Abstract
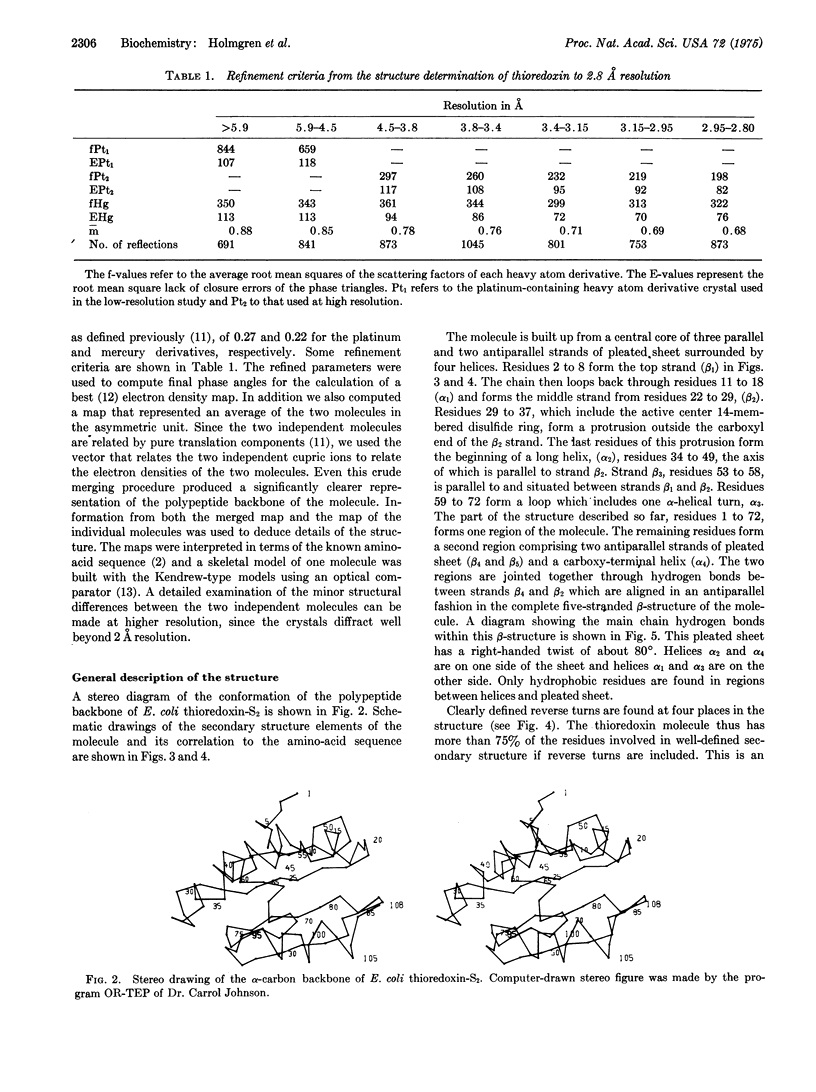
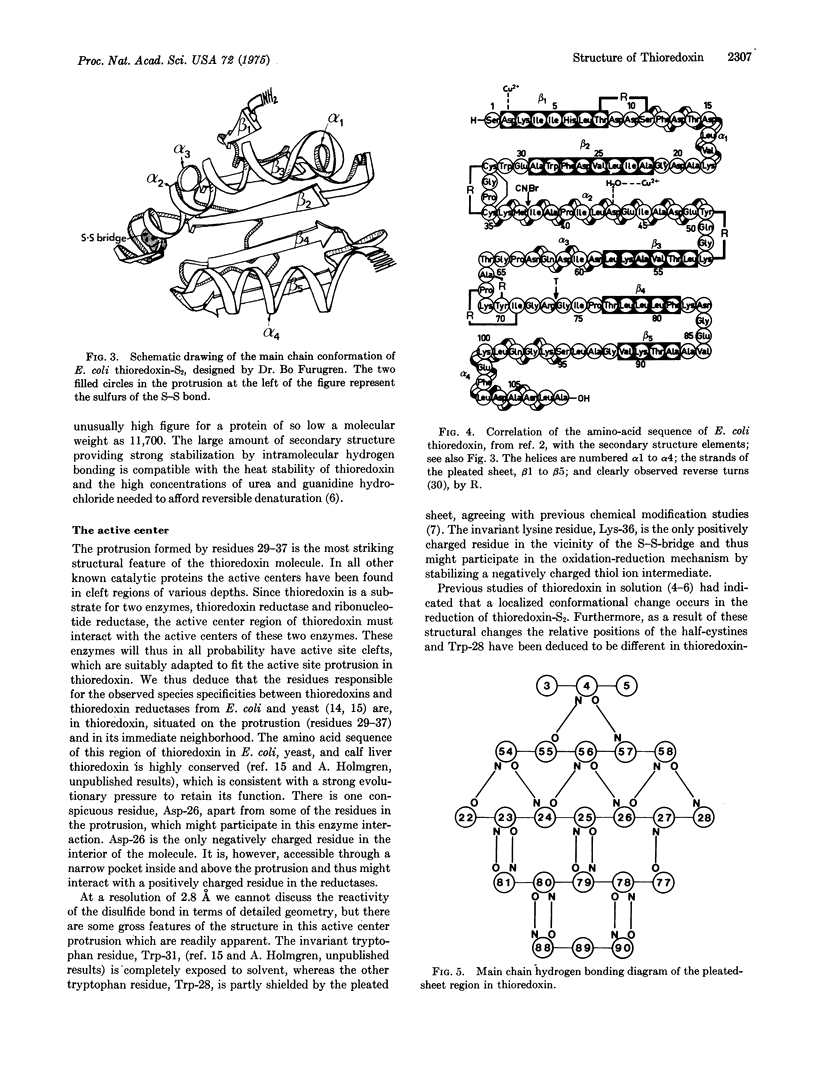
The three-dimensional structure of the electron transport protein thioredoxin-S2 from E. coli has been determined from a 2.8 A resolution electron density map. The molecule is built up of a central core of three parallel and two antiparallel strands of pleated sheet surrounded by four helices. Thr residues involved in the active center 14-membered disulfide ring of thioredoxin form a protrusion between one of the helices and the middle strand of the pleated sheet. This region of the molecule, comprising two parallel strands joined by the protrusion and a helix, is structurally very similar to corresponding functionally important regions in the nucleotide-binding domains of flavodoxin and the dehydrogenases. The molecule has about 75% of the residues in well-defined secondary structures. The structure indicates that the carboxy-terminal third of the molecule forms an independent folding unit consisting of two strands of antiparallel pleated sheet and a terminal alpha-helix. This agress with the noncovalent reconstitution experiments from thioredoxin peptide fragments. Thioredoxin is an example of a protein with the active center located on a protrusion rather than in a cleft, thus demonstrating the existence of male proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Ford G. C., Koekoek R., Lentz P. J., McPherson A., Jr, Rossmann M. G., Smiley I. E., Schevitz R. W., Wonacott A. J. Structure of lactate dehydrogenase at 2-8 A resolution. Nature. 1970 Sep 12;227(5263):1098–1103. doi: 10.1038/2271098a0. [DOI] [PubMed] [Google Scholar]
- Baril E. F., Jenkins M. D., Brown O. E., Laszlo J., Morris H. P. DNA polymerases I and II in regenerating rat liver and Morris hepatomas. Cancer Res. 1973 Jun;33(6):1187–1193. [PubMed] [Google Scholar]
- Blake C. C., Evans P. R. Structure of horse muscle phosphoglycerate kinase. Some results on the chain conformation, substrate binding and evolution of the molecule from a 3 angstrom Fourier map. J Mol Biol. 1974 Apr 25;84(4):585–601. doi: 10.1016/0022-2836(74)90118-1. [DOI] [PubMed] [Google Scholar]
