Abstract
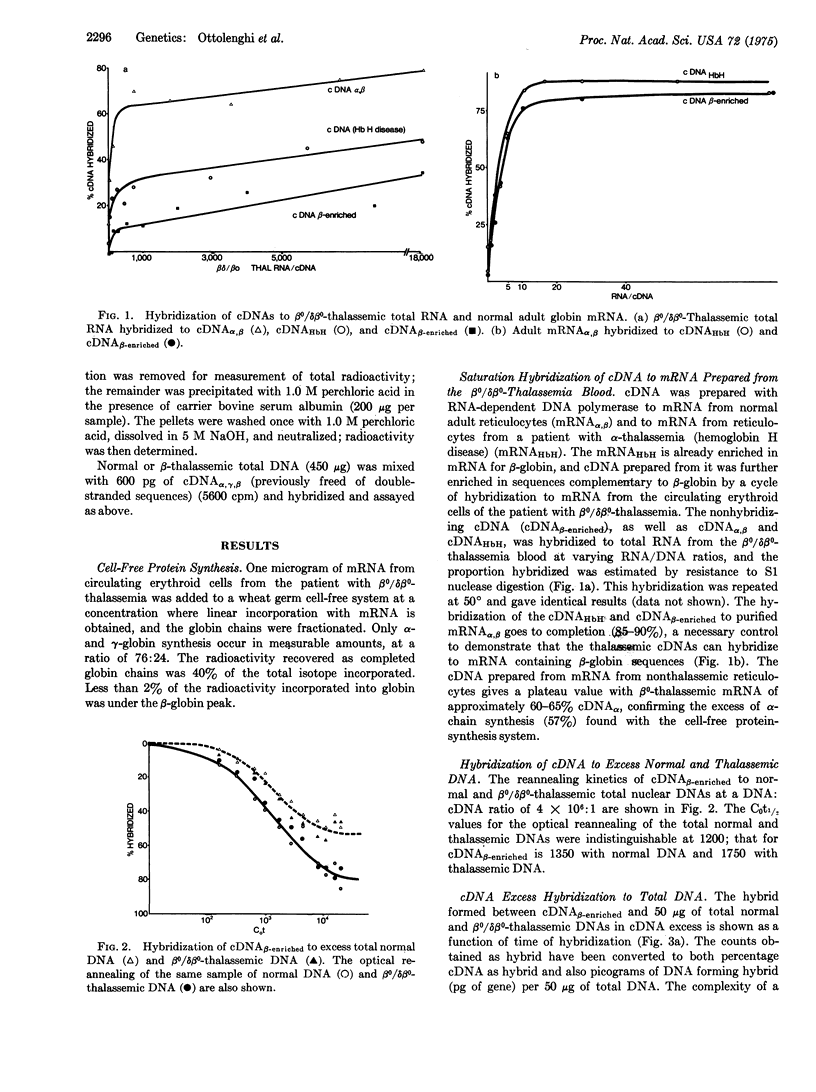
Complementary DNA (cDNA) was prepared with RNA-dependent DNA polymerase from human globin messenger RNA (mRNA). Annealing and translation experimenta with total mRNA from circulating cells from a patient with heterozygous beta/heterozygous beta-delta-o thalassemia (beta-o/delta beta-o-thalassemia) demonstrated no detectable mRNA for beta-globin. cDNA enriched in sequences homologous to beta-globin mRNA was prepared by hydroxylapatite fractionation of hybrids formed between beta-o/delta beta-o-thalassemic mRNA and cDNA made from mRNA from a patient with alpha-thalassemia (hemoglobin H disease). The rate of annealing of this beta-enriched cDNA to normal human nuclear DNA was that of a sequence present as only a single copy per haploid genome. The beta-enriched cDNA annealed to the beta-o-delta beta-o-thalassemia total DNA with approximately the same kinetics as to normal DNA, indicating that no total gene deletion of beta-globin genes from the diploid genome has occurred, although the accuracy of the technique could not exclude with certainty a partial deletion or a deletion of a beta-globin gene from only one of the haploid genomes. This demonstrates that at least one of the beta-o- or the delta beta-o-thalassemia haploid genomes in this case contains a substantially intact beta-globin gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. J., Jr, Forget B. G. Defect in messenger RNA for human hemoglobin synthesis in beta thalassemia. J Clin Invest. 1971 Dec;50(12):2755–2760. doi: 10.1172/JCI106778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Freeman K. B. DNA sequences neighboring the duck hemoglobin genes. Cold Spring Harb Symp Quant Biol. 1974;38:707–716. doi: 10.1101/sqb.1974.038.01.076. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Conconi F., Rowley P. T., Del Senno L., Pontremoli S., Volpato S. Induction of -globin synthesis in the -thalassaemia of Ferrara. Nat New Biol. 1972 Jul 19;238(81):83–87. doi: 10.1038/newbio238083a0. [DOI] [PubMed] [Google Scholar]
- Dow L. W., Terada M., Natta C., Metafora S., Grossbard E., Marks P. A., Bank A. Globin synthesis of intact cells and activity of isolated mRNA in -thalassaemia. Nat New Biol. 1973 May 23;243(125):114–116. [PubMed] [Google Scholar]
