Abstract
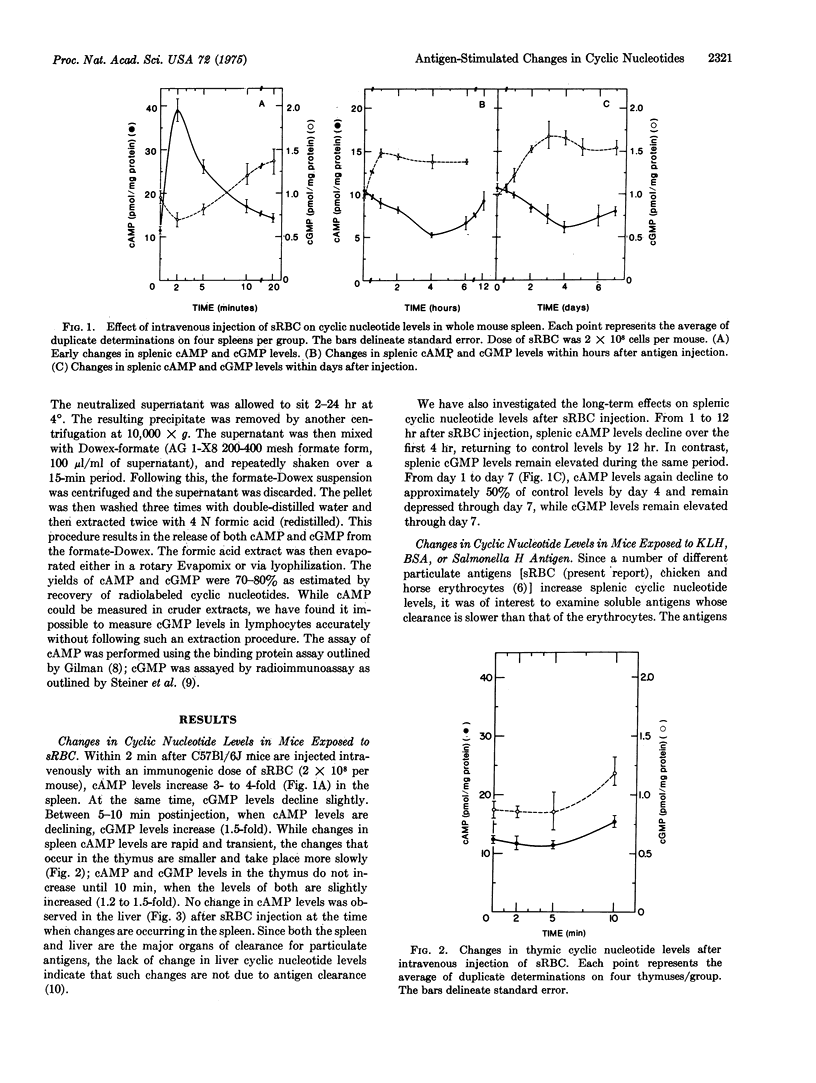
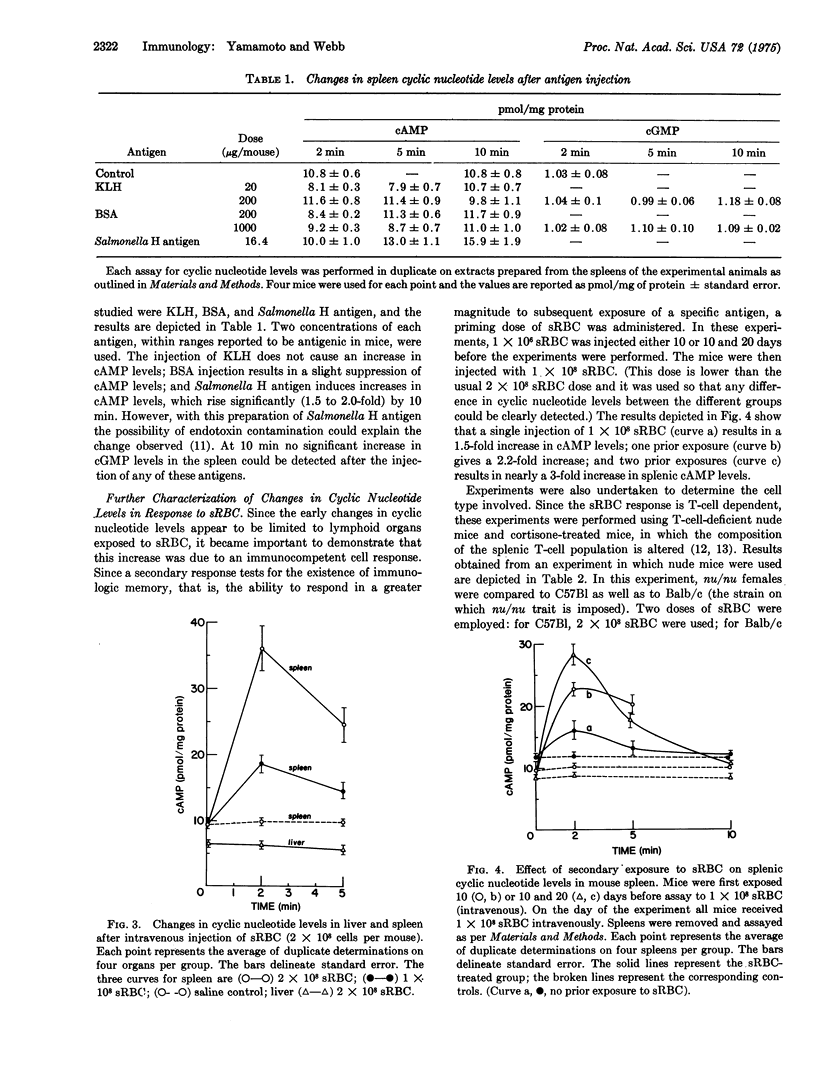
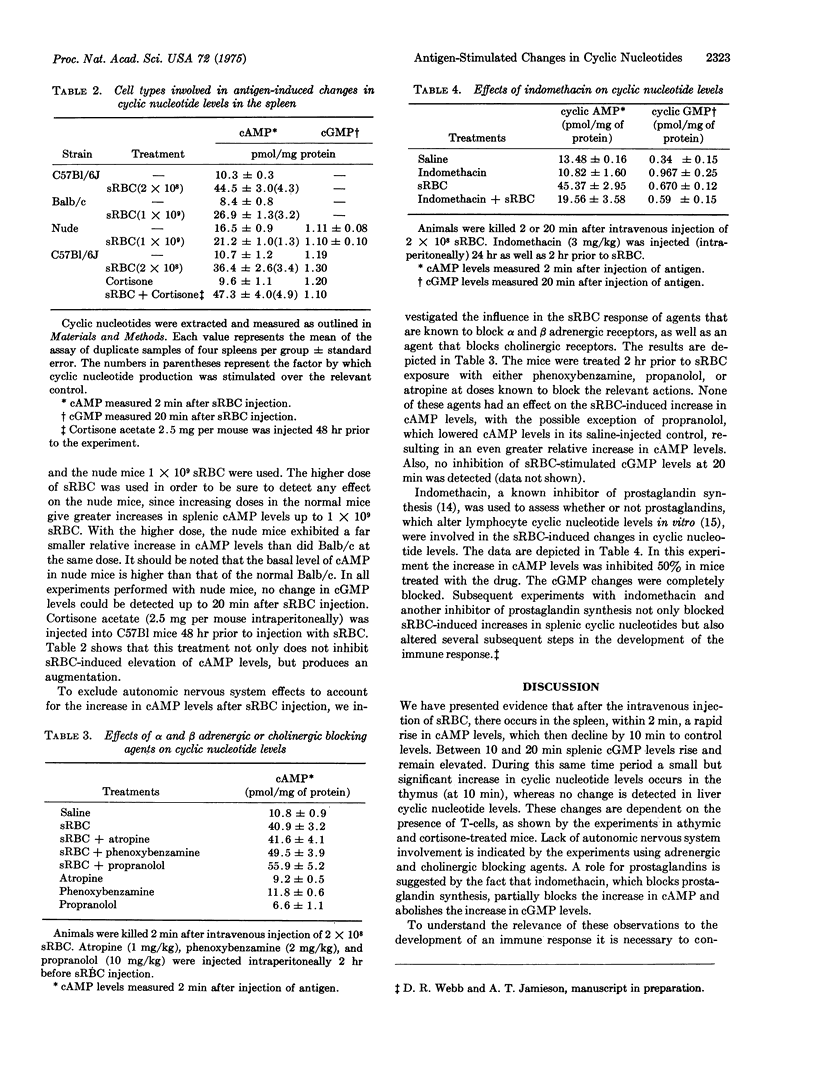
Mice injected intravenously with sheep erythrocytes (sRBC) demonstrate a transient increase in splenic cAMP levels (4-fold), which peak at 2 min after injection and return to basal levels by 20 min. In addition to the change in cAMP, an increase in splenic cGMP levels (1.5-fold) occurs beginning 5-10 min after sRBC injection, and persists for up to 7 days. During this period cAMP levels remain at or below control levels in the spleen. There is no change in 3':5' cyclic nucleotide levels in the liver and a small increase (1.2- to 1.3-fold) in the thymus at the time when splenic cyclic nucleotide levels are elevated. The changes in splenic cyclic nucleotide levels appear to be dependent on the presence of thymus-derived (T) lymphocytes, since little change occurs in cAMP and changes in cGMP are absent in athymic nude mice. In addition, cAMP levels were increased by pretreatment of normal mice with cortisone acetate, which selects for mature T lymphocytes. Agents that block autonomic nervous system functions have no effect on the early sRBC-induced changes in cyclic nucleotide levels.Indomethacin, an inhibitor of prostaglandin synthesis, reduces the change in cAMP level by 50% and blocks the change in cGMP levels completely. Secondary stimulation