Abstract
Little information is available on the pathogenesis of fluorosis during the fetal and initial postnatal period. In the present study, female rats received 0 (control), 7 or 100 ppm of sodium fluoride in drinking water, one week before breeding and throughout gestation and nursing periods. The hemimandibles of the offspring were collected at 0, 7 and 14 days of postnatal life (n = 5) and processed for morphological analyses by light and electron microscopy, immunohistochemical analysis for amelogenin and morphometric study of enamel matrix and ameloblasts of incisors. The results showed a decrease in matrix production at the secretory phase at all study periods for the 100 ppm group. In this same group, the secretory ameloblasts showed reduction of enamel matrix secretion, disorganization of mitochondrial crests, large vacuoles at the apical portion of the cytoplasm, retention of intracisternal material and dilatation of some cisterns in the rough endoplasmic reticulum. In the groups of animals aged 7 and 14 days, analysis of variance showed significant reduction (p<0.05) in cytoplasmic volume of 23.80% and 24.75%, respectively, in relation to the control group. The smooth-ended maturation ameloblasts exhibited a large number of vacuoles with electron-dense endocytic matrix, suggesting a delay in the resorption process. Immunohistochemical analysis showed no difference in the intensity and labeling pattern of the enamel matrix in any study group. Interestingly, in offspring at the age of 14 days for the 7 ppm group, there was an increase in the matrix length at the secretory phase. Therefore, part of the excessive dose of sodium fluoride given to the mother in drinking water can reach the offspring through the placenta and mother’s milk, causing morphological changes in ameloblasts and suggesting a reduction in secretion and a delay in matrix resorption.
Keywords: Fluoride, Dental Fluorosis, Ameloblasts
INTRODUCTION
Chronic and acute doses of fluoride have been shown to induce changes in the ultrastructure of ameloblasts of rats, such as atrophy of the distal portion, mitochondrial dilatation, distension and increase in secretion material in the endoplasmic reticulum11,24, accumulation of light vacuoles11,19,29 and dark bodies in the cytoplasm, increase of secretion granules at the apical portion, fragmentation and reduction of Tomes’ processes. Furthermore, there is a disorganization of the aspect of prismatic cells14,29 and a reduction in the number of smooth-ended ameloblasts during their modulation from the secretion phase to the maturation phase in rat incisors28. Studies on cultured ameloblasts in vitro indicate that the excess of fluoride can cause a stress in the rough endoplasmic reticulum (RER), changing the protein synthesis12 or interfering with the ameloblast cytoskeleton, damaging the synthesis as well as the resorption of enamel proteins13.
Most of these studies have been performed on adult animals. The fluoride administered during gestation can cross both human3,7 and rat placenta10, and is also present in mother’s milk3,4,6. Thus, fluoride can reach the dental tissues6,8, changing their development during the fetal or initial postnatal period15,16,18. However, further morphological information is necessary to explain the influence of fluoride on amelogenesis during gestation and nursing phases.
Therefore, the purpose of this study was to investigate the possible morphological changes in mandibular incisors of rats at 0, 7 and 14 days of postnatal life, whose mothers received deionized water with 0, 7 or 100 ppm of fluoride during the gestation and nursing phases.
MATERIAL AND METHODS
The animals were obtained from the Central Animal Laboratory of the Dental School of Bauru (FOB-USP), after review and approval by the Institutional Review Board for Animal Studies of FOB-USP. A total of 27 ninety-day old female rats (Rattus norvegicus, Wistar) were used. They received pelleted solid diet for rats (Purina, Purina, São Paulo, SP, Brasil) and deionized water containing 0, 7 or 100 ppm of sodium fluoride ad libitum one week before breeding. The mandibles of the offspring (n = 5) were collected at 0, 7 and 14 days of postnatal life.
The right hemimandible was submitted to light microscopy analysis. It was immersed in a fixative solution of 4% paraformaldehyde and 1% glutaraldehyde in 0.08 M sodium cacodylate buffer, pH 7.3, during 24 hours. After demineralization in 4.13% ethylenediaminetetraacetic acid (EDTA) solution, the hemimandibles were processed and embedded in Histosec (Merck, Darmstadt, Germany). Longitudinal semi-serial 5-µm-thick sections were stained with hematoxylin and eosin (H.E.) or by the immunohistochemical technique using primary polyclonal antibodies against the recombinant form of pig’s amelogenin obtained from transgenic Escherichia coli. These antibodies were supplied by Dr. James P. Simmer (Center for Craniofacial Molecular Biology, School of Dentistry, University of Southern California, Los Angeles, USA)25.