- Forget B. G., Benz E. J., Jr, Skoultchi A., Baglioni C., Housman D. Absence of messenger RNA for beta globin chain in beta(0) thalassaemia. Nature. 1974 Feb 8;247(5440):379–381. doi: 10.1038/247379a0. [DOI] [PubMed] [Google Scholar]
- Gambino R., Kacian D., O'Donnell J., Ramirez F., Marks P. A., Bank A. A limited number of globin genes in human DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3966–3970. doi: 10.1073/pnas.71.10.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummerson K. S., Williamson R. Sequence divergence of mammalian globin messenger RNA. Nature. 1974 Feb 1;247(5439):265–267. doi: 10.1038/247265a0. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Birnie G. D., Hell A., Humphries S., Young B. D., Paul J. Kinetic studies of gene frequency. I. Use of a DNA copy of reticulocyte 9 S RNA to estimate globin gene dosage in mouse tissues. J Mol Biol. 1974 Apr 25;84(4):539–554. doi: 10.1016/0022-2836(74)90115-6. [DOI] [PubMed] [Google Scholar]
- Hell A., Birnie G. D., Slimming T. K., Paul J. Controlled fragmentation of DNA by DNase I. Anal Biochem. 1972 Aug;48(2):369–377. doi: 10.1016/0003-2697(72)90089-9. [DOI] [PubMed] [Google Scholar]
- Housman D., Forget B. G., Skoultchi A., Benz E. J., Jr Quantitative deficiency of chain-specific globin messenger ribonucleic acids in the thalassemia syndromes. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1809–1813. doi: 10.1073/pnas.70.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Wrightstone R. N., Wilson J. B., Schroeder W. A., Kendall A. G. Hemoglobin Kenya, the product of fusion of amd polypeptide chains. Arch Biochem Biophys. 1972 Dec;153(2):850–853. doi: 10.1016/0003-9861(72)90408-0. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Gambino R., Dow L. W., Grossbard E., Natta C., Ramirez F., Spiegelman S., Marks P. A., Bank A. Decreased globin messenger RNA in thalassemia detected by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1886–1890. doi: 10.1073/pnas.70.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyon W. G., Ottolenghi S., Williamson R. Human globin gene expression and linkage in bone marrow and fetal liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):258–262. doi: 10.1073/pnas.72.