Abstract
Purpose
To compare the effect of cilostazol plus aspirin versus aspirin alone on the progression of intracranial arterial stenosis (IAS), and to compare ischemic and hemorrhagic events in patients with symptomatic IAS, an investigator-driven, nationwide multicenter cooperative randomized controlled trial (CATHARSIS; ClinicalTrials.gov Identifier 00333164) was conducted.
Methods
165 noncardioembolic ischemic stroke patients with >50% stenosis in the responsible intracranial artery after 2 weeks to 6 months from the onset were randomly allocated to receive either cilostazol 200 mg/day plus aspirin 100 mg/day (n = 83, CA group) or aspirin 100 mg/day alone (n = 82, A group). The primary endpoint was the progression of IAS on magnetic resonance angiography at 2 years after randomization. Secondary endpoints were any vascular events, any cause of death, serious adverse events, new silent brain infarcts, and worsening of the modified Rankin Scale score.
Results
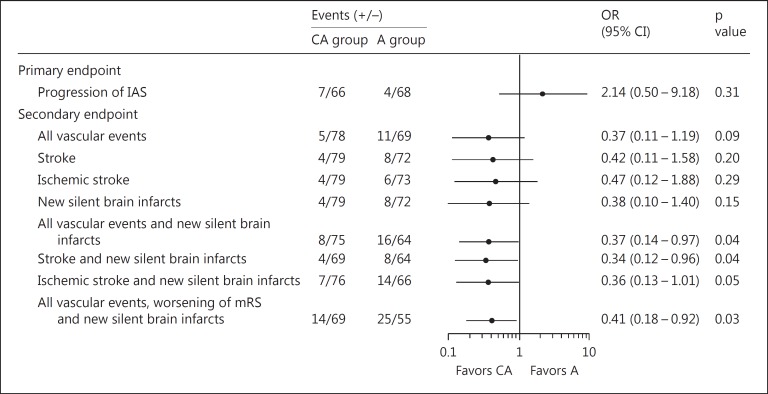
Progression of IAS was observed in 9.6% of the CA group patients and in 5.6% of the A group patients, with no significant intergroup difference (p = 0.53). The incidence of the secondary endpoints tended to be lower in the CA group compared with the A group, although the differences were not significant. By using exploratory logistic regression analysis adjusted for patient background characteristics, it was shown that the risk for certain combinations of secondary endpoints was lower in the CA group than in the A group [all vascular events and silent brain infarcts: odds ratio (OR) = 0.37, p = 0.04; stroke and silent brain infarcts: OR = 0.34, p = 0.04; all vascular events, worsening of modified Rankin Scale scores and silent brain infracts: OR = 0.41, p = 0.03]. Major hemorrhage was observed in 4 patients of the CA group and in 3 of the A group.
Conclusion
Progression of IAS during the 2-year observation period appears to be less frequent than previously reported in stroke patients on antiplatelet agents after the acute phase, which could be due to the adequate control of risk factors, and because patients with stroke within 2 weeks after the onset were excluded. The results of the CATHARSIS trial suggest a potential utility of pharmacotherapies with cilostazol plus aspirin as well as of strict control of risk factors for the management of symptomatic IAS. Larger studies with higher statistical power are required to obtain conclusive results.
Key Words: Intracranial arterial stenosis, Stroke prevention, Cilostazol, Aspirin
Introduction
Intracranial arterial stenosis (IAS) is more common in Asians, e.g. Japanese people, than in Caucasians [1,2] and is reported to be associated with 10-40% of ischemic stroke [3]. IAS is an independent predictor of poor outcome, and progression of IAS for 6 months reaches 29% despite aspirin therapy [4]; warfarin is not recommended because of an increased risk of intracranial hemorrhage and death when compared with aspirin [5]. IAS is commonly treated by pharmacotherapies, among which the most conventional one is antiplatelet therapy [6,7]. Neither bypass surgery nor endovascular therapy has been proven to be superior to medical management, including antiplatelet therapy, for recurrent stroke prevention with IAS [8]. The continued collection and evaluation of data on stroke prevention with antiplatelet agents is crucial to determine the value of new emerging options.
While conventional antiplatelet therapy to prevent progression of IAS primarily employs aspirin, attention is focused on the phosphodiesterase inhibitor cilostazol, which has both vasodilatory and antiatherogenic activities, and hence is expected to exert beneficial effects in patients with IAS. Results of the Cilostazol Stroke Prevention Study 2 (CSPS2) showed that cilostazol was more effective than aspirin for the prevention of stroke recurrence in Japanese patients with noncardioembolic ischemic stroke without increasing the risk of bleeding [9]. Additionally, in the 6-month follow-up study of cilostazol in Korean patients with symptomatic IAS (Trial of Cilostazol in Symptomatic Intracranial Steonosis, TOSS) [4], progression of IAS in the cilostazol plus aspirin group (CA group) was significantly lower than in the aspirin group (A group) (7 vs. 29%).
In the present study, we conducted a nationwide multicenter, prospective, open-labeled, randomized controlled trial to compare the effect of cilostazol plus aspirin versus aspirin alone on the progression of IAS, and to compare ischemic and hemorrhagic events in chronic stroke patients with IAS, in order to establish the best medical treatment and to provide information for future randomized controlled studies that compare medical treatment alone and intravascular intervention (intracranial artery stenting) in symptomatic IAS patients.
Methods
Patients
Noncardioembolic ischemic stroke patients with IAS of >50% were enrolled from 60 institutions in Japan between June 2006 and March 2010 and were treated for 2 years.
