Abstract
There are several studies about the cytotoxic effects of dental materials in contact with the pulp tissue, such as calcium hydroxide (CH), adhesive systems, resin composite and glass ionomer cements. The aim of this review article was to summarize and discuss the cytotoxicity and biocompatibility of materials used for protection of the dentin-pulp complex, some components of resin composites and adhesive systems when placed in direct or indirect contact with the pulp tissue. A large number of dental materials present cytotoxic effects when applied close or directly to the pulp, and the only material that seems to stimulate early pulp repair and dentin hard tissue barrier formation is CH.
Keywords: Cytotoxicity, Dental pulp capping, Fibroblasts, Odontoblasts
INTRODUCTION
The main purpose of restorative dentistry is to restore and maintain tooth health by an adequate restorative treatment in order to protect and re-establish pulp function. Pulp plays an important role in the formation and nutrition of dentin as well as in the inervation and defense of the teeth (Figure 1).
FIGURE 1. General view of the dentin-pulp complex. Below the tubular dentin (D) there is the predentin (Pd), which is underlined by the odontoblast layer (Od). Observe the cellfree zone of Weil – horizontal arrow) and the cell-rich zone (vertical arrows). HE, ×64.
The primary pulp function is dentin formation, which begins in the moment that the peripheric mesenchimal cells differentiate into odontoblasts and starts the deposition of collagen matrix, in a sequence of deposition/mineralization that ends with the complete tooth formation. Even after the initial formation, pulp continues to physiologically produce dentin due to the tooth aging. Reparative dentin may also be produced in response to physical and/or chemical injuries. Odontoblasts maintain their processes inside the newly formed tissue, thus creating real channels that are responsible for dentin nutrition. This is a continuous process while the pulp is biologically active. The transportation of fluids and nutrients maintains pulp vitality and the resilience necessary to neutralize the masticatory tension/stress of the dentin. Finally, the pulp is responsible for the response to different stimuli, which forms the defensive action and includes blood vessel dilatation and permeability, and the presence of inflammatory cells. When the stimulus does not exceed the pulp healing capacity, modification in the dentin-pulp complex may occur, including repair67.
The protection of the dentin-pulp complex consists of the application of one or more layers of specific material between the restorative material and dental tissue to avoid additional challenge to the pulp tissue caused by operative procedures, toxicity of restorative materials and bacteria penetration due to microleakage. Protection of the dentinpulp complex has also the function to recover pulp vitality8. The materials that can be used for this purpose are varnishes, calcium hydroxide (CH)-based products, glass ionomer cements (GICs) and adhesive systems.
The biological compatibility of dental materials is of paramount importance to avoid or limit pulp tissue irritation or degeneration. Cytotoxicity and biocompatibility of materials used in dentistry have been widely studied in different cell cultures or in deep cavities with or without pulp exposure. The aim of this review article was to summarize and discuss the cytotoxicity and biocompatibility of materials used for protection of the dentin-pulp complex, some components of resin composites and adhesive systems when in direct or indirect contact with the pulp tissue.
LITERATURE REVIEW
Pulp damage and repair
Pulp tissue is constantly subjected to environmental impacts. These potential aggressions include microbial toxins, heat, mechanical trauma, restorative materials, cleaning solutions and cavity liner cements. Following various injuries, pulp cells have the intrinsic capacity to repair, differentiate into odontoblasts and produce various dentin matrix proteins during wound healing11. Thus, not all the inflammatory reaction results in permanent damage.
Some studies have shown that gingival38,51 and pulp5,13,84 fibroblasts can secret many types of cytokines in response to various stimuli in the same manner as odontoblasts, endothelial cells and immune cells27,65,78,82. Cytokines are proteins secreted by innate or adaptative immune cells that act stimulating these cells. Different cytokines are produced in response to microorganisms and other stimuli, triggering several responses in immune and inflammatory cells. Some cytokines have structural homology that stimulate leukocyte dislocation and regulate the migration of blood to the tissue, which are denominated chemoattractant cytokines. These are produced by various cell types in response to inflammatory stimuli, and consequently recruit leukocytes to the inflammation area1. Some studies have shown that pulp fibroblasts are real sources of chemokines59,61,85.
When the injury is removed before pulp damage, a repair process begins81 and collagen synthesis is accellerated in this phase 62. The deposition of collagen in the pulp tissue is increased by the action of cytokines. Collagen synthesis can be improved by transforming growth factor (TGF)-β1 and TGF-β2 and interleukin (IL)-1β5,11. Fibroblasts at an inflammatory microenviroment deposit collagen in a remarkable amount during the inflammatory process as opposed to normal conditions5. The collagen synthesis by fibroblasts during the inflammatory process is a key event for human pulp repair11.
CH and mineral trioxide aggregate (MTA)
CH solutions have been largely used with increased frequency, due to their property of stimulating sclerotic and reparative dentin formation and protecting the pulp against termal stimuli and antibacterial action30,76. The first formulation of CH was introduced in dentistry by Hermann (1920), and presented the capacity to induce pulp tissue to form a mineralized barrier, blocking the exposed surface. CH is a multipurpose agent and there are several indications for its clinical application. Some of its indications include direct and indirect pulp capping, apexogenesis, apexification and treatment of root resorption, iatrogenic root perforations, root fractures, replanted teeth and interappointment intracanal dressing28. CH is certainly one of the most studied dental materials and it is classically used as the gold standard in biocompatibility tests due to its direct or indirect effect on exposed pulp repair. It is the material of choice for all pulp conservative treatment because of its biological and therapeutic potential25.
CH, in dry powder, suspension or cement form, has been recommended for the treatment of exposed pulp due to its beneficial properties, such as induction of mineralization and inhibition of bacterial growth9. Many studies indicate pulp repair and hard tissue barrier formation when exposed pulp tissue is directly capped with different CH formulations (Figures 2 and 3). In the clinical practice, the presence of hard tissue barrier after capping can be considered an asset, since it provides natural protection against the infiltration of bacteria and chemical products44.
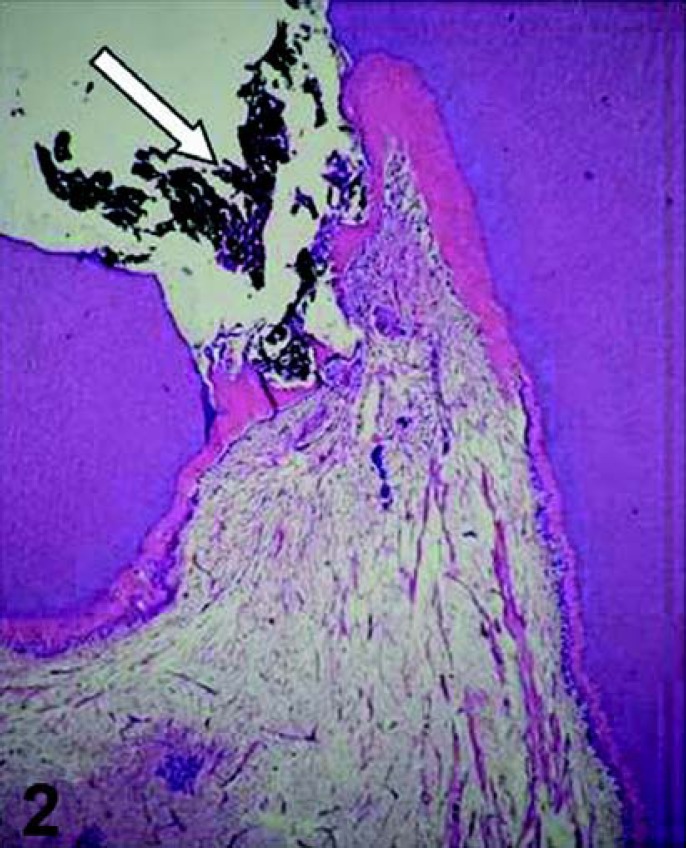
FIGURE 2. Pulp exposure capped with calcium hydroxide (arrow). Observe that 30 days after the pulp therapy, a partial hard tissue barrier was formed adjacent to the capping agent. HE, ×32.