The left hemimandible was submitted to scanning electron microscopy analysis. It was immersed in a fixative solution of 2.5% glutaraldehyde in 0.08 M sodium cacodylate buffer, pH 7.3, during 24 hours under refrigerator. Afterwards, the piece was demineralized in 4.13% EDTA solution. Incisors’ fragments containing areas of secretion and maturation ameloblasts were embedded in Spurr resin (Electron Microscopy Sciences, Fort Washington, PA, USA). Semithin sections (0.5-µm) were obtained and stained with 1% methylene blue and 1% Azur II aqueous solution. These sections were used for morphometric analysis of cell height and volume by light microscopy. Ultrathin sections (70-100 nm) were analyzed by Zeiss EM900 transmission electron microscopy (belonging to the Research Support Center in Electron Microscopy Applied to Agricultural Research (NAP/MEPA), Luiz de Queiroz Agriculture College, University of São Paulo, Brazil). Ten images were obtained with Kodak Electron Microscopy Film (4489) and digitized with a scanner (Genius ColorPage-HR7X Slim; 300 dpi resolution) connected to a computer.
Morphometric analysis
Quantitative microscopic analysis was performed in a Carl Zeiss KS300 Image Analyzer (Kontron Elektronik, Eching, Germany) equipped with a CCD – IRIS RGB – Sony camera installed in a Zeiss Axioskop 2 trinocular microscope. The matrix length was obtained with aid of 10x objective lens in sections of hemimandibles included in Histosec. For this measurement, sections presenting the incisors cut through their long axes were selected, avoiding sections where the tooth or ameloblasts were sectioned in oblique position. The length was obtained at the central region of enamel, and not on the enamel surface. The matrix was measured dividing it into small straight line segments because the software employed is unable to measure the length of a curve.
The cell height was calculated by direct measurement of 50 secretory ameloblasts and 50 maturation ameloblasts on semithin sections, at 100x magnification. These sections were randomly selected by group.
For cell volume measurement, the major (D) and minor (d) diameters of nuclear transections of the same cell oriented in longitudinal direction were measured from 50 nuclei of secretory ameloblasts and 50 nuclei of maturation ameloblasts for each study group. These nuclei were analyzed from 10 images of each of the five semithin sections randomly selected for each animal. These images were enlarged with the aid of a 100x objective. The nucleus volume (Vn) was calculated by the formula , as indicated by Pardini and Taga19 (1996). The