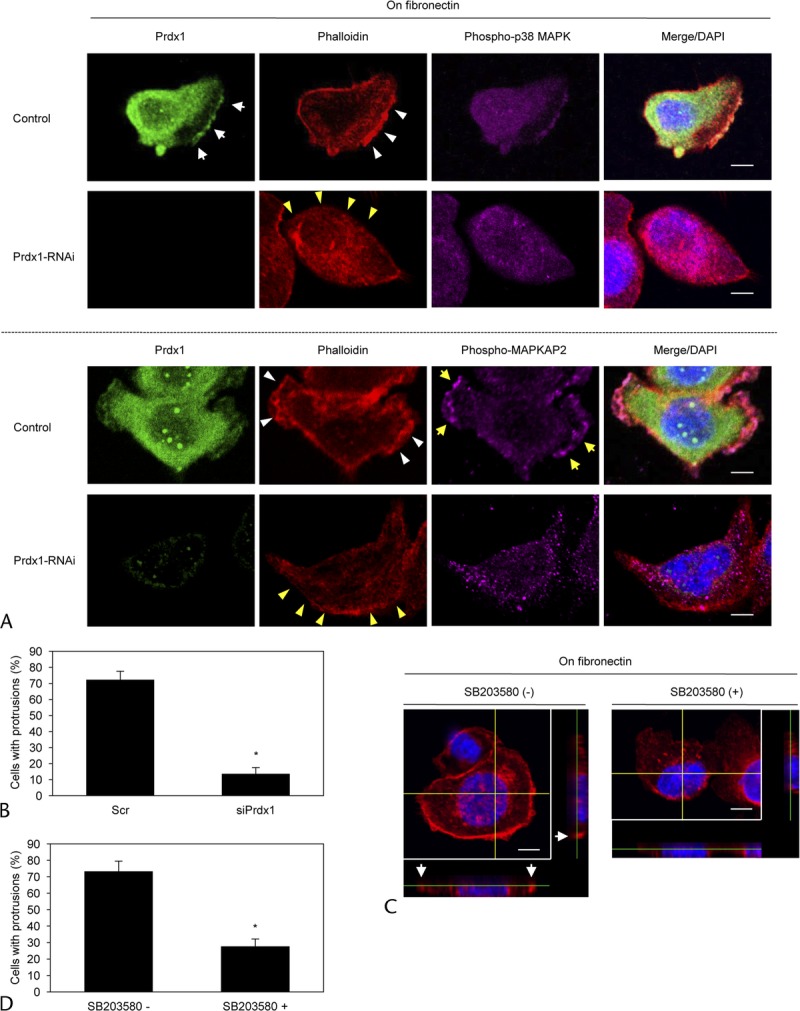
Prdx1 and phospho-p38 MAPK accumulated in cell protrusions induce the further formation of membrane protrusions. A, Scrambled control S2-013 cells or Prdx1-knockdown S2-013 cells were incubated on fibronectin and immunocytochemically stained with anti-Prdx1 antibody (green), phalloidin (red), and antibody against anti–phospho-p38 MAPK (upper panels) or anti–phospho-MAPKAP2 (lower panels) (violet). Actin filaments were labeled by phalloidin. Arrows, Prdx1 colocalized with phospho-p38 MAPK in cell protrusions of control cells. Yellow arrows, Phospho-MAPKAP2 accumulated in cell protrusions. White arrowheads, Fibronectin-mediated peripheral actin structures in control cells. Yellow arrowheads, Decreased peripheral actin structures by suppression of Prdx1. Blue, DAPI staining (bars, 10 μm). B, Quantification of data shown in A; the values represent the number of scrambled control S2-013 cells or Prdx1-knockdown S2-013 cells with cell protrusions in which peripheral actin structures were increased. All cells in 4 fields per group were scored. Data were derived from 3 independent experiments. Columns, Mean; bars, SD. *P < 0.001 compared with control cells (Student t test). C, Fibronectin-stimulated S2-013 cells were treated with SB203580. Actin filaments of the cells were immunocytochemically labeled by phalloidin. The confocal Z stack shows phalloidin-labeled peripheral actin structures (red) and DAPI-labeled nuclei (blue). The lower and right panels in the confocal Z stack show a vertical cross section (yellow lines) through the cells. Arrows, cell protrusions in which peripheral actin structures were increased (bars, 10 μm). D, Quantification of data shown in C; the values represent S2-013 cells with cell protrusions in which peripheral actin structures were increased. All cells in 4 fields per group were scored. Data were derived from 3 independent experiments. Columns, Mean; bars, SD. *P < 0.002 compared with control cells (Student t test).