FIGURE 3. Sixty days after applying calcium hydroxide on the pulp tissue, a complete hard tissue barrier (HB) is formed. Note the tunnel defect (horizontal arrow) and cellular inclusions (vertical arrows) within the hard tissue barrier, which is underlined by a new layer of odontoblast-like cells. HE, ×125.
However, the importance of calcified hard tissue barrier formation after capping has been challenged by other studies, which have shown multiple tunnel defects and cell inclusions in bridges following pulp capping with CH. This may lead to leakage and bacteria penetration into pulp tissue unlike the permanent seal produced by bonding agents37,69. These tunnels not caused by the CH itself but are rather a consequence of the severity of the trauma to the pulp and the number of vessels injured during the mechanical exposure. It has been observed that inside the tunnels there are blood vessels, which maintain the calcium source to the necrotic tissue. The calcium ions in the necrotic layer are responsible for partial dystrophic calcification of the coagulation necrosis. Another type of defect in hard tissue barriers, when present, is represented by cellular inclusions generally situated between the coagulation necrosis and the calcification zone69. The presence of a hard tissue barrier must be recognized not only as a structural barrier against future injuries, but also as a sign of biological recovery, represented by odontoblast activity76. Yoshiba, et al.86 reported findings that support the hypothesis that the differenciation of pulp cells into odontoblasts during reparative dentinogenisis is mediated by fibronectin, which is associated with the initially formed calcified layer after pulp capping with CH86.
Capping of contaminated and inflamed dental pulps results in a remarkably high degree of healing, characterized by resolution of inflammation, reorganization of soft tissue, and formation of new hard tissue at the exposed site76.
The mechanism of pulp repair using CH as a direct pulp capping agent is still not well understood. However, it has been reported that the high alkaline pH of CH solutions can solubilize and release some proteins and growth factors from dentin. These events may be responsible for the pulp repair and hard tissue barrier formation40. Due to its high pH, CH induces a coagulation necrosis layer when in direct contact with pulp tissue43. CH products do not act as bioestimulators neither are biocompatible to the pulp tissue. On the contrary, cells in contact with CH are killed due its alkaline pH, forming a necrotic layer (cauterization zone) of variable thickness. Then, rather than the pulp capping agent (CH), the subjacent pulp tissue is responsible for the pulpal healing associated with hard tissue barrier formation. Similar effects on pulp exposures are caused by cements that present high pH, such as different formulations of MTA cements32.
Classical microscopic studies have shown that CH produces a superficial pulp necrosis and forms calcium carbonate, whose globules act, in a first moment, as dystrophic calcification nucleous, in the margin and in the interior of the dense reticular fiber deposition, immediately beneath the granular zone43, where odontoblast-like cells differentiate and organize to produce dentin. The cauterization effect of CH is essential for the repair of exposed pulp68.
It has been suggested that the pH increases due to the presence of free hydroxyl ions may initiate mineralization80, although other alkaline compounds, such as barium hydroxide and calcium phosphate, failed in this process55. The alkaline pH can neutralize the lactic acid secreted by osteoclasts and may help preventing mineral tissue destruction. CH can act as a local buffer against the acid reactions produced by the inflammatory process42. Heithersay31 (1975) suggested that the calcium ions can reduce capillary permeability, thus low intercellular fluid produces increasing calcium ions concentration at the mineralization area42.
In spite of all these advantages, CH is soluble in water and acid and its physical properties are deficient76. The multiple tunnel defects present a morphological disruption of the dentin bridge barrier, in that they fail to provide not only a solid barrier, but also a long-term biological seal against bacterial infection. Consequently, the tunnels permit oral contaminants, such as bacteria and their toxic factors, to eventually gain access to the pulp tissue through the marginal gap formed at tooth/restoration interface76. The presence of bacteria and their products that penetrate via microleakage, and not the medicament per se, is the main factor responsible for pulp inflammation and necrosis76.
MTA materials are a mixture of a refined Portland cement and bismuth oxide, and are reported to contain trace amounts of SiO2, CaO, MgO, K2SO4, and Na2SO4 24. Although it may be inferred that Portland cement could serve as a MTA substitute, it is important to emphasize Portland cement and MTA are not identical materials70. Few reports exist on the clinical applicability of Portland cement14,71. MTA products have been reported to have a smaller mean particle size, contain fewer toxic heavy metals, has a longer working time, and undergo additional processing/purification than regular Portland cements47. Up to 2002, only one MTA cement, consisting of a graycolored powder, was commercially available. In the same year, white MTA (WMTA) was introduced as ProRoot-MTA (Dentsply, Tulsa, OK, USA) to address esthetic concerns24. After that time, two forms of MTA materials were categorized: the traditional gray MTA (GMTA) and WMTA. Scanning electron microscopy (SEM) and electron probe microanalysis characterized the differences between GMTA and WMTA, and revealed that the major difference between them is in the concentrations of Al2O3, MgO, and FeO24. WMTA was also reported to possess an overall smaller particle size than GMTA26, while it was also suggested that the reduction in magnesium could also contribute to the lighter color of WMTA24.
Calcium release from MTA materials diminishes slightly over time26. MTA materials were reported to form a porous matrix characterized by internal capillaries and water channels in which increased liquid/powder mixing ratio produced more porosity and increased solubility33. GMTA solubility levels have been reported to be stable over time, but the usually reported pH between 11 or 12 may slightly decrease32. The high pH level of MTA materials has led some authors to theorize that the biologic activity and its biocompatibility is due to the formation of CH26,32,33. Both WMTA and GMTA have been shown to possess antibacterial and antifungal activities, which are presumably due to its pH.