cytoplasm area (Scit) and the total area (Scel) of the ameloblast were used to determine the cytoplasmic volume density (Svcit) as . This way, the cytoplasmic volume density (Vvcit) was obtained, as follows: (principle of Delesse5). The nuclear volume density (Vvn) was calculated by the ratio: . Then, the cytoplasmic volume (Vcit) was obtained by the relation: . The cell volume was calculated from the nuclear volume and cytoplasmic volume.
The data for each morphometric dimension evaluated were compared by analysis of variance and mean difference (Tukey’s Test) using Microsoft Excel software to investigate the presence of statistical differences among groups at a significance level of 5% (p<0.05). The means and standard deviations were calculated to indicate variations within each group.
RESULTS
Immunohistochemical Results for Amelogenin
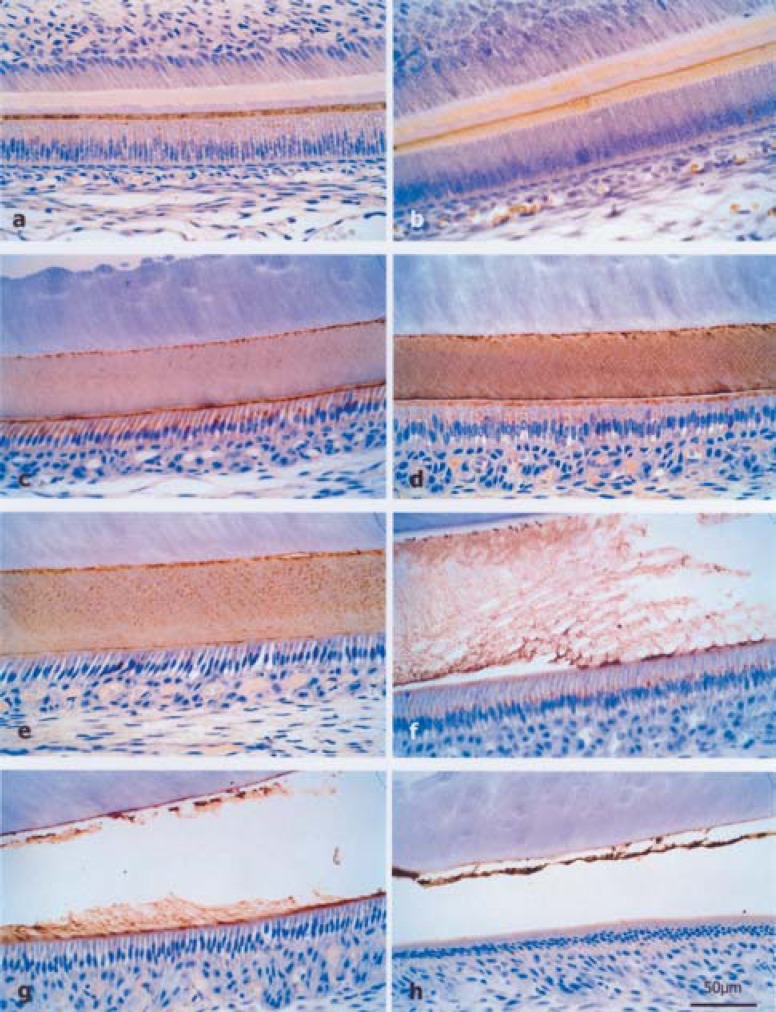
The morphological aspects observed by light microscopy in animals at 0, 7 or 14 days, whose mothers received 0, 7 or 100 ppm of fluoride in drinking water, were similar. At the region of the initial secretory phase, immunohistochemical staining was punctiform and intense in the enamel matrix (Figure 1a). At the other regions of the secretory phase, labeling was homogeneous with a higher intensity only at the enamel-dentin junction (Figure 1b). During most of the secretory phase, the enamel matrix showed less staining (Figure 1c). At the apical portion of the ameloblasts, there was moderate punctiform labeling (Figure 1d). At the maturation phase, staining was intense with some areas showing dotty labeling (Figure 1e). In enamel maturation, the remaining matrix was resorbed (Figure 1f-g). At the final part of this phase, it was represented only by a little fillet of organic matrix, which was highly reactive to the immunohistochemical technique (Figure 1h).
FIGURE 1. (a–h)- Histological sections of rat incisor buds stained by immunohistochemical reaction for amelogenin. a) Punctiform staining pattern in the secretory enamel matrix of the 7 ppm group at the age of 7 days, b) Homogeneous labeling with higher intensity only at the dentinoenamel junction of newborn rats from the 0 ppm group, c) Less staining of enamel matrix for most of the secretory phase, d) Moderate punctiform labeling at the apical portion of ameloblasts of the 0 ppm group at the age of 7 days, e) Punctiform staining pattern during the maturation phase of the 0 ppm group at the age of 14 days, f-g) Resorption of the enamel matrix during maturation phase of the 7 ppm and 100 ppm groups at the age of 14 days, respectively, h) Remaining maturation matrix represented by a little fillet of highly reactive organic matrix of the 7 ppm group at the age of 7 days.
