Abstract
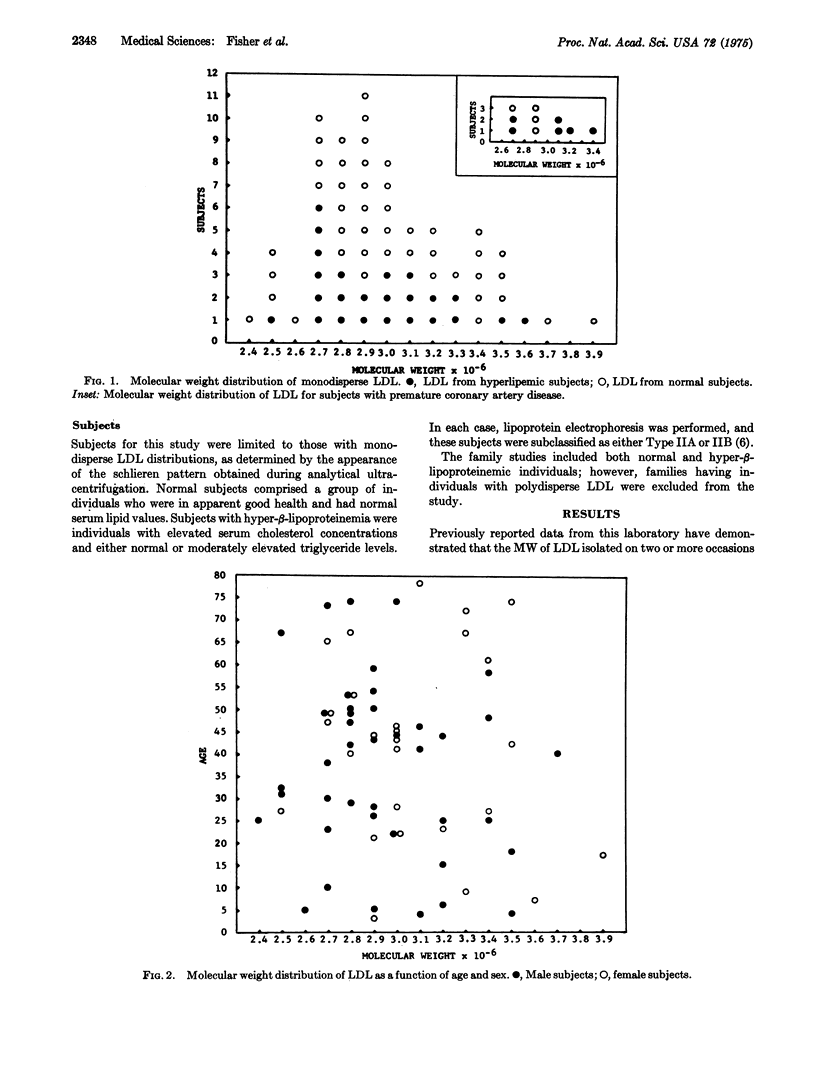
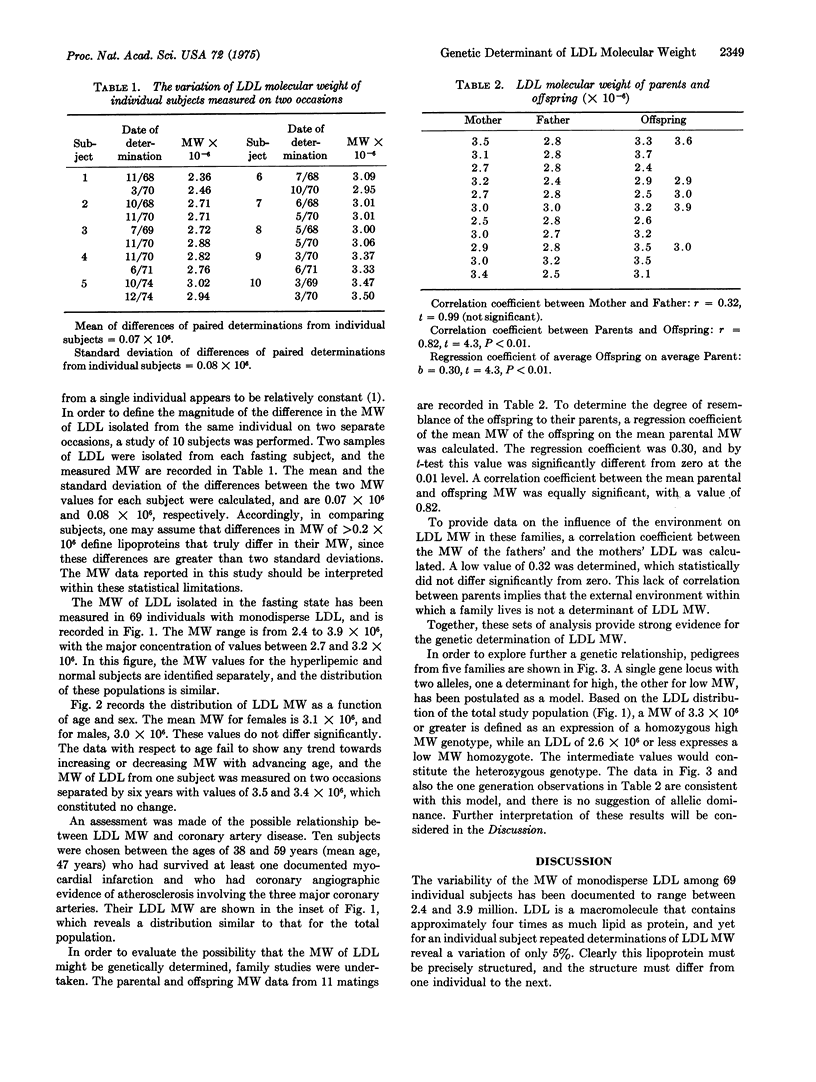
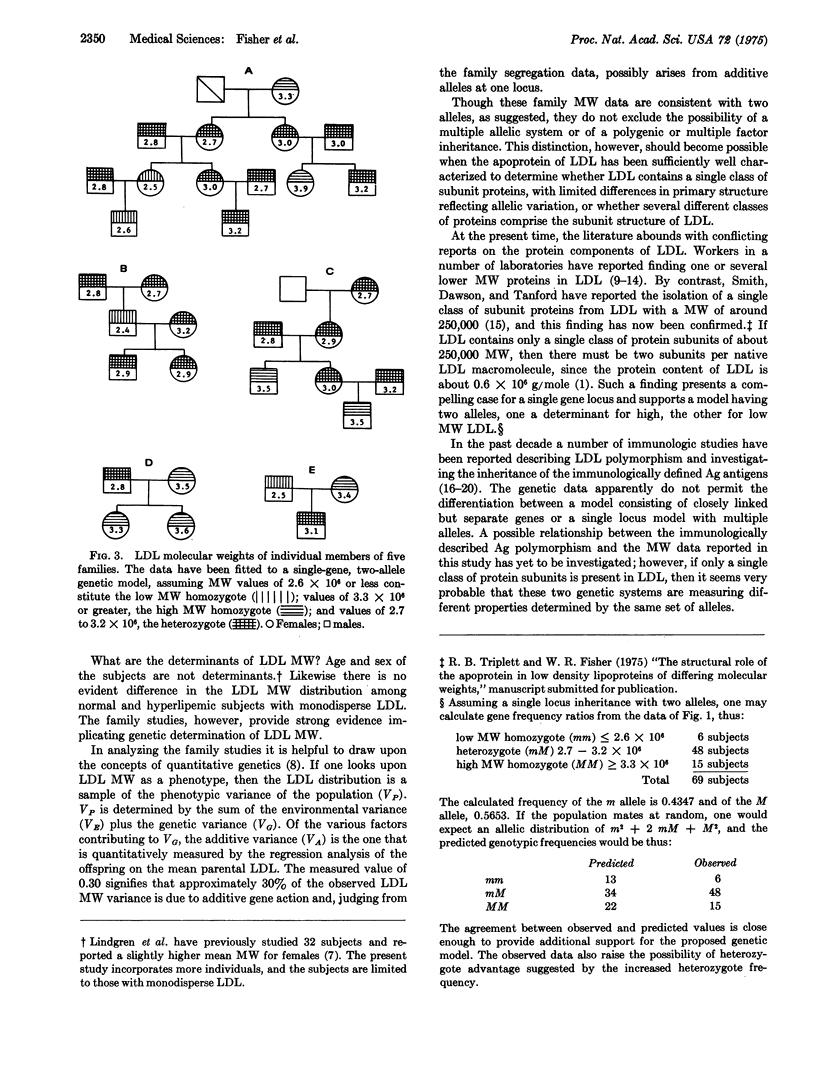
The molecular weight of monodisperse human plasma low densitylipoprotein has been measured in 69 individuals and found to vary over the range of 2.4 to 3.9 X 10-6. By contrast, the molecular weight of low density lipoprotein measured on two separate occasions for specific individuals shows a mean difference of 0.07 X 10-6 and a standard deviation of 0.08 X 10-6; hence low density lipoprotein differing in molecular weight by greater than 0.2 X 10-6 may be considered different macomolecules. The distribution of the molecular weight of low density lipoprotein does not differ as a function of age or sex. Hyperlipemic subjects having monodisperse low density lipoprotein show similar molecular weight distribution to normal subjects, as do subjects with premature coronary artery disease. Family studies reveal a correlation coefficient of 0.82 between average molecular weights of parents and offspring, with significance at 0.01. In order to assess the influence of environment on molecular weight of low density lipoprotein, the correlation coefficient between the fathers' and mothers' low density lipoprotein was measured and no statistically significant correlation was found. These data are interpreted as strong evidence for a genetic determination of molecular weight of low density lipoprotein. A study of individuals in five families yields molecular weight data consistent with a single gene locus genetic mode of inheritance without dominance. The regression coefficient of the mean low denisty lipoprotein parental molecular weight on the offspring molecular weight is 0.30. If the variability of molecular weight is considered as an expression of phenotypic variance, then the regression analysis identified 30% of this phenotypic variance as arising from additive gene action presumably at a single locus. Segregation in the family data is consistent. Since the differences in molecular weight of low density lipoprotein arise from differences in the amount of lipid bound to the apoprotein, it is likely that an additional portion of the phenotypic variance of the molecular weight results from individual variations in the metabolism of low density lipoprotein, which yield differences in lipid content. The individual variation in molecular weight is only approximately 5%; hence those metabolic sequences that influence molecular weight of low density lipoproteins must be precisely controlled.