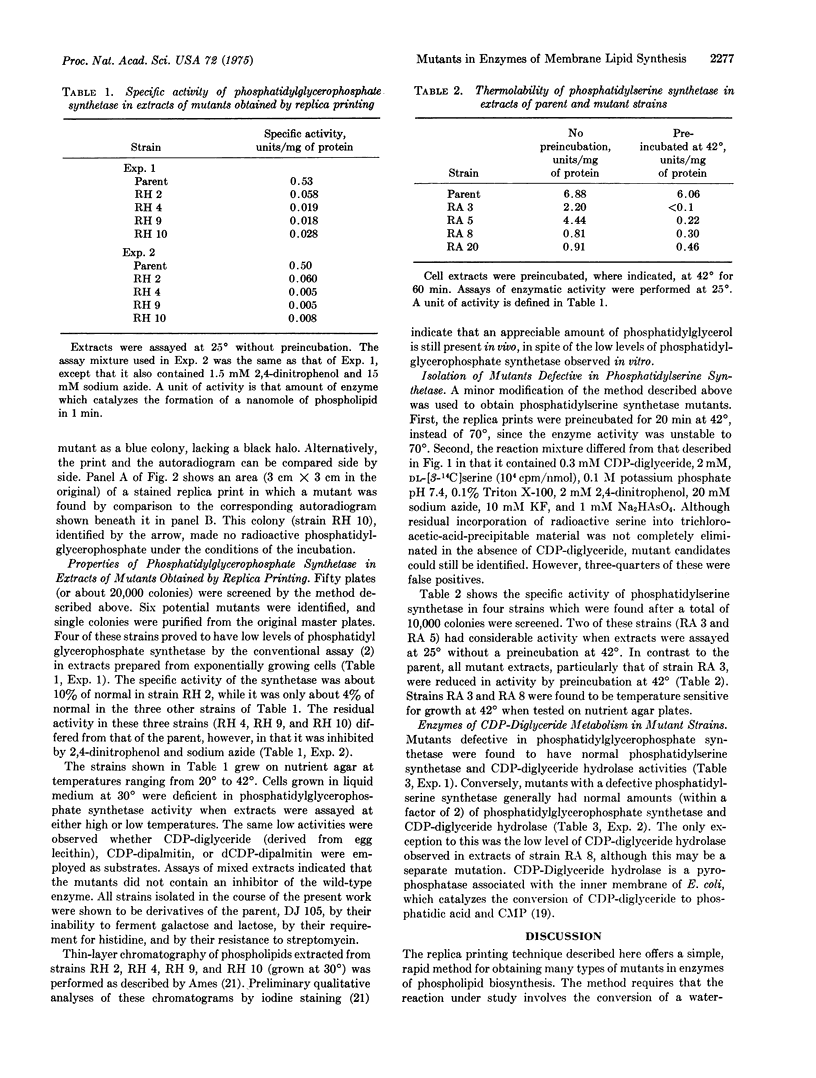
Abstract
A new method has been developed which permits the rapid screening of E. coli colonies for mutants with defective enzymes of phospholipid metabolism. In this procedure, a disc of filter paper is pressed down on an agar plate containing several hundred colonies of mutagen-treated cells, after which the paper is lifted off. In the process the colonies are transferred to the paper, giving rise to a replica print of the master plate. The few cells from each colony left on the master keep growing in the original pattern. The pattern of colonies is also retained on the filter paper, even after the cells are rendered permeable with lysozyme and EDTA. Colonies treated in this manner remain absorbed to the paper, where they can convert sn-(U-14-C)glycero-3-P to phosphatidyl(U-14-C)glycerophosphate, dependent on added CDP-diglyceride. Unrelated reactions of sn-(U-14-C)glycero-3-P that may obscure the synthesis of phosphatidyl-glycerophosphate are inhibited by the addition of reagents poisoning energy generation. The radioactive phospholipid that forms around each colony on the paper is precipitated in situ with trichloroacetic acid, and unreacted sn-(U-14-C)glycero-3-P is washed away. After autoradiography, the colonies on the filter paper are stained with Coomassie blue. When the autoradiogram is superimposed on the strained paper, mutants are identified as blue colonies lacking a black halo. With this method, 20,000 colonies were screened in several days. Four mutants were identified with low levels of CDP-diglyceride:snglycero-3-P phosphatidyl transferase (EC 2.7.8.5, GLYCEROL-PHOSPHATE PHOSPHATIDYLTRANSFERASE, PHOSPHATIDYLGLYCEROPHOSPHATE SYNTHETASE) IN EXTRACTS. With a similar assay, 10,000 additional colonies were screened for mutants with altered CDP-diglyceride:L-serine O-phosphatidyltransferase (EC 2.7.8.8, phosphatidylserine synthetase), and four strains were found in which the enzyme is thermolabile. The screening technique described here is termed replica printing and should be applicable not only to studies of phospholipid metabolism but also to nucleic acid and protein synthesis.