Morphological Results of Scanning Electron Microscopy
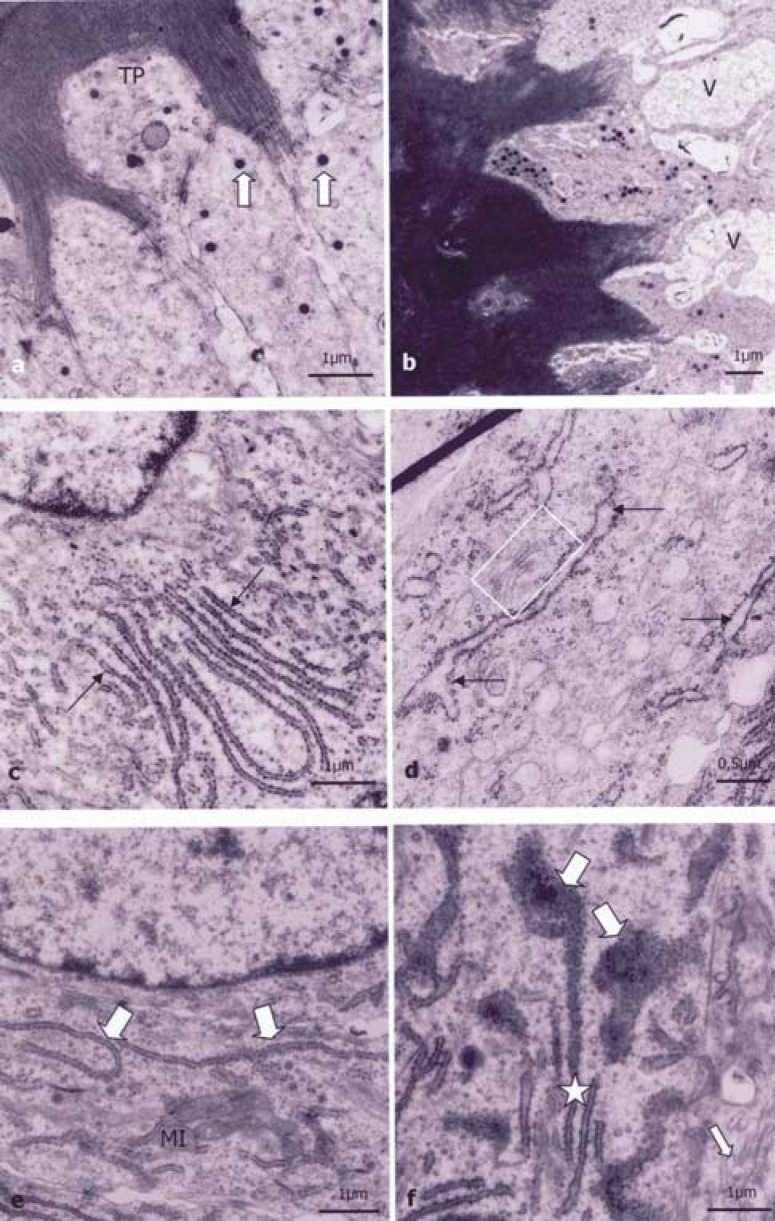
The secretory ameloblasts of control and 7 ppm groups at 0, 7 and 14 days showed normal morphology. The secretory ameloblast presented RER cisterns and some small secretion granules at the apical region (Figure 2a), especially in the Tomes’ processes. The recently synthesized matrix was distributed in a homogeneous manner around the process. At the middle region, the Golgi complexes were constituted by several dictyosomes arranged in few and small saccules near the intense RER net (Figure 2c and 2e). The elongated nuclei with euchromatin and evident nucleoli occupied most of the suprabasal portion. A large number of little mitochondria concentrated between the nuclei and the basal region of the cell membrane and a small amount was spread out in the remaining cytoplasm.
FIGURE 2. (a-f)- Ultrathin sections of the secretory ameloblast of rat incisor buds. a) Some small secretion granules (arrows) at the apical region and in the Tomes’ process (TP) region of the 0 ppm group at the age of 0 days, b) Large vacuoles (V) in the TP region of the newborn rat from the 100 ppm group; c) In the middle region, homogeneous and parallelly distributed RER cisterns of the 0 ppm group at the age of 0 days, d) In supranuclear area, dilated RER cisterns (arrows) and small saccules of Golgi complex (▭) of newborn rat from the 100 ppm group; e) Preserved RER cisterns (large arrow) of the 7 ppm group at the age of 14 days, f) Preserved RER cisterns (star) and dilated RER cisterns filled with intracisternal material (big arrow), and Golgi complex saccules (small arrow) of the 100 ppm group at the age of 14 days.
In the Tomes’ processes of secretory ameloblasts of the 100 ppm groups, there were large vacuoles similar to cystic formations, sometimes with electron-dense material (Figure 2b). Although this vacuolation occurs, most of the interfaces between the Tomes’ processes and the recently synthesized matrix showed preserved morphology similar to the control group. Secretion granules did not present morphological changes, though in a smaller number compared to the control group. In all supranuclear regions, the RER showed dilated (Figure 2d) and disorganized cisterns filled with electron-dense material (Figure 2f). Most mitochondria were more dilated and had less disorganized and more spaced crests than the control group, especially at the basal portion (Figure 3b).