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Dray S. Identification and genetic control of two rabbit low-density lipoprotein allotypes. Biochem Genet. 1968 Jun;2(1):25–35. doi: 10.1007/BF01458449. [DOI] [PubMed] [Google Scholar]
- BLUMBERG B. S., BERNANKE D., ALLISON A. C. A human lipoprotein polymorphism. J Clin Invest. 1962 Oct;41:1936–1944. doi: 10.1172/JCI104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont J. L., Carlson L. A., Cooper G. R., Fejfar Z., Fredrickson D. S., Strasser T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull World Health Organ. 1970;43(6):891–915. [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Aladjem F. Subunit structure of the apoprotein of human serum low density lipoproteins. Biochem Biophys Res Commun. 1974 Sep 23;60(2):549–554. doi: 10.1016/0006-291x(74)90275-7. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Granade M. E., Mauldin J. L. Hydrodynamic studies of human low density lipoproteins. Evaluation of the diffusion coefficient and the preferential hydration. Biochemistry. 1971 Apr 27;10(9):1622–1629. doi: 10.1021/bi00785a019. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Hammond M. G., Warmke G. L. Measurements of the molecular weight variability of plasma low density lipoproteins among normals and subjects with hyper- -lipoproteinemia. Demonstration of macromolecular heterogeneity. Biochemistry. 1972 Feb 15;11(4):519–525. doi: 10.1021/bi00754a006. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Levy R. I., Fredrickson D. S. Preparation and properties of an apoprotein derivative of human serum beta-lipoprotein. Lipids. 1968 Nov;3(6):463–470. doi: 10.1007/BF02530887. [DOI] [PubMed] [Google Scholar]
- Hammond M. G., Fisher W. R. The characterization of a discrete series of low density lipoproteins in the disease, hyper-pre-beta-lipoproteinemia. Implications relating to the structure of plasma lipoproteins. J Biol Chem. 1971 Sep 10;246(17):5454–5465. [PubMed] [Google Scholar]