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Lodish H. F., Kan Y. W., Housman D. Beta thalassemia and translation of globin messenger RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2514–2518. doi: 10.1073/pnas.68.10.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Isolation and translation of hemoglobin messenger RNA from thalassemia, sickle cell anemia, and normal human reticulocytes. J Clin Invest. 1971 Nov;50(11):2458–2460. doi: 10.1172/JCI106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi S., Lanyon W. G., Paul J., Williamson R., Weatherall D. J., Clegg J. B., Pritchard J., Pootrakul S., Boon W. H. The severe form of alpha thalassaemia is caused by a haemoglobin gene deletion. Nature. 1974 Oct 4;251(5474):389–392. doi: 10.1038/251389a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Roberts A. V., Weatherall D. J., Clegg J. B. The synthesis of human haemoglobin A 2 during erythroid maturation. Biochem Biophys Res Commun. 1972 Apr 14;47(1):81–87. doi: 10.1016/s0006-291x(72)80013-5. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley P. T., Kosciolek B. Distinction between two types of beta-thalassaemia by inducibility of the cell-free synthesis of beta-chains by nonthalassaemic soluble fraction. Nat New Biol. 1972 Oct 25;239(95):234–235. doi: 10.1038/newbio239234a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Effects of sequence divergence on the reassociation properties of repetitive DNAs. Nat New Biol. 1971 Jul 21;232(29):82–83. doi: 10.1038/newbio232082a0. [DOI] [PubMed] [Google Scholar]
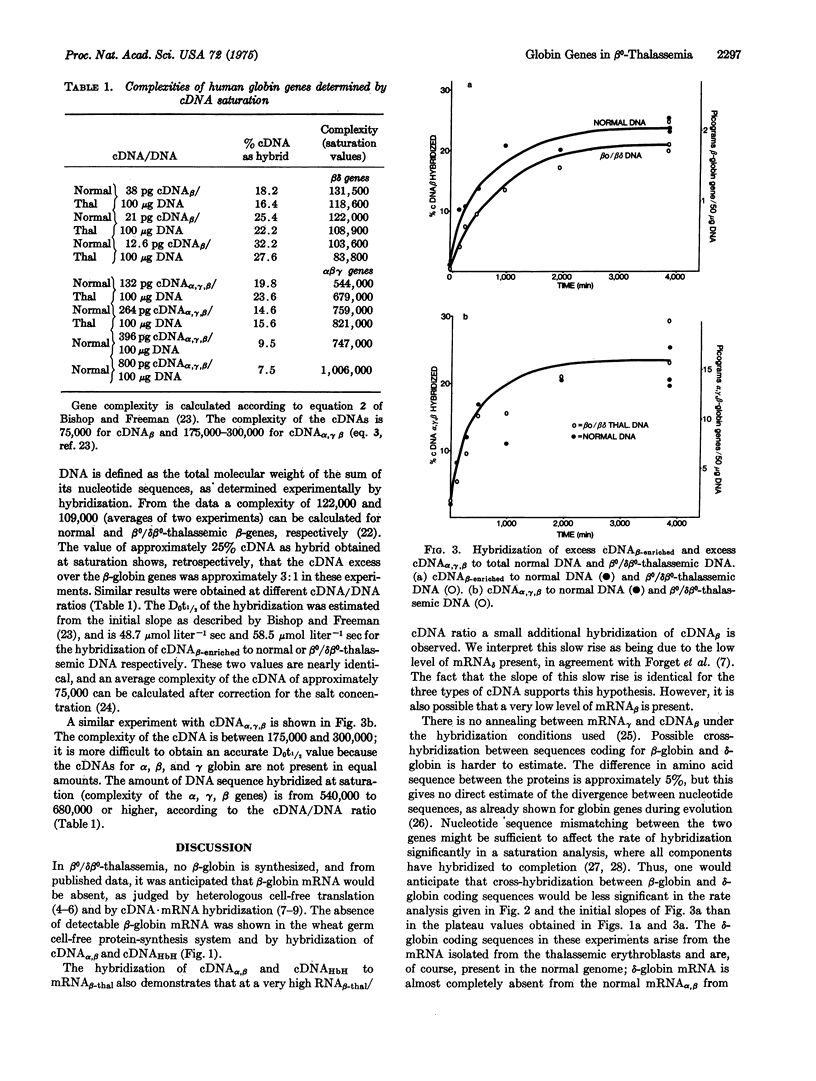
- Taylor J. M., Dozy A., Kan Y. W., Varmus H. E., Lie-Injo L. E., Ganesan J., Todd D. Genetic lesion in homozygous alpha thalassaemia (hydrops fetalis). Nature. 1974 Oct 4;251(5474):392–393. doi: 10.1038/251392a0. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Na-Nakorn S., Wasi P. The pattern of disordered haemoglobin synthesis in homozygous and heterozygous beta-thalassaemia. Br J Haematol. 1969 Mar;16(3):251–267. doi: 10.1111/j.1365-2141.1969.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Naughton M. A. Globin synthesis in thalassaemia: an in vitro study. Nature. 1965 Dec 11;208(5015):1061–1065. doi: 10.1038/2081061a0. [DOI] [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]