DISCUSSION
Here, we report a novel function for Prdx1 in the regulation of cell motility and invasion in PDAC. Peroxiredoxin 1 played a role in p38 MAPK phosphorylation, and the complexes of Prdx1 and phopho-p38 MAPK assembled in cell protrusions of PDAC cells. Suppression of Prdx1 or decreased activity of p38 MAPK by pretreatment with a specific p38 MAPK inhibitor inhibited the formation of cell protrusions and thereby decreased cell motility and invasion of PDAC cells.
Peroxiredoxin 1 regulates senescence signaling specifically through redox-sensitive p38 MAPK phosphorylation in H2O2 phosphatases, MAPK phosphatase 1, and MAPK phosphatase 5 in breast cancer cells,25 indicating that Prdx1 mediates cell signaling through redox-specific protein-protein interactions. On the other hand, Prdx1 suppresses ionizing radiation-induced JNK activation and apoptosis through interaction with the S-transferase pi–JNK complex, but the antioxidant activity of Prdx1 is not essential for JNK inhibition by Prdx1 in HEK293 and HeLa cells.13 Similar to the latter finding, our result showing that Prdx1C52A/C173A increased phosphorylated p38 MAPK to the same levels as Prdx1WT in Prdx1-knockdown S2-013 cells (Fig. 5D) suggests that the peroxidase activity of Prdx1 was not necessary for modulating p38 MAPK activity. In addition, we showed that Prdx1 failed to modulate the activities of an upstream protein, ASK1, of p38 MAPK; however, the activities of a downstream protein, MAPKAP2, were modulated. These results suggest that among the signaling components of the p38 MAPK pathway, p38 MAPK was the only binding partner of Prdx1 in PDAC cells.
We showed that suppression of Prdx1 decreased the expression of phospho-p38 MAPK and phospho-MAPKAP2 in cell protrusions (Fig. 6A). The finding that phospho-p38 MAPK and phospho-MAPKAP2 were still present in the cytoplasm of the cell bodies after knockdown of Prdx1 suggested that Prdx1 can associate with phospho-p38 MAPK localized in cell protrusions. In addition, the finding that Prdx1 did not colocalize with phospho-MAPKAP2 in cell protrusions of S2-013 (Fig. 6A) suggested that MAPKAP2 might be activated by phospho-p38 MAPK in the protrusions, but not by Prdx1. These findings indicated that the expression of membranous Prdx1 in human PDAC tissues and in the penumbra of xenograft tumors derived from S2-013 cells may be interrelated. At the front of tumor invasion in the penumbra regions, PDAC cells are endowed with increased migratory capacity and augmented invasiveness.26 Therefore, cell populations that overexpress Prdx1 near cell membranes may have a powerful advantage with regard to invasiveness.
Dynamic, actin-based plasma membrane protrusions that control growth cone pathfinding include lamellipodia, in which the actin cytoskeleton assumes a cross-linked and branched meshwork, and filopodia, which consist of parallel bundles of actin filaments protruding from the growth cone or lamellipodial margin.27 Migratory competence of tumor cells requires activation of the motile cycle, the first step of which is actin remodeling, which drives the formation of cell protrusions, defines the direction of migration, and initiates the growth of the lamellipodium.28 Our results indicate that Prdx1 is a physiological activator that induces actin redistribution, leading to peripheral actin rearrangements. Interestingly, peripheral actin rearrangements were strongly inhibited when Prdx1 RNAi S2-013 cells were plated on fibronectin. P38 MAPK has been implicated in peripheral actin rearrangements and cellular migration in various cell types.29–31 Given that the p38 MAPK inhibitor also prevented peripheral actin rearrangements in fibronectin-stimulated S2-013 cells, the Prdx1 and phospho-p38 MAPK complex was associated with peripheral actin rearrangement, resulting in the formation of membrane protrusions.