The cytotoxicity of GMTA, amalgam and ZOE was measured using a cell viability assay for mitochondrial dehydrogenase activity in human periodontal ligament fibroblasts after 24 h of exposure to extracts of varying concentrations of the tested materials, in both freshly mixed and 24-h set states49. In the freshly mixed state, the sequence of toxicity was amalgam > Super-EBA > MTA. In the 24-h set state, the sequence of toxicity at a low extract concentration was Super-EBA > MTA, amalgam; while at higher extract concentrations it was Super-EBA> amalgam > MTA49. Similarly, another report reinforced that GMTA did not affect negatively human periodontal ligament fibroblast mitochondrial dehydrogenase activity52. WMTA, as well as CH and a ZOE sealer, was shown not to affect the cell viability or the prostaglandin E2 synthesis of murine macrophages and fibroblasts54. WMTA effect on dental pulp cell viability and proliferation has been evaluated using mouse MDPC-23 odontoblast-like cells and OD-21 undifferentiated pulp cells. After 24 h of exposure to WMTA, apoptosis was not induced in either cell line, and WMTA was reported to cause increase in DNA synthesis, suggesting a positive effect on cellular proliferation56. This was corroborated by another report that suggested that WMTA had a more stimulating effect on human dental pulp cells than a commercial CH preparation79. Some reports speculated that MTA material biocompatibility was derived from CH formation24,33.
A prospective study compared CH and GMTA as permanent dentition pulp-capping medicaments and concluded that CH specimens were hallmarked by tissue inflammation with a 0.15-mm thick dentinal bridge with adjacent pulp tissue necrosis noted at 6 months2. These findings were in contrast with those for GMTA specimens displaying mild tissue reactions with a 0.28-mm and 0.43mm dentin bridge noted at 2 and 6 months, respectively, as well as absence of pulp tissue inflammation, associated with a near-regular odontoblastic layer2. However, the authors did acknowledge a small sample size and the need for further studies. A second prospective study compared WMTA and a CH preparation as direct pulp cap medicaments and concluded that at 30 post-treatment days, WMTA group had 20 teeth with clinically normal pulpal status while three were diagnosed with reversible pulpal disease48. CH group showed 17 teeth with normal pulpal signs, 6 exhibited signs of reversible pulpal disease, and 1 was diagnosed with irreversible pulpal disease48. At the 136th recall day, all 23 teeth present for the WMTA group were clinically diagnosed as successful as well as 22 teeth of the CH group48. For use of MTA materials as direct pulp cap medicaments, the two clinical prospective studies suggest that both GMTA and WMTA may perform equally in comparison to traditional CH in non-carious mechanical pulp exposures in teeth with normal pulp tissue70. Although the initial results are positive, further clinical studies are needed, especially in more clinically relevant situations involving carious pulp exposures before MTA materials can be unequivocally indicated as direct pulp-capping agents70. The histologic pulpal response comparing WMTA to CH as pulpotomy dressings was investigated in premolars extracted for orthodontic purposes, reporting that WMTA induced a more homogenous and continuous dentin bridge with less pulpal inflammation than CH at both 4 and 8 weeks after treatment10.
Reports have strongly suggested that the favorable biologic performance exhibited by MTA materials is due to hydroxyapatite formation when these materials are exposed to physiologic solutions. Although the overall results in human studies involving MTA materials are very positive, further longitudinal studies are encouraged, as at present insufficient well-designed and controlled clinical studies exist that allow systematic and meta-analysis review of MTA materials in all of its suggested clinical indications57,70.
Adhesive systems, resin composite and cell response
Although physical properties of resin composites are being improved constantly, in vivo studies have shown that the use of resins as restorative materials is occasionally associated with irritation and necrosis of the pulp6,75,77 periodontal tissues60. Most components of the adhesive systems and resin composites, such as bisphenol A-glycidyl methacrylate (Bis-GMA), urethane dimethacrylate (UDMA), triethylene glycol dimethacrylate (TEG-DMA), camphoroquinone, 2-hydroxyethyl methacrylate (HEMA) and others, have been shown to have definite cytotoxicity when in direct contact with mammalian fibroblasts39. In that study39, the most cytotoxic monomers were Bis-GMA and UDMA, which caused irreversible effects on the cellular metabolism39. These monomers, when applied on dentin discs, even in the presence of internal pressure, are able to diffuse through the dentinal tubules and reach the pulpal space in concentrations directly proportional to the molecular weight of the monomeric materials7. Resin composite components can be leachable when the degree of conversion is not reached29 or when the resin is degraded by esterase from saliva or when hydrolytic degradation occurs31.
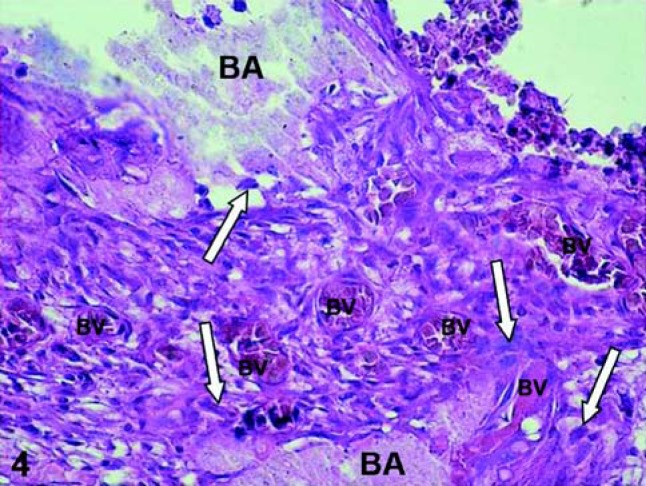
Adhesive resin systems are used to enhance retention, reduce microleakage, and decrease postoperative sensitivity of composite resin restorations. In vivo studies have demonstrated that the application of an adhesive resin directly onto a site of pulp exposure, or to a thin layer of dentin (less than 0.5 mm), causes dilatation and congestion of blood vessels as well as chronic inflammatory pulpal response (Figure 4)40,41. Complete polymerization of adhesive resins might be unachievable during direct pulp-capping procedures due to the presence of the pulpal edema. In additiom, it has benn shown that the oxygen prevents complete polymerization of adhesive resin monomers34. Consequently, unpolymerized monomers released from the resin-based material can diffuse directly into the pulp at the exposure site, as well as diffuse through the dentinal tubules to cause cytotoxic effects to the pulp cells66.
FIGURE 4. Exposed human pulp tissue capped with an adhesive system. Sixty days after the capping procedure the tooth was extracted and processed for histological evaluation of the pulp tissue. Note the presence of bonding agent (BA) on the pulp exposure site and inside of the connective tissue (BA – arrow). Intense chronic inflammatoty reaction mediated by macrophages and a number of dilated and congested blood vessles (BV) is observed. HE, ×320.
There is a dramatic difference in the responses of cells to the three conditions of polymerization (light curing for 0, 10 or 40 s). While unpolymerized and partially polymerized adhesive resin induced apoptosis very rapidly in macrophages, undifferentiated pulp cells (OD-21) and mouse odontoblast-like cells (MDPC-23), polymerized adhesive resin induced significant apoptosis only in macrophages. These findings might be explained by the lower leaching of toxic elements from polymerized as compared with unpolymerized adhesive resins, and underline the importance of thorough polymerization of the adhesive system before placement of the resin composite53.
Although many resins present excellent physical and mechanical properties, when irradiated for short periods of time or with a low light intensity, inadequate conversion of the monomers may interfere negatively on mechanical properties and increase the resin cytotoxicity. Costa, et al.18, 2003, in an in vitro study evaluated the cytotoxic effects of a restorative resin composite applied to an immortalized odontoblast cell line. The results suggest that the amount of unconverted monomers on dentin should be reduced to avoid diffusion of the residual monomers to the pulpal space, resulting in chemical damage to the pulp tissue. This may be accomplished by treating appropriated increments of restorative resin with adequate light intensity and the time of light curing, which will play an important role in converting uncured monomers into polymers18.