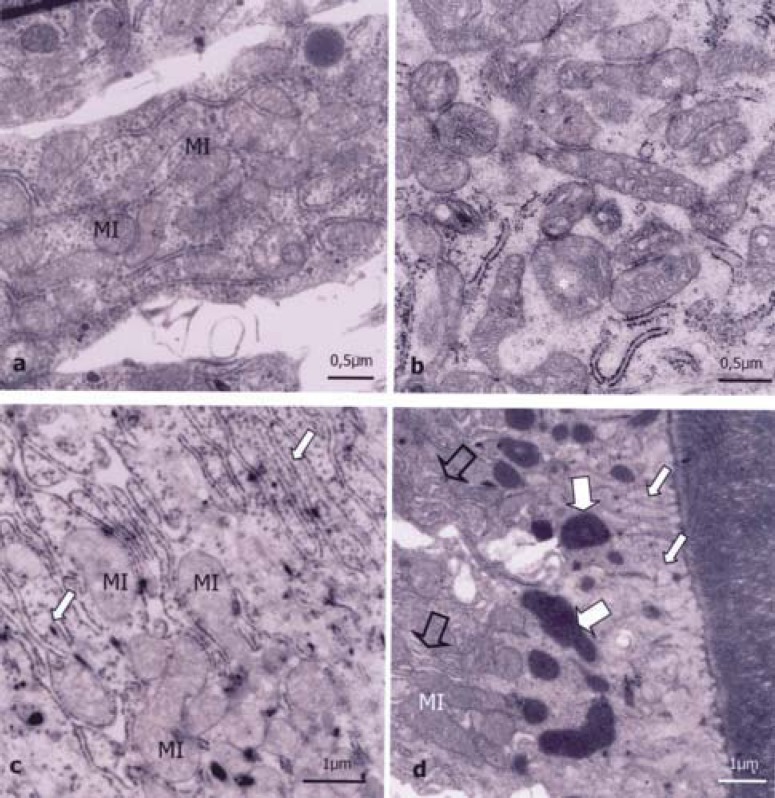
FIGURE 3. (a-d)- Ultrathin section of the secretory and maturation ameloblast of rat incisor buds. Observe: a) Preserved mitochondria (MI) of the smooth-ended ameloblast of 0 ppm group at the age of 7 days, b) At the basal region of the secretory ameloblast of newborn rat from the 100 ppm group, most mitochondria (*) are dilated with crests that are less organized than those of the control group, c) At the apical portion of ruffled-ended ameloblasts from rats of 100 ppm group at the age of 14 days, large amount of dilated mitochondria (MI) and preserved RER cisterns (arrow); d) At the apical region next to the matrix of smooth-ended ameloblasts of rats of the 100 ppm group at the age of 7 days, there are preserved RER cisterns (empty arrows), mitochondria (MI) and increased endocytic light microvesicles (small arrows) and big endocytic vacuoles (large arrows).
The maturation ameloblasts were present only in 7-day-old and 14-day-old animals. The control and 7 ppm groups showed normal morphological characteristics. The smooth-ended cells had many elongated and rounded mitochondria at the basal region among the invaginations of the cell membrane (Figure 3a). The nuclei had less dense chromatin and were smaller or less elongated than those observed in secretory ameloblasts. At the middle region of the smooth-ended ameloblasts, there was less RER than in secretory cells. At this region there were more evident mitochondria, lysosomes, digestive vacuoles and microfilaments.
Maturation ameloblasts of 7-day-old and 14-day-old animals for the 100 ppm group did not exhibit ultrastructural changes in ruffled-ended cells, except for the presence of dilated mitochondria between and under the apical invaginations (Figure 3c). The smooth-ended cells had many little light pinocytic vesicles and electron-dense endocytic figures at the apical region close to the enamel matrix (Figure 3d). The RER and mitochondria at this region did not present differences in relation to the control group. At the basal area, the mitochondria, microfilaments, nuclei and nucleoli presented normal morphological characteristics.
Morphometric Results
With regard to the enamel matrix length, there were no statistically significant differences either at the secretory phase in newborn animal groups or in maturation phase groups at all study periods (Table 1). However, in 7-day-old animals, there was a significant reduction (p<0.05) of 14.38% in the matrix length at the secretory phase of the 100 ppm group in relation to the control animals. In 14-day-old animals, there was an increase (p<0.05) of 16.47% in the 7 ppm group in relation to the control group.
TABLE 1. Means and standard deviation of morphometric parameters from groups of 0, 7 and 14 days of postnatal life that received fluoride treatment (n = 5).