We showed that suppression of Prdx1 inhibited cell motility and invasion of S2-013 cells. Pretreatment of scrambled control S2-013 cells with SB203580 significantly inhibited cell invasion, whereas this treatment did not alter the invasiveness of Prdx1-knockdown S2-013 cells (Fig. 5B). Because suppression of Prdx1 decreased p38 MAPK activity, it is possible that SB203580 significantly failed to inhibit the invasiveness of Prdx1-knockdown S2-013 cells. These results suggest that p38 MAPK interdependently associated with Prdx1 in modulating invasiveness of PDAC cells. Furthermore, inhibition of peroxidase activity using mercaptosuccinate did not contribute to invasiveness of S2-013 cells (Fig. 5F). These results suggest that the peroxidase activity of Prdx1 was likely not associated with cell invasion in PDAC. Among the 6 mammalian members of the Prdx family, Prdx6 possesses phospholipase A2 activity in addition to peroxidase activity.32,33 Peroxiredoxin 6 promotes cell growth and invasiveness via phospholipase A2 activity, but not via peroxidase activity in lung cancer cells.34 In prostate cancers, mercaptosuccinate treatment has no significant effect on invasiveness.35 Therefore, it is possible that the peroxidase activity of the Prdx family is not associated with invasiveness in cancer cells from multiple organs. We showed that treatment with the p38 MAPK inhibitor prevented invasiveness of S2-013 cells, similar to the effects of suppression of Prdx1. This result indicates that Prdx1 played a role in promoting cell invasion through its influence on p38 MAPK activity.
The findings presented in this study are supportive of the pivotal roles of Prdx1 in the coordinated regulation of cortical actin changes via the regulation of the level of p38 MAPK activity. Inhibition of binding of Prdx1 with active p38 MAPK may be effective for targeted molecular therapy, because any such therapy would inhibit the formation of cell protrusions and consequently limit cell motility and invasion of pancreatic cancer cells.
ACKNOWLEDGMENTS
The authors thank Aki Tanouchi, Chiaki Okura, and Shunichi Manabe for their excellent technical assistance.
Footnotes
This study was supported by the Grants-in-Aid for Scientific Research (KAKENHI) (to K.T. and S.I.), by the Pancreas Research Foundation of Japan (to K.T.), and by the Japanese Foundation for Multidisciplinary Treatment of Cancer (to K.T.).
The authors declare no conflict of interest.