Bonding agents have been found to release camphoroquinone, a photoinitiator and photosensitizer widely used to generate free radicals including reactive oxygen species46. It has been documented that the camphoroquinone acts not only as a cytotoxic agent, but also as a mutagen and its lixiviation may partly explain why these kind of resinous products are considered as toxic agents46.
Although clinical and in vivo studies have shown a low incidence of unfavorable effects of dentin bonding systems, pathological changes of pulpal tissues, such as dilatation and congestion of blood vessels, inflammatory responses and production of irregular dentin as well as odontoblastic displacement or tooth sensitivity can occur after placement of composite restorations75. The monomers in contact with oxygen are not converted into polymers and will remain at the outer layer of the adhesive systems29. The unconverted monomers of the adhesive systems and resin composite in the cavity may diffuse to the pulp through dentinal fluid66 and may cause adverse effects35. In addition, dentin bonding agents may be responsible for undesirable pulpal reactions when placed directly on the pulp or in deep cavities, due to the presence of Bis-GMA, which is the major component of most current bonding systems. Bis-GMA is easily solubilized from polymerized resins by solvents such as ethanol39. These pulp reactions can be related to components of the adhesive resins (TEGDMA and HEMA) that were shown to be soluble in aqueous solutions and cytotoxic to immortalized 3T3-fibroblast cultures36.
The high concentration of HEMA present in primers and adhesive resins available in the dental market may promote remarkable cytopathic effects on cultured pulp cells, even after curing and rinsing the experimental materials in order to decrease the concentration of acidic and non-acidic agents, which are common ingredients of dentin adhesive resins22. When the adhesive system (Scotchbond MP; 3M/ESPE-Dental Products, St Paul, MN, USA) and the monomer HEMA (Polysciences, Inc., Warrington, PA, USA) were implanted in subcutaneous tissue of rats, persistent inflammatory reaction was observed adjacent to the material. This persistent inflammatory response was mediated by macrophages and giant cells and may be caused by partially or unpolymerized resin particulates that remain in contact with the connective tissue21. In another study in subcutaneous tissue of rats, the authors concluded that the adhesive systems applied directly on the tissue promote persistent inflammatory reaction that interfere with the healing, which indicates that these materials cannot be considered as biocompatible16. In addition, when the adhesive system is applied directly on exposed human pulps, it has been shown that there is no formation of hard tissue barrier or pulp tissue repair, but rather a persistent foreign-body reaction characterized by macrophages and giant cells adjacent to the pulp exposure site3.
Several studies have suggested that an etch-and-rinse/bonding treatment of pulp exposures may also lead to tissue repair23. These studies support the hypothesis that pulps can heal after placement of acidic restorative system in deep cavities or even on exposed pulps, as long as the hemorrhage is controlled before placement of the adhesive system64 and a hermetic seal against bacterial infiltration is guaranteed69. This concept is popular despite the fact that several in vitro and in vivo studies have shown the definite cytotoxicity of resin-based composites and their components applied to cell culture or in direct contact with subcutaneous tissue or pulp tissue in animals69. In human pulps, direct capping with bonding systems has shown different degrees of pulp inflammation, regardless of the presence of bacteria40,69. These studies describe a persistent chronic inflammation with a considerable number of giant cells surrounding residues of adhesive scattered into the pulp tissue close to the exposure after a postoperative period of 180 days.
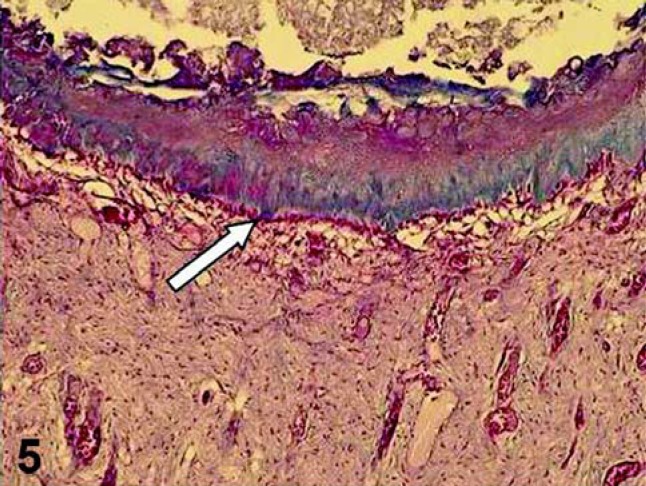
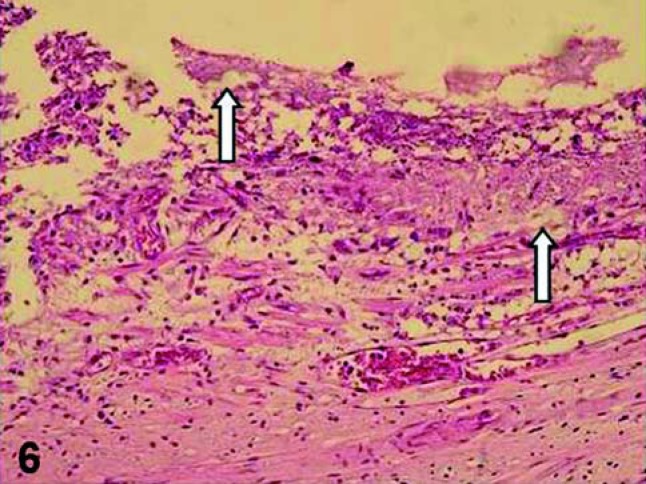
There are remarkable differences between the pulp responses to capping procedures with adhesive system and dental products with high pH, such as MTA and CH (Figures 5 and 6). These differences are related to the quality of pulp tissue, the nature and the degree of the inflammatory process and the quality of tissue repair after pulp capping. In short-term periods, pulps capped with the adhesive systems exhibited different degrees of inflammation in which mononucleated inflammatory cells predominate and the odontoblastic layer subjacent to the pulp exposure site is disrupted or sometimes absent, indicating a low tolerance of these cells close to the adhesive system69.
FIGURE 5. Pulp capped with a dental material that present high pH (MTA). Sixty days after the pulp therapy, a defined hard tissue barrier was formed, which was underlined by a new layer of odontoblast-like cells (arrow). Masson's Trichrome, ×86.
FIGURE 6. Pulp exposure capped with an adhesive system. Note that no hard tissue barrier was formed 60 days after the pulp therapy. Fragments of resinous material (arrows) are observed displaced to the pulp exposure site, which exhibits persistent inflammatory response. HE, ×125.
In a previous study69, the histological features of pulps capped with CH were different, with the migration of pulp cells subjacent to the pulp exposure site as early as 9 days after capping. The adjacent odontoblast layer, cell-free zone of Weil and the cell-rich zone were well preserved as well as the deeper structures of the pulp. These early manifestations of pulp repair coincided with a high rate of success found at the longest periods, when a normal pulp and a complete bridge formation were common events69.