with sRBC results in a larger increase in cAMP levels than that seen with a primary injection of sRBC, indicating the presence of specific antigen-sensitive memory cells. Changes in splenic cyclic nucleotide levels cannot be detected at early times after the injection of soluble protein antigens such as bovine serum albumin and keyhole limpet hemocyanin. Salmonella H antigen stimulates a 2-fold increase in cAMP levels, the increase occurring more slowly than with sRBC. The in vivo changes incyclic nucleotide levels are correlated with known changes in cyclic nucleotide levels which have been documented in vitro in both T-cells and T-cell-dependent-B-cell (bone-marrow derived) antibody responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Melmon K. L. Adenyl cyclase in human leukocytes: evidence for activation by separate beta adrenergic and prostaglandin receptors. J Pharmacol Exp Ther. 1971 Jul;178(1):1–7. [PubMed] [Google Scholar]
- Cohen I. R., Stavy L., Feldman M. Glucocorticoids and cellular immunity in vitro. Facilitation of the sensitization phase and inhibition of the effector phase of a lymphocyte anti-fibroblast reaction. J Exp Med. 1970 Dec 1;132(6):1055–1070. doi: 10.1084/jem.132.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Claman H. N. Thymus-marrow immunocompetence. V. Hydrocortisone-resistant cells and processes in the hemolytic antibody response of mice. J Exp Med. 1971 May 1;133(5):1026–1034. doi: 10.1084/jem.133.