| 0 days | |||
| Secretion Phase | 0ppm | 7ppm | 100ppm |
| Cell Height (in µm) | 63.94 ± 2.38 | 63.89 ± 2.64 | 63.35 ± 2.35 |
| Cell Volume (in µm3) | 1642. 75 ± 113.88 | 1614.76 ± 58.17 | 1589.24 ± 91.59 |
| Matrix Length (in µm) | 1833.31 ± 299.46 | 1856.30 ± 181.79 | 1615.91 ± 198.01 |
| 7 days | |||
| Secretion Phase | 0ppm | 7ppm | 100ppm |
| Cell Height (in µm) | 69.50 ± 4.37 | 70.08 ± 4.76 | 69.50 ± 3.78 |
| Cell Volume (in µm3) | 2311.91 ± 157.91 | 2167.16 ± 191.72 | 1859.28 ± 88.27 |
| a | a | b | |
| Matrix Length (in µm) | 4733.57 ± 393.50 | 4683.87 ± 177.82 | 4052.92 ± 311.94 |
| a | a | b | |
| Maturation Phase | 0ppm | 7ppm | 100ppm |
| Cell Height (in µm) | 45.50 ± 3.40 | 44.40 ± 2.80 | 43.94 ± 2.58 |
| Cell Volume (in µm3) | 1431.74 ± 73.88 | 1390.14 ± 142.89 | 1306.46 ± 121.51 |
| Matrix Length (in µm) | 2852.43 ± 222.85 | 2841.48 ± 275.74 | 3094.39 ± 157.41 |
| 14 days | |||
| Secretion Phase | 0ppm | 7ppm | 100ppm |
| Cell Height (in µm) | 74.28 ± 3.42 | 73.71 ± 3.64 | 72.63 ± 4.18 |
| Cell Volume (in µm3) | 2334.24 ± 187.36 | 2134.30 ± 190.10 | 1850.24 ± 46.39 |
| a | a | b | |
| Matrix Length (in µm) | 4622.38 ± 351.43 | 5383.64 ± 471.77 | 4541.83 ± 249.80 |
| a | b | a | |
| Maturation Phase | 0ppm | 7ppm | 100ppm |
| Cell Height (in µm) | 42.07 ± 3.88 | 41.53 ± 3.90 | 38.58 ± 4.18 |
| Cell Volume (in µm3) | 1304.54 ± 121.92 | 1400.71 ± 157.95 | 1284.12 ± 41.97 |
| Matrix Length (in µm) | 3080.11 ± 348.38 | 3313.09 ± 515.90 | 3326.43 ± 171.85 |
Same letters or the absence of letters indicate no statistical difference (p>0.05). Different letters indicate statistically significant difference (p<0.05)
In relation to cell height, there were no significant changes (p>0.05) in all study groups (Table 1). On the other hand, the 100 ppm dose caused a reduction (p<0.05) of 24.34% and 26.16% in the cell volume of secretory ameloblasts, respectively, in 7-day-old and 14-day-old animals in relation to the control animals.
DISCUSSION
The amelogenins compose more than 90% of the matrix secreted by ameloblast. The correlation between amelogenin properties and the mechanism of dental fluorosis became the key to understand its pathogenesis. Standardization and reproducibility of experimental fluorosis were necessary, which led to proposition of different experimental models, as rodents26, respecting their metabolism and adjusting the fluoride doses, which must be 10 to 50 times higher than in humans1.
In immunohistochemical analysis, the high dose (100 ppm) of fluoride in mother’s drinking water or mother’s milk seemed to cause no changes in the labeling pattern and intensity of amelogenins in the enamel matrix of offspring at 0, 7 or 14 days of postnatal life.
In subjective investigation by transmission electron microscopy (TEM), the secretory animals of 100 ppm groups showed more dilated mitochondria than observed in the control group. They had large light spaces between mitochondrial crests that were similar to those described in the literature for adult animals11,22. Light vacuoles were also observed at the apical portion of the cytoplasm before the Tomes’ processes, as previously described in the literature for adult rats receiving acute and chronic doses of fluoride2,11,19,29. Especially in 14-day-old animals, the RER presented dilated cisterns, which sometimes were filled with electron-dense material, as described by Kruger11 (1968), Patterson, et al.20 (1976) and Matsuo, et al.17 (1996). This can suggest that fluoride interferes with the intracellular transport mechanism and exocytosis, causing a delay in protein material inside RER cisterns. In smooth-ended ameloblasts, there were also dilated mitochondria, as previously described by Pergolizzi, et al.21 (1995) in adult rat incisors exposed to chronic doses of fluoride in drinking water. In ruffled-ended ameloblasts, there were dense vacuoles at the apical region similar to those described by Aoki2 (1989) in rats, which were subjected to a fluorosis condition.
In relation to the matrix length at the secretory phase, there were no significant statistical differences between 0-day-old and 7-day-old animals exposed to the 7 ppm dose. This suggests that, although this dose of fluoride (offered to mothers during gestation or nursing phases) can reach the offspring through the placenta or mother’s milk, it does not affect the enamel matrix synthesis or secretion at these ages. On the other hand, in relation to the 100 ppm group, there was a decrease (p<0.05) of 14.38% in matrix length at the secretory phase compared to the control group. This may suggest that the fluoride received from the mother’s milk can reduce enamel production, anticipating enamel maturation at this age. At 14 days of postnatal life, the secretion phase matrix of the 7 ppm group was 16.47% longer (p<0.05) than the control group. The retention and consequent increase of enamel matrix length in rats receiving fluoride in drinking water have also been detected by Smith, et al.27 (1993) and Zhou, et al.30 (1996). An increase in tooth length due to fluoride administration has been described by Glenn, et al.9 (1997), by using pregnant hamsters that received 10 and 20 ppm of fluoride in drinking water. The authors suggested that fluoride can be an essential nutrient for dental development and administration of this ion is important during odontogenesis of deciduous tooth. Anyway, the statement that an ideal fluoride dose can favor tooth development or increase the enamel matrix retention is not well supported so far.