Only a few studies with human teeth have been performed to indicate whether or not dental materials can be used for pulp capping of dental cavities with or without pulp exposure. An adhesive system and a CH paste were placed directly on exposed pulps40 and after seven days, a large area of neutrophil infiltrate underlying the adhesive system and death of adjacent odontoblasts were observed. The neutrophil reaction was replaced by fibroblast proliferation with macrophages and giant cells surrounding globules of resin scattered in the coronal pulp tissue. The persistent inflammatory reaction and hyaline alteration of extracellular matrix inhibited complete pulp repair or dentin bridging. In contrast, at the 7th day, the pulp tissue capped with CH exhibited odontoblast-like cells organized underneath a zone of coagulation necrosis, pulp repair and apparent complete dentin bridge formation after 60 days. These findings suggeste that adhesive systems seem to be indicated for direct pulp capping of human teeth40.
There is a controversy concerning the use of animals for evaluation of the biocompatibility of dental materials. However, these studies remain the only acceptable manner of testing the biocompatibility19. The self-etching adhesive system Clearfil Liner Bond 2 V (Kuraray Co., Tokyo, Japan) and the resin-modified glass-ionomer cement Vitrebond (3M/ESPE) allowed pulpal healing characterized by cell-rich fibrodentin and tertiary dentin deposition as well as calcified barrier formation19. These results were obtained when these materials were placed on pulp exposures in class I cavities prepared on the occlusal surface of maxillary first molars of rats and compared with CH as control group. In the control group, it was observed an intense deposition of tubular reparative dentin continuous with the reactionary dentin deposited by primary odontoblasts around the pulp exposure site that gave rise to a defined calcified barrier19. The histological features observed in this specific in vivo study performed in rat teeth confirmed that the results observed in animal pulps cannot be directly extrapolated to human beings.
GICs and cell response
GIC were developed by Wilson and Kent, in 1971, and introduced in the market in the early 1970s. Their popularity is due to the fact that these materials present several important properties such as fluoride release, coefficient of thermal expansion and modulus of elasticity similar to dentin, bonding to both enamel and dentin and biocompatibility61. Despite these advantages, conventional GICs possess limitations as restorative materials, which are related to their susceptibility to dehydration12 and poor physical properties, such as high solubility and slow setting rate58. Developments in the field of GICs have led to the introduction of light-activated hybrid GIC versions creating the resin-modified GICs (RMGICs)72. The incorporation of polymerizable water-compatible monomers such as HEMA to the formulation of conventional GICs resulted in enhanced flexural strength, diametral tensile strength, elastic modulus and wear resistance83, although they may not be as biocompatible as conventional GICs74. The incorporation of HEMA to the formulation of conventional cements has been proven to increase their toxic effects and as a consequence, RMGICs have been shown to be more cytotoxic than conventional GICs4. Although the degree of monomer conversion of the RMGICs has not been determined, several studies have demonstrated that measurable quantities of HEMA are released into the storage solutions used4. Leached residual HEMA can easily diffuse through the dentinal tubules due to its hydrophilicity and low molecular weight, thus reaching dental pulp cells4. The magnitude of the damage that may be caused by residual monomers to the pulp cells is inversely proportional to the remaining dentin thickness between the cavity floor and the pulp tissue4. One may expect that RMGICs might trigger an inflammatory reaction when applied directly in contact to the connective tissue73.
To compare the cytotoxicity of five RMGICs and one metal-reinforced GIC, Stanislawski, et al.74 carried out an in vitro pulp cell viability assay. The most toxic materials were the metal-reinforced GIC and RMGIC Vitremer (3M/ESPE), while the least toxic were the RMGICs Compoglass (Ivoclar Vivadent Ltda., São Paulo, SP, Brasil) and Photac Fil (3M/ESPE). This toxicity was due to the presence of unpolymerized monomers, such as HEMA and TEGDMA, and polyacrilic acid, which were leached from resin modified materials and metal-reinforced GIC. The main elements responsible for the toxicity of the metal-reinforced GIC were Cu2+ and Ag+ present in toxic concentrations. It was further analyzed the possible cytotoxicity of some ions that are present in significant amounts in GICs, such as F-, Al3+, Zn2+ and Sr2+. The zinc was the only component that was found to be of a sufficiently high concentration to induce cytotoxicity. These elements were present in the metal-reinforced GIC and may have contributed to its cytotoxicity74.
Complementing the results presented above, the cytotoxic effects of five RMGICs and conventional GICs on an odontoblast cell line were studied. The GICs were the least cytotoxic experimental materials and the RMGICs caused intense cytophatic effects on the cultured cells decreasing significantly the cell metabolism as well as causing remarkable cell death. The high cytotoxic effects observed for RMGICs in that study may be caused by unreacted resin monomers rather than by other compounds such as F-, Al3+, Sr2+, Zn2+ which are present in the conventional GICs17.
Although a true RMGIC must be capable of setting without being light-activated45, higher levels of released HEMA are found when these cements are only allowed to cure chemically4. The liner RMGIC Vitrebond (3M/ESPE) releases a high concentration of HEMA monomer before immersion in distilled water, even when polymerized according to the manufacturer's recommendations, when compared to the amount released from the restorative RMGIC Vitremer (3M/ESPE)4. Other RMGIC toxic components, such as fluoride, aluminum, silver, siliceous, strontium, zinc and silicate, may also be released during the setting reaction or solubilization of the cement in a wet environment4, but it was already mentioned that the ions released from the RMGIC are not sufficiently high to cause cytotoxic effects, except for Zn2+ 74.
Other studies affirm that conventional GICs are less cytotoxic than the RMGICs Vitrebond (3M/ESPE) and Vitremer (3M/ESPE), which caused intense cytophatic effects in cell cultures decreasing significantly the cell metabolism as well as causing remarkable cell death (Figures 7)17,63
FIGURE 7. Deep cavity was prepared in human premolar. In this figure it is shown the pulp tissue related to the cavity floor in which total etch was applied and an adhesive system and composite resin were used for cavity restoration. Sixty days after the clinical procedure the tooth was extracted and processed for histological evaluation. Note the intense inflammatory response as well as the zone of inner dentinal resorption (arrow). HE, ×160.
The adverse effect caused by Vitremer (3M/ESPE) was attributed to the leaching of at least two components of polyacidic phase (HEMA) as well as unidentified acidic species63. Other authors compared the toxicity of 9 types of GICs on cultured human dental pulp cells and concluded that RMGICs are more toxic to pulp cells than conventional GICs. Consequently, the application of RMGICs directly onto dental pulp cells is not recommended50. However, current in vivo studies performed in human teeth have demonstrated that the RMGIC Vitrebond (3M/ESPE) applied as a liner in very deep class V cavities caused no inflammatory pulp response15,20. In this way, it seems that the presence of a dentin barrier between this kind of light-cured RMGIC and the pulp cells may prevent pulpal damage.
Before initiating any restorative procedure, it is important to select the most appropriate dental materials to be used for each case. Attention should be taken not only to the handling characteristics of the materials, but also to the possible cytotoxic effects that they may cause to the oral mucosa and teeth. Within the experimental limitations of the ISO recommendations for in vivo testing of dental materials, which do not allow direct extrapolation of the results to clinical situations, researchers and clinicians must be aware that all clinical procedures in dentistry must be carried out based on scientific evidence.