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Franzl R. E. The primary immune response in mice. 3. Retention of sheep red blood cell immunogens by the spleen and liver. Infect Immun. 1972 Oct;6(4):469–482. doi: 10.1128/iai.6.4.469-482.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook A. I., Trainin N. Hormone-like activity of a thymus humoral factor on the induction of immune competence in lymphoid cells. J Exp Med. 1974 Jan 1;139(1):193–207. doi: 10.1084/jem.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes E., Shearer G. M., Melmon K. L., Bourne H. R. In vitro correction of antigen-induced immune suppression: effects of poly(A) poly(U) and prostaglandin E. Cell Immunol. 1973 Nov;9(2):226–233. doi: 10.1016/0008-8749(73)90073-7. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Yamamoto I., Shimamura T., Feit C. Early cellular events in the response of mice to sheep red blood cells reflected by changes in the spleen level of cyclic amp. Ann N Y Acad Sci. 1975 Feb 28;249:362–369. doi: 10.1111/j.1749-6632.1975.tb29084.x. [DOI] [PubMed] [Google Scholar]
- Watson J., Epstein R., Cohn M. Cyclic nucleotides as intracellular mediators of the expression of antigen-sensitive cells. Nature. 1973 Dec 14;246(5433):405–409. doi: 10.1038/246405a0. [DOI] [PubMed] [Google Scholar]