At the secretory phase, the ameloblasts reached a mean height of 63.35 µm at birth, increasing to 69.50 µm on day 7 and reaching 72.63 µm on day 14. At the maturation phase, the ameloblast height was 45.50 µm on day 7 and 42.07 µm on day 14, without difference between groups. These values were similar to those observed in other studies with animals that did not receive intentional administration of fluoride from diet23,27. In relation to the cell volume, the 100 ppm dose of fluoride caused a significant reduction (p<0.05) in secretory ameloblasts in the 7-day-old and 14-day-old animals. The volume of maturation ameloblasts was not reduced, as described by Ribeiro23 (2001), who observed a decrease (p<0.05) of 31% in the 100 ppm group compared to the control group.
CONCLUSIONS
Based on the morphological and morphometric results, it may be concluded that the 7 ppm dose administered during the gestation and nursing phases in rats does not cause significant morphological changes in ameloblasts. However, it can increase the matrix length at the secretory phase. Fluoride excess (100ppm) caused changes in the incisor buds of rats at 0, 7 and 14 days of postnatal life, especially at the secretory phase. Thus, the excessive dose of sodium fluoride administered in drinking water during gestation can cross the placenta and milk, altering the matrix secretion velocity and resorption.
REFERENCES
- 1.Aoba T, Fejerskov O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med. 2002;13(2):155–170. doi: 10.1177/154411130201300206. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H. Ultrastructural changes induced in rat ameloblasts and enamel by NaF administration, especially the stages of transition and maturation. Shikwa Gakuho. 1989;89(10):1605–1637. [PubMed] [Google Scholar]
- 3.Avery JK. Avery JK. Desenvolvimento e histologia bucal. Porto Alegre: Artmed; 2005. Agentes que afetam o desenvolvimento dentário e ósseo; pp. 156–165. [Google Scholar]
- 4.Buttner G, Muhler JC. Fluoride placental transfer in the rat. J Dent Res. 1958;37(2):326–329. doi: 10.1177/00220345580370021901. [DOI] [PubMed] [Google Scholar]
- 5.Delesse A. Procédé mécanique pour déterminer la composition des roches. C R Acad Sci (Paris) 1847;25:544–544. apud Weibel29. [Google Scholar]
- 6.Drinkard CR, Deaton TG, Bawden JW. Enamel fluoride in nursing rats with mothers drinking water with high fluoride concentrations. J Dent Res. 1985;64(6):877–880. doi: 10.1177/00220345850640060301. [DOI] [PubMed] [Google Scholar]
- 7.Fassman DK. Prenatal fluoridation: a literature review. N Y State Dent J. 1993;59(6):47–51. [PubMed] [Google Scholar]
- 8.Gedalia I, Shapira L. Effect or prenatal and postnatal fluoride on the human deciduous dentition: a literature review. Adv Dent Res. 1989;3(2):168–176. doi: 10.1177/08959374890030021601. [DOI] [PubMed] [Google Scholar]
- 9.Glenn FB, Glenn WD, Burdi AR. Prenatal fluoride for growth and development: part X. J Dent Child. 1997;64(5):317–321. [PubMed] [Google Scholar]
- 10.Katz S, Stookey GK. Further studies concerning the placental transfer of fluoride in the rat. J Dent Res. 1973;52(2):206–210. doi: 10.1177/00220345730520020301. [DOI] [PubMed] [Google Scholar]
- 11.Kruger BJ. Ultrastructural changes in ameloblasts from fluoride treated rats. Arch Oral Biol. 1968;13(8):969–977. doi: 10.1016/0003-9969(68)90012-5. [DOI] [PubMed] [Google Scholar]
- 12.Kubota K, Lee DH, Tsuchiya M, Young CS, Everetti ET, Martinez-Mier EA, et al. Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J Biol Chem. 2005;280(24):23194–23202. doi: 10.1074/jbc.M503288200. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Decker S, Yuan ZA, Denbesten PK, Aragon MA, Jordan-Sciutto K, et al. Effects of sodium fluoride on the actin cytoskeleton of murine ameloblast. Arch Oral Biol. 2005;50(8):681–688. doi: 10.1016/j.archoralbio.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Lyaruu DM, Jong M, Bronckers ALJJ, Woltgens JHM. Ultrastructural study of fluoride-induced in vitro hypermineralization of enamel in hamster tooth germs explanted during the secretory phase of amelogenesis. Arch Oral Biol. 1986;31(2):109–117. doi: 10.1016/0003-9969(86)90034-8. [DOI] [PubMed] [Google Scholar]