CONCLUSION
Based on this literature review, it may be concluded that:
Calcium hydroxide products are the best choice for conservative treatment of the pulp due to their therapeutic and biological potential and the property of stimulating the formation of sclerotic and reparative dentin as well as protecting the pulp against thermal stimuli.
Monomers present in resin composites and adhesive systems (e.g.: BISGMA, UDMA, TEGDMA, HEMA) have been shown to have cytotoxic effects as a consequence of direct contact with fibroblasts and may be leached during the polymerization when the conversion degree is not fully reached.
In human pulps, direct pulp capping with adhesive systems produces different degrees of pulp inflammation, even without bacterial presence and absence of dentin bridge formation as well as pulp repair. Some studies support the idea that when hermetic seal of cavity is obtained, the dentinpulp complex protection materials are unnecessary and they not influence the pulp repair, but hermetic seal of the restoration is difficult to be obtained.
4RMGICs are more cytotoxic to the pulp cells than conventional GICs due to the presence of unpolymerized monomers, and should not be applied directly to the pulp tissue.
REFERENCES
- 1.Abbas A, Lichtman A. Cellular and molecular immunology. In: Abbas A, Lichtman A, editors. Cytokines. 5 ed. Philadelphia: Saunders; 2003. pp. 243–274. [Google Scholar]
- 2.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2002;36:225–231. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 3.Akimoto N, Momoi Y, Kohno A, Suzuki S, Otsuki M, Suzuki S, et al. Biocompatibility of Clearfil Liner Bond 2 and Clearfil AP-X system on nonexposed and exposed primate teeth. Quintessence Int. 1998;29(3):177–188. [PubMed] [Google Scholar]
- 4.Aranha AMF, Giro EMA, Souza PPC, Hebling J, Costa CAS. Effect of curing regime on the cytotoxicity of resin-modified glass-ionomer lining cements applied to an odontoblast-cell line. Dent Mater. 2006;22:864–869. doi: 10.1016/j.dental.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Barkhordar R, Ghani Q, Russell T, Hussain M. Interleukin-1beta activity and collagen synthesis in human dental pulp fibroblasts. J Endod. 2002;28(3):157–159. doi: 10.1097/00004770-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Baume LJ, Fiore-Donno G. Response of the human pulp to a new restorative material. J Am Dent Assoc. 1968;76:1018–1022. doi: 10.14219/jada.archive.1968.0150. [DOI] [PubMed] [Google Scholar]
- 7.Bouillaguet S, Wataha J, Hanks CT, Ciucci B, Holz J. In vitro cytotoxicity and dentin permeability of HEMA (2-hydroxyethyl methacrylate) J Endod. 1996;22:244–248. doi: 10.1016/s0099-2399(06)80141-x. [DOI] [PubMed] [Google Scholar]
- 8.Briso ALF, Rahal V, Mestrener SR, Dezan E., Jr Biological response of pulps submitted to different capping materials. Braz Oral Res. 2006;20(3):219–225. doi: 10.1590/s1806-83242006000300007. [DOI] [PubMed] [Google Scholar]
- 9.Cavalcanti BN, Rode SM, Marques MM. Cytotoxicity of substances leached or dissolved from pulp capping materials. Int Endod J. 2005;38:505–509. doi: 10.1111/j.1365-2591.2005.00967.x. [DOI] [PubMed] [Google Scholar]
- 10.Chacko V, Kurikose S. Human pulpal response to mineral trioxide aggregate (MTA): a histologic study. J Clin Pediatr Dent. 2006;30:203–209. doi: 10.17796/jcpd.30.3.38h13g5p84651652. [DOI] [PubMed] [Google Scholar]
- 11.Chan C, Lan W, Chang M, Chen Y, Lan W, Chang H. Effects of TGFbeta s on the growth, collagen synthesis and collagen lattice contraction of human dental pulp fibroblasts in vitro. Arch Oral Biol. 2005;50(5):469–479. doi: 10.1016/j.archoralbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Cho E, Kopel H, White SN. Moisture susceptibility of resin-modified glass-ionomer materials. Quintessence Int. 1995;26:351–358. [PubMed] [Google Scholar]
- 13.Coil J, Tam E, Waterfield J. Proinflammatory cytokine profiles in pulp fibroblasts stimulated with lipopolysaccharide and methyl mercaptan. J Endod. 2004;30(2):88–91. doi: 10.1097/00004770-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Conti TR, Sakai VT, Fornetti APC, Moretti ABS, Oliveira TM, Lourenço N, Neto, et al. Pulpotomies with Portland cement in human primary molars. J Appl Oral Sci. 2009;17(1):66–69. doi: 10.1590/S1678-77572009000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa CA, Giro EM, Nascimento AB, Teixeira HM, Hebling J. Short-term evaluation of the pulpo-dentin complex response to a resin-modified glass-ionomer cement and a bonding agent applied in deep cavities. Dent Mater. 2003;19(8):739–746. doi: 10.1016/s0109-5641(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 16.Costa CA, Teixeira HM, Nascimento AB, Hebling J. Biocompatibility of two current adhesive resins. J Endod. 2000;26(9):512–516. doi: 10.1097/00004770-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Costa CAS, Hebling J, Garcia-Godoy F, Hanks CT. In vitro cytotoxicity of five glass-ionomer cements. Biomaterials. 2003;24:3853–3858. doi: 10.1016/s0142-9612(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 18.Costa CAS, Hebling J, Hanks CT. Effects of light-curing time on the cytotoxicity of a restorative resin composite applied to an immortalized odontoblast-cell line. Oper Dent. 2003;28(4):365–370. [PubMed] [Google Scholar]
- 19.Costa CAS, Oliveira MF, Giro EMA, Hebling J. Biocompatibility of resin-based materials as pulp-capping agents. Int Endod J. 2003;36:831–839. doi: 10.1111/j.1365-2591.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 20.Costa CAS, Teixeira HM, Lopes do Nascimento AB, Hebling J. Biocompatibility of resin-based dental materials applied as liners in deep cavities prepared in human teeth. J Biomed Mater Res B Appl Biomater. 2007;81(1):175–184. doi: 10.1002/jbm.b.30651. [DOI] [PubMed] [Google Scholar]
- 21.Costa CAS, Teixeira HM, Nascimento ABL, Hebling J. Biocompatibility of an adhesive system and 2-hydroxyethylmethacrylate. ASDC J Dent Child. 1999;66(5):337–342. [PubMed] [Google Scholar]
- 22.Costa CAS, Vaerten MA, Edwards CA, Hanks CT. Cytotoxic effects of current dental adhesive systems on immortalized odontoblast cell line MDPC-23. Dent Mater. 1999;15:434–441. doi: 10.1016/s0109-5641(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 23.Cox CF, Hafez AA, Akimoto N, Otsuki M, Suzuki S, Tarim B. Biocompatibility of primer, adhesive and resin composite systems on nonexposed and exposed pulps of non-human primate teeth. Am J Dent. 1998;11(Sp issue):S55–S63. [PubMed] [Google Scholar]