- Hirschfeld J., Rittner C. Inheritance of the Ag(x), Ag(y), (a1) and Ag(z) antigens. Vox Sang. 1969 Feb;16(2):146–154. doi: 10.1111/j.1423-0410.1969.tb04727.x. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Richards E. G., Havel R. J. Subunit heterogeneity in human serum beta lipoprotein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1075–1082. doi: 10.1073/pnas.66.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. M., Alaupovic P. Studies of the composition and structure of plasma lipoproteins. Isolation, composition, and immunochemical characterization of low density lipoprotein subfractions of human plasma. Biochemistry. 1970 May 26;9(11):2244–2252. doi: 10.1021/bi00813a004. [DOI] [PubMed] [Google Scholar]
- Lindgren F. T., Jensen L. C., Wills R. D., Freeman N. K. Flotation rates, molecular weights and hydrated densities of the low-density lipoproteins. Lipids. 1969 Sep;4(5):337–344. doi: 10.1007/BF02531003. [DOI] [PubMed] [Google Scholar]
- Morganti G., Beolchini P. E., Bütler R., Brunner E., Vierucci A. Contributions to the genetics of serum beta-lipoproteins in man. IV. Evidence for the existence of the Ag-a1-d and Ag-c-g loci, closely linked to the Ag-x-y locus. Humangenetik. 1970;10(3):244–253. doi: 10.1007/BF00295787. [DOI] [PubMed] [Google Scholar]
- Rapacz J., Grummer R. H., Hasler J., Shakelford R. M. Allotype polymorphism of low density beta-lipoproteins in pig serum (LDLpp I, LDLpp 2). Nature. 1970 Mar 7;225(5236):941–942. doi: 10.1038/225941a0. [DOI] [PubMed] [Google Scholar]
- Scanu A., Pollard H., Reader W. Properties of human serum low density lipoproteins after modification by succinic anhydride. J Lipid Res. 1968 May;9(3):342–349. [PubMed] [Google Scholar]
- Shore B., Shore V. The protein moiety of human serum beta-lipoproteins. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1003–1007. doi: 10.1016/0006-291x(67)90080-0. [DOI] [PubMed] [Google Scholar]
- Smith R., Dawson J. R., Tanford C. The size and number of polypeptide chains in human serum low density lipoprotein. J Biol Chem. 1972 Jun 10;247(11):3376–3381. [PubMed] [Google Scholar]