- Bryant T. N., Watson H. C., Wendell P. L. Structure of yeast phosphoglycerate kinase. Nature. 1974 Jan 4;247(5435):14–17. doi: 10.1038/247014a0. [DOI] [PubMed] [Google Scholar]
- Brändén C. I., Eklund H., Nordström B., Boiwe T., Söderlund G., Zeppezauer E., Ohlsson I., Akeson A. Structure of liver alcohol dehydrogenase at 2.9-angstrom resolution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2439–2442. doi: 10.1073/pnas.70.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehner M., Ford G. C., Moras D., Olsen K. W., Rossman M. G. D-glyceraldehyde-3-phosphate dehydrogenase: three-dimensional structure and evolutionary significance. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3052–3054. doi: 10.1073/pnas.70.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett R. M., Darling G. D., Kendall D. S., LeQuesne M. E., Mayhew S. G., Smith W. W., Ludwig M. L. The structure of the oxidized form of clostridial flavodoxin at 1.9-A resolution. J Biol Chem. 1974 Jul 25;249(14):4383–4392. [PubMed] [Google Scholar]
- Engström N. E., Holmgren A., Larsson A., Söderhäll S. Isolation and characterization of calf liver thioredoxin. J Biol Chem. 1974 Jan 10;249(1):205–210. [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. Purification of a thioredoxin system from yeast. J Biol Chem. 1970 May 10;245(9):2363–2370. [PubMed] [Google Scholar]
- Hall D. E., Baldesten A., Holmgren A., Reichard P. Yeast thioredoxin. Amino-acid sequence around the active-center disulfide of thioredoxin I and II. Eur J Biochem. 1971 Nov 11;23(2):328–335. doi: 10.1111/j.1432-1033.1971.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Effects of oxidation of tryptophan residues in thioredoxin from Escherichia coli by N-bromosuccinimide. J Biol Chem. 1973 Jun 10;248(11):4106–4111. [PubMed] [Google Scholar]
- Holmgren A., Soderberg B. Crystallization and preliminary crystallographic data for thioredoxin from Escherichia coli B. J Mol Biol. 1970 Dec 14;54(2):387–390. doi: 10.1016/0022-2836(70)90437-7. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin-C': Reconstitution of an active form of Escherichia coli thioredoxin from two noncovalently linked cyanogen bromide peptide fragments. FEBS Lett. 1972 Aug 15;24(3):351–354. doi: 10.1016/0014-5793(72)80389-2. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Tryptophan fluorescence study of conformational transitions of the oxidized and reduced form of thioredoxin. J Biol Chem. 1972 Apr 10;247(7):1992–1998. [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Ohlsson I., Nordström B., Brändén C. I. Structural and functional similarities within the coenzyme binding domains of dehydrogenases. J Mol Biol. 1974 Oct 25;89(2):339–354. doi: 10.1016/0022-2836(74)90523-3. [DOI] [PubMed] [Google Scholar]
- Rao S. T., Rossmann M. G. Comparison of super-secondary structures in proteins. J Mol Biol. 1973 May 15;76(2):241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. An immunologic approach to the conformational equilibria of polypeptides. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3790–3794. doi: 10.1073/pnas.69.12.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz G. E., Elzinga M., Marx F., Schrimer R. H. Three dimensional structure of adenyl kinase. Nature. 1974 Jul 12;250(462):120–123. doi: 10.1038/250120a0. [DOI] [PubMed] [Google Scholar]
- Slaby I., Holmgren A. Reconstitution of Escherichia coli thioredoxin from complementing peptide fragments obtained by cleavage at methionine-37 or arginine-73. J Biol Chem. 1975 Feb 25;250(4):1340–1347. [PubMed] [Google Scholar]
- Soderberg B. O., Holmgren A., Branden C. I. Structure of oxidized thioredoxin to 4 with 5 A resolution. J Mol Biol. 1974 Nov 25;90(1):143–152. doi: 10.1016/0022-2836(74)90262-9. [DOI] [PubMed] [Google Scholar]
- Stryer L., Holmgren A., Reichard P. Thioredoxin. A localized conformational change accompanying reduction of the protein to the sulfhydryl form. Biochemistry. 1967 Apr;6(4):1016–1020. doi: 10.1021/bi00856a009. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K. D., Sieker L. C., Jensen L. H. The binding of riboflavin-5'-phosphate in a flavoprotein: flavodoxin at 2.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3857–3860. doi: 10.1073/pnas.70.12.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]