Abstract
Several rather different models of the Fe—O2 bond in oxyhemoglobin have previously been proposed, none of which provide a satisfactory explanation of several properties. We propose a new model for the bonding of an O2 to the Fe of myoglobin and hemoglobin and report ab initio generalized valence bond and configuration interaction calculations on FeO2 that corroborate this model. Our model is based closely upon the bonding in ozone which recent theoretical studies have shown to be basically a biradical with a singlet state stabilized by a three-center four-electron pi bond. In this model, the facile formation and dissociation of the Fe—O2 bond is easily rationalized since the O2 always retains its triplet ground state character. The ozone model leads naturally to a large negative electric field gradient (in agreement with Mössbauer studies) and to z-polarized (perpendicular to the heme) charge transfer transitions. It also suggests that the 1.3 eV transition, present in HbO2 and absent in HbCO, is due to a porphyrin-to-Fe transition, analogous to that of ferric hemoglobins (e.g., HbCN).
Keywords: electronic structure, generalized valence bond, configuration interaction, excited electronic states, electric field gradient
Full text
PDF

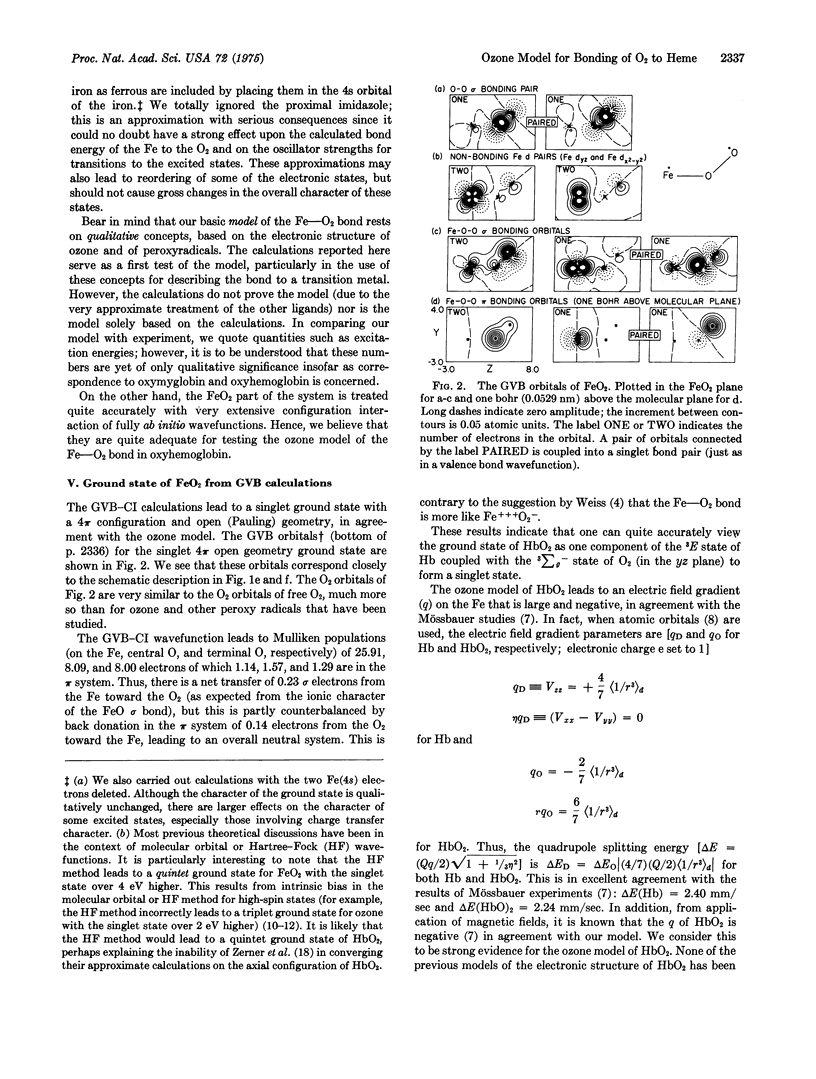


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collman J. P., Gagne R. R., Reed C. A., Robinson W. T., Rodley G. A. Structure of an iron(II) dioxygen complex; a model for oxygen carrying hemeproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1326–1329. doi: 10.1073/pnas.71.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G., Marshall W. Mössbauer effect in some haemoglobin compounds. J Mol Biol. 1966 Jul;18(3):385–404. doi: 10.1016/s0022-2836(66)80032-3. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Polarized single crystal absorption spectra of carboxy- and oxyhemoglobin. Ann N Y Acad Sci. 1973;206:210–222. doi: 10.1111/j.1749-6632.1973.tb43213.x. [DOI] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodley G. A., Robinson W. T. Structure of a monomeric oxygen-carrying complex. Nature. 1972 Feb 25;235(5339):438–439. doi: 10.1038/235438a0. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- Weissbluth M., Maling J. E. Interpretation of quadrupole splittings and isomer shifts in hemoglobin. J Chem Phys. 1967 Nov 15;47(10):4166–4172. doi: 10.1063/1.1701594. [DOI] [PubMed] [Google Scholar]