Full text
PDF
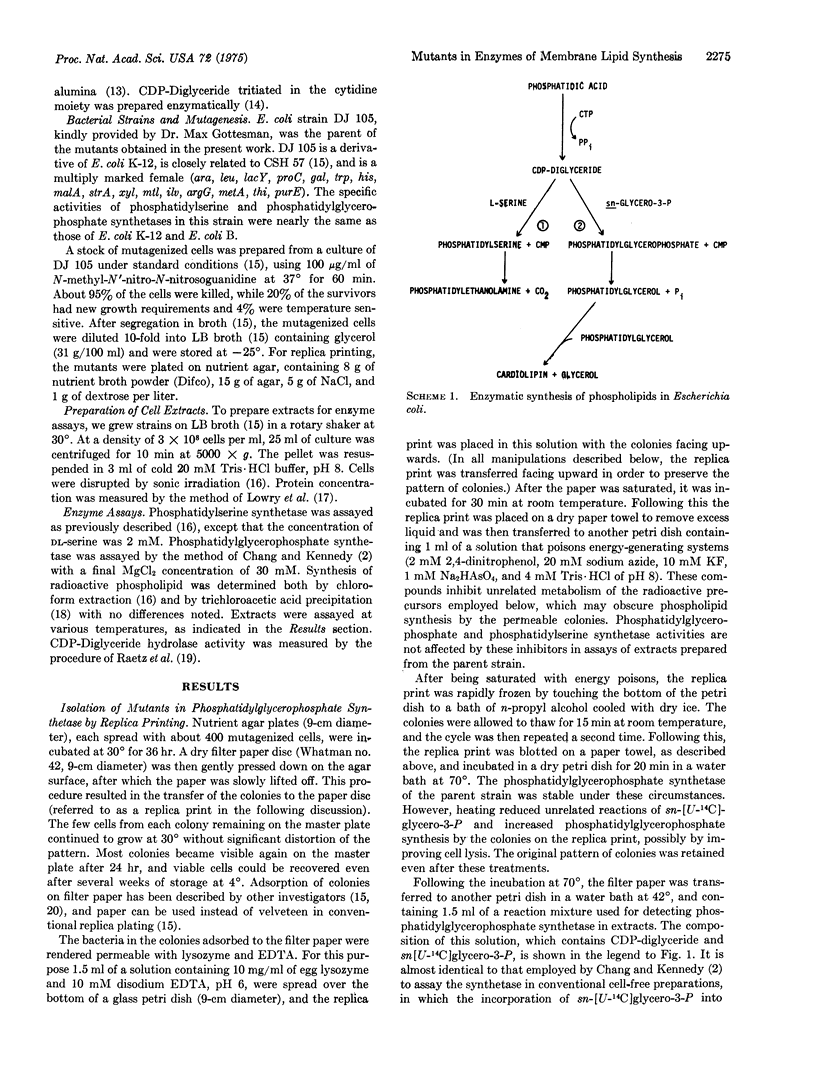
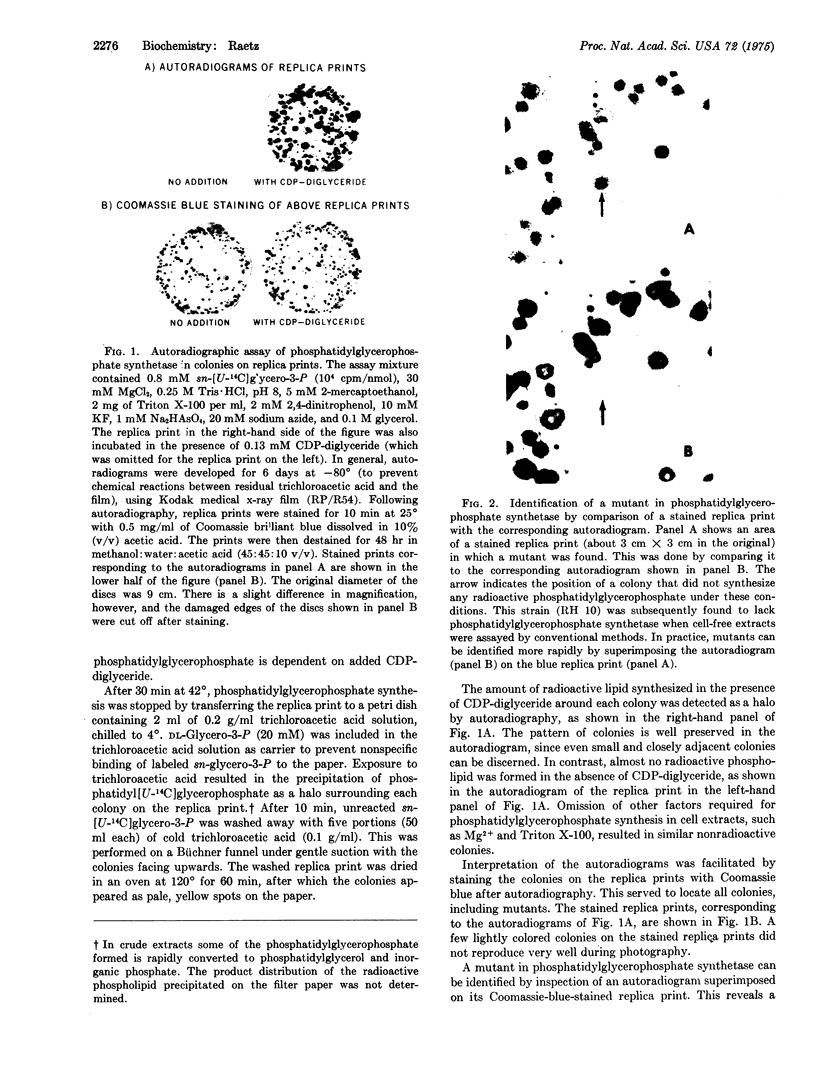

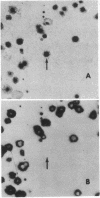
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Pathways for the synthesis of glycerophosphatides in Escherichia coli. J Biol Chem. 1967 Feb 10;242(3):516–519. [PubMed] [Google Scholar]
- Cronan J. E., Jr A new method for selection of Escherichia coli mutants defective in membrane lipid synthesis. Nat New Biol. 1972 Nov 1;240(96):21–22. doi: 10.1038/newbio240021a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Olivera R. M., Bonhoeffer E. Replication of Escherichia coli requires DNA polymerase I. Nature. 1974 Aug 9;250(5466):513–514. doi: 10.1038/250513a0. [DOI] [PubMed] [Google Scholar]
- Ota A., Shibuya I., Maruo B., Ishinaga M., Kito M. An extremely labile phosphatidylserine synthetase of an Escherichia coli mutant with the temperature-sensitive formation of phosphatidylethanolamine. Biochim Biophys Acta. 1974 Jun 26;348(3):449–454. [PubMed] [Google Scholar]
- Raetz C. R., Hirschberg C. B., Dowhan W., Wickner W. T., Kennedy E. P. A membrane-bound pyrophosphatase in Escherichia coli catalyzing the hydrolysis of cytidine diphosphate-diglyceride. J Biol Chem. 1972 Apr 10;247(7):2245–2247. [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):1098–1105. [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Partial purification and properties of phosphatidylserine synthetase from Escherichia coli. J Biol Chem. 1974 Aug 25;249(16):5083–5045. [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]