- 15.Maciejewska I, Adamowicz-Klepalska B. Influence of low and high doses of fluoride on tooth germ development in rats. Folia Morphol (Warsz) 2000;59(4):307–310. [PubMed] [Google Scholar]
- 16.Maciejewska I, Adamowicz-Klepalska B, Kmiec Z, Dziewiatkowski J. Influence of diet and fluoride on dentin and enamel deposition and maturation in rats. Folia Morphol (Warsz) 2000;59(2):131–136. [PubMed] [Google Scholar]
- 17.Matsuo S, Inai T, Kurisu K, Kiyomiya K, Kurebe M. Influence of fluoride on secretory pathway of the secretory ameloblast in rat incisor tooth germs exposed to sodium fluoride. Arch Toxicol. 1996;70(7):420–429. doi: 10.1007/s002040050294. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira DT. Efeito crônico do flúor no esmalte e nos ameloblastos secretores de incisivos de ratos. Piracicaba (SP): Faculdade de Odontologia de Piracicaba, Universidade Estadual de Campinas; 1988. [dissertação] [Google Scholar]
- 19.Pardini LC, Taga R. Stereological study of the sexual dimorphism in mouse submandibular glands. Okajimas Folia Anat Jpn. 1996;73(2/3):19–24. doi: 10.2535/ofaj1936.73.2-3_119. [DOI] [PubMed] [Google Scholar]
- 20.Patterson CM, Basford KE, Kruger BJ. The effect of fluoride on the immature enamel matrix protein of the rat. Arch Oral Biol. 1976;21(2):131–132. doi: 10.1016/0003-9969(76)90083-2. [DOI] [PubMed] [Google Scholar]
- 21.Pergolizzi S, Santoro A, Santoro G, Trimarchi F, Anastaso G. Enamel fluorosis in rat’s incisor: S.E.M. and T.E.M. investigation. Bull Group Int Rech Sci Stomatol Odontol. 1995;38(3/4):95–105. [PubMed] [Google Scholar]
- 22.Pindborg JJ, Weinmann JP. Morphologic and functional correlations in the enamel organ of the rat incisor during amelogenesis. Acta Anat (Basel) 1969;36(4):367–381. doi: 10.1159/000141450. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro DA. Estudo morfológico e estereológico ultraestrutural do ameloblasto na fase de maturação do esmalte de dentes incisivos de ratos submetidos à fluorose. Bauru (SP): Faculdade de Odontologia de Bauru, Universidade de São Paulo; 2001. [dissertação] [Google Scholar]
- 24.Richards A. Nature and mechanisms of dental fluorosis in animals. J Dent Res. 1990;69:701–705. doi: 10.1177/00220345900690S136. sp. issue. [DOI] [PubMed] [Google Scholar]
- 25.Simmer JP, Hu CC, Lau EC, Sarte P, Slavkin HC, Fincham AG. Alternative splicing of the mouse amelogenin primary RNA transcript. Calcif Tissue Int. 1994;55(4):302–310. doi: 10.1007/BF00310410. [DOI] [PubMed] [Google Scholar]
- 26.Smith CE, Nanci A. A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat Rec. 1989;225(3):257–266. doi: 10.1002/ar.1092250312. [DOI] [PubMed] [Google Scholar]
- 27.Smith CE, Nanci A, Denbesten PK. Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat Rec. 1993;237(2):243–258. doi: 10.1002/ar.1092370212. [DOI] [PubMed] [Google Scholar]
- 28.Walton RE, Eisenmann DR. Ultrastructural examination of various stages of amelogenesis in the rat following parenteral fluoride administration. Arch Oral Biol. 1974;19(2):171–182. doi: 10.1016/0003-9969(74)90212-x. [DOI] [PubMed] [Google Scholar]
- 29.Weibel ER. Stereological principles of morphometry in electron microscopy cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou R, Zaki AE, Eisenmann DR. Morphometry and autoradiographiy of altered rat enamel protein processing due to chronic exposure to fluoride. Arch Oral Biol. 1996;41(8/9):739–747. doi: 10.1016/s0003-9969(96)00078-7. [DOI] [PubMed] [Google Scholar]