- 24.Dammaschke T, Gerth HUV, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21:731–738. doi: 10.1016/j.dental.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Demarco FF, Tarquinio SBC, Jaeger MMM, Araújo VC, Matson E. Pulp response and citotoxicity evaluation of 2 dentin bonding agents. Quintessence Int. 2001;32(3):211–220. [PubMed] [Google Scholar]
- 26.Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, Fraga SdC. pH and calcium ion release of two root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:345–347. doi: 10.1067/moe.2003.12. [DOI] [PubMed] [Google Scholar]
- 27.Durand SH, Flacher V, Roméas A, Carrouel F, Colomb E, Vincent C, et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176(5):2880–2887. doi: 10.4049/jimmunol.176.5.2880. [DOI] [PubMed] [Google Scholar]
- 28.Farhad A, Mohammadi Z. Calcium hydroxide: a review. Int Dent J. 2005;55(5):293–301. doi: 10.1111/j.1875-595x.2005.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferracane JL, Condon JR. Rate of elution of leachable components from composite. Dent Mater. 1990;6:282–287. doi: 10.1016/S0109-5641(05)80012-0. [DOI] [PubMed] [Google Scholar]
- 30.Foreman PC, Barnes IE. A review of calcium hydroxide. Int Endod J. 1990;23(6):283–297. doi: 10.1111/j.1365-2591.1990.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 31.Freund M, Munksgaard EC. Enzymatic degradation of Bis-GMA/TEGDMA polymers causing decreased microhardeness and greater wear in vitro. Scand J Dent Res. 1990;98:351–355. doi: 10.1111/j.1600-0722.1990.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 32.Fridland M, Rosado R. MTA solubility: a long term study. J Endod. 2005;(31):376–379. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 33.Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder rations. J Endod. 2003;29:814–817. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Gerzina TM, Hume WR. Diffusion of monomers from bonding resinresin composite combinations through dentine in vitro. J Dent. 1996;24:125–128. doi: 10.1016/0300-5712(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 35.Geurtsen W, Lehman F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–480. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Geurtsen W, Spahl W, Muller K, Leyhausen G. Aqueous extracts from dentine adhesives contain cytotoxic chemicals. J Biomed Mater Res. 1999;48:772–777. doi: 10.1002/(sici)1097-4636(1999)48:6<772::aid-jbm2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg F, Massone EJ, Spielberg C. Evaluation of the dentinal bridge after pulpotomy and calcium hydroxide dressing. J Endod. 1984;10(7):318–320. doi: 10.1016/S0099-2399(84)80186-7. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez-Venegas G, Maldonado-Frias S, Ontiveros-Granados A, Kawasaki-Cardenas P. Role of p38 in nitric oxide synthase and cyclooxygenase expression, and nitric oxide and PGE2 synthesis in human gingival fibroblasts stimulated with lipopolysaccharides. Life Sci. 2005;77(1):60–73. doi: 10.1016/j.lfs.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70(11):1450–1455. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 40.Hebling J, Giro EM, Costa CA. Biocompatibility of an adhesive system applied to exposed human dental pulp. J Endod. 1999;25(10):676–682. doi: 10.1016/s0099-2399(99)80354-9. [DOI] [PubMed] [Google Scholar]
- 41.Hebling J, Giro EMA, Costa CA. Human pulp response after an adhesive system application in deep cavities. J Dent. 1999;27:557–564. doi: 10.1016/s0300-5712(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 42.Heithersay GS. Calcium hydroxide in the treatment of pulpless teeth with associated pathology. J Brit Endod Soc. 1975;8:74–93. doi: 10.1111/j.1365-2591.1975.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 43.Holland R. Histochemical response of amputes pulps to calcium hydroxide. Rev Bras Pesq Med Biol. 1971;4(1-2):83–95. [Google Scholar]
- 44.Holland R, Souza V, Mello W, Nery MJ, Bernabé PF, Otoboni JA., Filho Permeability of the hard tissue bridge formed after pulpotomy with calcium hydroxide: a histologic study. J Am Dent Assoc. 1979;99(3):472–475. doi: 10.14219/jada.archive.1979.0317. [DOI] [PubMed] [Google Scholar]
- 45.Hse KMY, Leung SK, Wei SHY. Resin-ionomer restorative materials for children: a review. Aust Dent J. 1999;44:1–11. doi: 10.1111/j.1834-7819.1999.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang FM, Chang YC. Cytotoxicity of dentine-bonding agents on human pulp cells in vitro. Int Endod J. 2002;35:905–909. doi: 10.1046/j.1365-2591.2002.00589.x. [DOI] [PubMed] [Google Scholar]
- 47.Islam I, Chng HK, Yap AUJ. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod. 2006;32:193–197. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 48.Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, Jeffries S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent. 2006;19:85–90. [PubMed] [Google Scholar]
- 49.Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod. 2000;26:288–291. doi: 10.1097/00004770-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Lan WH, Lan W, Wang TM, Lee YL, Tseng WY, Lin CP. Cytotocixity of conventional and modified glass ionomer cements. Oper Dent. 2003;28:251–259. [PubMed] [Google Scholar]
- 51.Letzelter C, Croute F, Pianezzi B, Roques C, Soleilhavoup J. Supernatant cytotoxicity and proteolytic activity of selected oral bacteria against human gingival fibroblasts in vitro. Arch Oral Biol. 1998;43(1):15–23. doi: 10.1016/s0003-9969(97)00095-2. [DOI] [PubMed] [Google Scholar]
- 52.Lin CP, Chen YJ, Lee YL, Wang JS, Chang MC, Lan WH, et al. Effects of root-end filling materials and eugenol on mitochondrial dehydrogenase activity and cytotoxicity to human periodontal ligament fibroblasts. J Biomed Mater Res B Appl Biomater. 2004;71:429–440. doi: 10.1002/jbm.b.30107. [DOI] [PubMed] [Google Scholar]
- 53.Mantellini MG, Botero TM, Yaman P, Dennison JB, Hanks CT, Nör JE. Adhesive resin induces apoptosis and cell-cycle arrest of pulp cells. J Dent Res. 2003;82(8):592–596. doi: 10.1177/154405910308200804. [DOI] [PubMed] [Google Scholar]
- 54.Melegari KK, Botero TM, Holland GR. Prostaglandin E2 production and viability of cells cultured in contact with freshly mixed endodontic materials. Int Endod J. 2006;39:357–362. doi: 10.1111/j.1365-2591.2006.01070.x. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell OF, Shankwalker GB. Osteogenic potencial of calcium hydroxide and other materials in soft tissue and bone wounds. J Dent Res. 1958;37:1157–1163. doi: 10.1177/00220345580370061501. [DOI] [PubMed] [Google Scholar]
- 56.Moghaddame-Jafari S, Mantellini MG, Botero TM, McDonald NJ, Nör JE. Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod. 2005;31:387–391. doi: 10.1097/01.don.0000145423.89539.d7. [DOI] [PubMed] [Google Scholar]