REFERENCES
- 1. Kinnula VL, Lehtonen S, Sormunen R, et al. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002; 196: 316– 323. [DOI] [PubMed] [Google Scholar]
- 2. Kang SW, Rhee SG, Chang TS, et al. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005; 11: 571– 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005; 38: 1543– 1552. [DOI] [PubMed] [Google Scholar]
- 4. Karihtala P, Mäntyniemi A, Kang SW, et al. Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003; 9: 3418– 3424. [PubMed] [Google Scholar]
- 5. Qi Y, Chiu JF, Wang L, et al. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics. 2005; 5: 2960– 2971. [DOI] [PubMed] [Google Scholar]
- 6. Ai J, Tan Y, Ying W, et al. Proteome analysisof hepatocellular carcinoma by laser capture microdissection. Proteomics. 2006; 6: 538– 546. [DOI] [PubMed] [Google Scholar]
- 7. Cheng Y, Zhang J, Li Y, et al. Proteome analysis of human gastric cardia adenocarcinoma by laser capture microdissection. BMC Cancer. 2007; 7: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009; 8: 4072– 4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim YJ, Lee WS, Ip C, et al. Prx1 suppresses radiationinduced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006; 66: 7136– 7142. [DOI] [PubMed] [Google Scholar]
- 10. Wen ST, Van Etten RA. The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes Dev. 1997; 11: 2456– 2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao J, Schulte J, Knight A, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009; 28: 1505– 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997; 275: 90– 94. [DOI] [PubMed] [Google Scholar]
- 13. Kim SY, Kim TJ, Lee KY. A novel function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating kinase 1 (ASK1)–mediated signaling pathway. FEBS Lett. 2008; 582: 1913– 1918. [DOI] [PubMed] [Google Scholar]
- 14. Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003; 424: 561– 565. [DOI] [PubMed] [Google Scholar]
- 15. Chen MF, Keng PC, Shau H, et al. Inhibition of lung tumor growth and augmentation of radiosensitivity by decreasing peroxiredoxin I expression. Int J Radiat Oncol Biol Phys. 2006; 64: 581– 591. [DOI] [PubMed] [Google Scholar]
- 16. Baumgart M, Heinmöller E, Horstmann O, et al. The genetic basis of sporadic pancreatic cancer. Cell Oncol. 2005; 27: 3– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001; 114: 2713– 2722. [DOI] [PubMed] [Google Scholar]
- 18. Totsukawa G, Wu Y, Sasaki Y, et al. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004; 164: 427– 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000; 29: 545– 576. [DOI] [PubMed] [Google Scholar]
- 20. Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT-2) producing carcinoembryonic antigen and carbohydrate antigen 19-9. Jpn J Cancer Res. 1987; 78: 54– 62. [PubMed] [Google Scholar]
- 21. Taniuchi K, Yokotani K, Saibara T. BART inhibits pancreatic cancer cell invasion by Rac1 inactivation through direct binding to active Rac1. Neoplasia. 2012; 14: 440– 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAPK-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006; 25: 2987– 2998. [DOI] [PubMed] [Google Scholar]
- 23. McLaughlin MM, Kumar S, McDonnell PC, et al. Identification of mitogen-activated protein (MAP) kinase–activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996; 271: 8488– 8492. [DOI] [PubMed] [Google Scholar]
- 24. Rhee SG, Kang SW, Jeong W, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005; 17: 183– 189. [DOI] [PubMed] [Google Scholar]
- 25. Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006; 7: 833– 846. [DOI] [PubMed] [Google Scholar]
- 26. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119: 1420– 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004; 58: 92– 102. [DOI] [PubMed] [Google Scholar]
- 28. Eiseler T, Döppler H, Yan IK, et al. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009; 11: 545– 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kavurma MM, Khachigian LM. ERK, JNK, and p38 MAP kinases differentially regulate proliferation and migration of phenotypically distinct smooth muscle cell subtypes. J Cell Biochem. 2003; 89: 289– 300. [DOI] [PubMed] [Google Scholar]
- 30. Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003; 278: 21989– 21997. [DOI] [PubMed] [Google Scholar]
- 31. Mudgett JS, Ding J, Guh-Siesel L, et al. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci U S A. 2000; 97: 10454– 10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim TS, Sundaresh CS, Feinstein SI, et al. Identification of a human cDNA clone for lysosomal type Ca2+−independent phospholipase A2 and properties of the expressed protein. J Biol Chem. 1997; 272: 2542– 2550. [DOI] [PubMed] [Google Scholar]
- 33. Chen JW, Dodia C, Feinstein SI, et al. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem. 2000; 275: 28421– 28427. [DOI] [PubMed] [Google Scholar]
- 34. Ho JN, Lee SB, Lee SS, et al. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol Cancer Ther. 2010; 9: 825– 832. [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Altuwaijri S, Yeh S. RRR-alpha-tocopheryl succinate inhibits human prostate cancer cell invasiveness. Oncogene. 2004; 23: 3080– 3088. [DOI] [PubMed] [Google Scholar]