- 57.Moretti AB, Sakai VT, Oliveira TM, Fornetti AP, Santos CF, Machado MA, et al. The effectiveness of mineral trioxide aggregate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int Endod J. 2008;41(7):547–555. doi: 10.1111/j.1365-2591.2008.01377.x. [DOI] [PubMed] [Google Scholar]
- 58.Mount GJ. Glass-ionomer cements: past, present and future. Oper Dent. 1994;19:82–90. [PubMed] [Google Scholar]
- 59.Nagaoka S, Tokuda M, Sakuta T, Taketoshi Y, Tamura M, Takada H. Interleukin-8 gene expression by human dental pulp fibroblast in cultures stimulated with Prevotella intermedia lipopolysaccharide. J Endod. 1996;22(1):9–12. doi: 10.1016/S0099-2399(96)80228-7. [DOI] [PubMed] [Google Scholar]
- 60.Nasjleti CE, Castelli WA, Caffesse RG. Effects of composite restorations on the periodontal membrane of monkeys. J Dent Res. 1983;62:75–78. doi: 10.1177/00220345830620011801. [DOI] [PubMed] [Google Scholar]
- 61.Ogura N, Tobe M, Sakamaki H, Nagura H, Abiko Y, Kondoh T. Tumor necrosis factor-alpha increases chemokine gene expression and production in synovial fibroblasts from human temporomandibular joint. J Oral Pathol Med. 2005;34(6):357–363. doi: 10.1111/j.1600-0714.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 62.Okiji T. Seltzer and Bender's dental pulp. In: Hargreaves K, Goodis H, editors. Pulp as a connective tissue. Carol Stream: Quintessence; 2002. pp. 95–122. [Google Scholar]
- 63.Oliva A, Ragione D, Salerno A, Riccio V, Tarataro G, Gozzolino A. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials. 1996;17:1351–1356. [PubMed] [Google Scholar]
- 64.Onoe N, Inokoshi S, Yamada T. Proceedings of the international Conference of Dentin/Pulp Complex. In: Shimono M, Maeda T, Suda H, editors. Histopathological evaluation of adhesive resins for direct pulp capping. Tokyo: Quintessence; 1996. pp. 221–226. [Google Scholar]
- 65.Pääkkönen V, Vuoristo J, Salo T, Tjäderhane L. Effects of TGF-beta 1 on interleukin profile of human dental pulp and odontoblasts. Cytokine. 2007;40(1):44–51. doi: 10.1016/j.cyto.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Pashley DH. Consideration of dentin permeability in cytotocixity testing. Int Endod J. 1988;21:143–154. doi: 10.1111/j.1365-2591.1988.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 67.Pashley DH. Dynamics of the pulpo-dentin comples. Crit Rev Oral Biol Med. 1996;7(2):104–133. doi: 10.1177/10454411960070020101. [DOI] [PubMed] [Google Scholar]
- 68.Pereira JC, Bramante CM, Berbet A, Mondelli J. Effect of calcium hydroxide in power or in paste form on pulp-capping procedures: histophatologic and radiographic analysis in dog's pulp. Oral Surg. 1980;50(2):176–186. doi: 10.1016/0030-4220(80)90207-8. [DOI] [PubMed] [Google Scholar]
- 69.Pereira JC, Segala AD, Costa CA. Human pulpal response to direct pulp capping with an adhesive system. Am J Dent. 2000;13(3):139–147. [PubMed] [Google Scholar]
- 70.Robertsa HW, Tothb JM, Berzinsc DW, Charltond DG. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent Mater. 2008;24:149–164. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Sakai VT, Moretti ABS, Oliveira TM, Fornetti APC, Santos CF, Machado MAAM, et al. Pulpotomy of human primary molars with MTA and Portland cement: a randomized controlled trial. Br Dent J. 2009;207(3):E5–E5. doi: 10.1038/sj.bdj.2009.665. [DOI] [PubMed] [Google Scholar]
- 72.Sidhu SK, Watson TF. Resin-modified glass ionomer materials. A status report for the American Journal of Dentistry. Am J Dent. 1995;8:59–67. [PubMed] [Google Scholar]
- 73.Souza PPC, Aranha AMF, Hebling J, Giro EMA, Souza Costa CA. In vitro cytotoxicity and in vivo biocompatibility of contemporary resin-modified glass-ionomer cements. Dent Mater. 2006;22:838–844. doi: 10.1016/j.dental.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Stanislawski L, Daniau X, Lauti A, Goldberg M. Factors responsible for pulp cell cytotoxicity induced by resin-modified glass-ionomer cements. J Biomed Mater Res. 1999;48:277–288. doi: 10.1002/(sici)1097-4636(1999)48:3<277::aid-jbm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 75.Stanley HR, Going RE, Chauncey HH. Human pulp response to acid pretreatment of dentin and to composite restoration. J Am Dent Assoc. 1975;91:817–825. doi: 10.14219/jada.archive.1975.0455. [DOI] [PubMed] [Google Scholar]
- 76.Stanley HR, Pameijer CH. Dentistry's friend: calcium hydroxide. Oper Dent. 1997;22:1–3. [PubMed] [Google Scholar]
- 77.Stanley HR, Swerdlow H, Buonocore MG. Pulp reactions to anterior restorative materials. J Am Dent Assoc. 1967;75:132–141. doi: 10.14219/jada.archive.1967.0193. [DOI] [PubMed] [Google Scholar]
- 78.Staquet MJ, Durand SH, Colomb E, Roméas A, Vincent C, Bleicher F, et al. Different roles of odontoblasts and fibroblasts in immunity. J Dent Res. 2008;87(3):256–261. doi: 10.1177/154405910808700304. [DOI] [PubMed] [Google Scholar]
- 79.Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39:415–422. doi: 10.1111/j.1365-2591.2006.01097.x. [DOI] [PubMed] [Google Scholar]
- 80.Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod. 1981;7:17–21. doi: 10.1016/S0099-2399(81)80262-2. [DOI] [PubMed] [Google Scholar]
- 81.Trowbridge H. Seltzer and Bender's Dental Pulp. In: Hargreaves K, Goodis H, editors. Histology of pulpal inflammation. Carol Stream: Quintessence; 2002. pp. 227–245. [Google Scholar]
- 82.Veerayutthwilai O, Byers MR, Pham TT, Darveau RP, Dale BA. Differential regulation of immune responses by odontoblasts. Oral Microbiol Immunol. 2007;22(1):5–13. doi: 10.1111/j.1399-302X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 83.Xie D, Brantley BM, Culbertson G, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000;16:129–138. doi: 10.1016/s0109-5641(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Huang F, Lin C, Liu C, Lai C, Chang Y. Induction of interleukin-8 gene expression by black-pigmented Bacteroides in human pulp fibroblasts and osteoblasts. Int Endod J. 2003;36(11):774–779. doi: 10.1046/j.1365-2591.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 85.Yang L, Tsai C, Huang F, Liu C, Lai C, Chang Y. Induction of interleukin-6 gene expression by pro-inflammatory cytokines and black-pigmented Bacteroides in human pulp cell cultures. Int Endod J. 2003;36(5):352–357. doi: 10.1046/j.1365-2591.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 86.Yoshiba K, Yoshiba N, Nakamura H, Iwaku M, Ozawa H. Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J Dent Res. 1996;75(8):1590–1597. doi: 10.1177/00220345960750081101. [DOI] [PubMed] [Google Scholar]