Inclusion and Exclusion Criteria
Inclusion criteria were ischemic stroke 2 weeks to 6 months prior to entry, with the responsible lesion identified on magnetic resonance imaging (MRI), IAS of >50% in the supraclinoid internal carotid artery, the M1 portion of the middle cerebral artery or the basilar artery identified on magnetic resonance angiography (MRA), age of 45-85 years, and able to visit an outpatient clinic.
Patients were excluded if they had potential cardiac sources of embolism, a history of symptomatic intracranial hemorrhage, other hemorrhagic diseases (such as active peptic ulcer), hemophilia or coagulation abnormalities, hypersensitivity to cilostazol or aspirin, congestive heart failure or uncontrollable angina pectoris, thrombocytopenia (<100,000/mm3), liver dysfunction (AST or ALT >100 IU/l), or renal dysfunction (creatinine >2.0 mg/dl). Patients who could not be followed up during the study period were excluded as well, and so were those on cilostazol or warfarin, those in whom MRI could not be performed, those who were scheduled to undergo percutaneous transluminal coronary angioplasty or bypass surgery, or who were enrolled in other clinical trials. The primary physician at each institution judged the eligibility of each patient. No restriction was imposed on diet or exercise therapy.
Ethics
The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. It was approved by the institutional review board of each participating institution. Written informed consent was obtained from all patients or their families.
Randomization
Patients were randomly allocated to either receive cilostazol 200 mg/day plus aspirin 100 mg/day (CA group) or aspirin 100 mg/day alone (A group). Concomitant use of any antiplatelet and anticoagulant agents other than the study drugs was prohibited. Age (≥70 vs. <70 years) and stenosis location (supraclinoid internal carotid artery/middle cerebral artery vs. basilar artery) were used as stratification factors for randomization.
Primary and Secondary Endpoints
Patients were assessed at baseline, at months 3 and 6, and at years 1 and 2. At each visit, hematological and biochemical laboratory analyses and blood pressure measurements were conducted. The primary endpoint was the progression of IAS on MRA at 2 years after randomization. Secondary endpoints were all vascular events (ischemic stroke, myocardial infarct, and other vascular events), death (stroke death, vascular death other than stroke, and any death other than due to a vascular cause), serious adverse events, new silent brain infarcts, and worsening of the modified Rankin Scale (mRS) score (1 or more scores). All adverse events were recorded.
IAS was assessed by MRA in accordance with the standardized method for measuring IAS [10]. In brief, the diameters (D) of normal and stenotic arteries in a segment proximal to the stenosis were measured from MRA and the percent stenosis calculated as [D (normal) – D (stenosis)/D (normal)]. The measurement was performed at the site of the most severe stenosis, using MRAs from at least two directions. If the diameter of a proximal normal artery could not be measured, a distal normal artery was measured instead. Using the calculated percent stenosis, the severity of IAS was classified into 5 grades: normal, mild (<50%), moderate (≥50%), severe (absence of the blood flow signal at the stenotic lesion and presence of the signal at the distal portion of the stenosis), and occluded (absence of the blood flow signal both at the stenotic lesion and its distal portion) [4]. Changes by ≥1 severity grade were defined as progressed or improved. Worsening of the mRS score was defined as an increase in mRS score by ≥1 during the 2-year period. Serious adverse events included death, cardiovascular events, symptomatic intracranial hemorrhage, and major extracranial hemorrhage requiring hospitalization.
Statistical Estimation and Analysis
The TOSS study by Kwon et al. [4] reported IAS progression in 29% of patients in the A group and in 7% of patients in the CA group over the 6-month treatment period. Exponential distribution probability calculation of these data yielded 2-year progression rates of 75 and 25%, respectively. Therefore, the 2-year rates were provisionally set to be 60 and 35%, respectively, and the minimum number of subjects in the two groups was calculated using a two-tailed Fisher's exact probability test to achieve a power of 80% at the 5% level to detect a significant between-group difference in the frequency of progression. The number of patients required was estimated to be 70 in each group, totaling 140. When 30% of the patients were assumed nonevaluable for statistical analysis due to the lack of MRA data, ineligibility revealed after the registration, or due to other reasons during the 2-year period, the minimum target patient population was calculated to be 100 per group, totaling 200.
All patients who received the study drugs at least once were included in the intention-to-treat (ITT) population. With regard to baseline parameters of patients, data for continuous variables were expressed as median values and compared between groups by the Wilcoxon rank sum test; intergroup differences in the distribution of categorical variables were analyzed by Fisher's exact test.
The primary endpoint was compared between the treatment groups with Fisher's exact test. For the secondary endpoints, data on ischemic stroke, myocardial infarction, all vascular events, stroke, all vascular deaths, and all deaths were compared between the groups by the log rank test. An intergroup comparison of event-free time was performed using the log rank test, after estimating the event-free probability by the Kaplan-Meier method in each group. The Clopper and Pearson method and the Greenwood formula were used for the calculation of the hazard ratio with 95% confidence interval (CI) for the incidence ratio and the interval for event-free survival ratio in event-free time analysis, respectively. The data on new silent cerebral infarcts were compared between groups by Fisher's exact test and worsening of mRS scores by the Wilcoxon rank sum test. No control for multiplicity of analyses was applied. Additionally, exploratory logistic regression analyses were performed to investigate whether the risk for any combinations of each secondary endpoint differs between the CA group and the A group, by adjusting patient background characteristics.
The analyses were performed by two-tailed tests with a p value <0.05 considered significant. Data analyses were performed by using SAS version 9.1.3 (service pack 2; SAS Institute Inc., Cary, N.C., USA).
Results
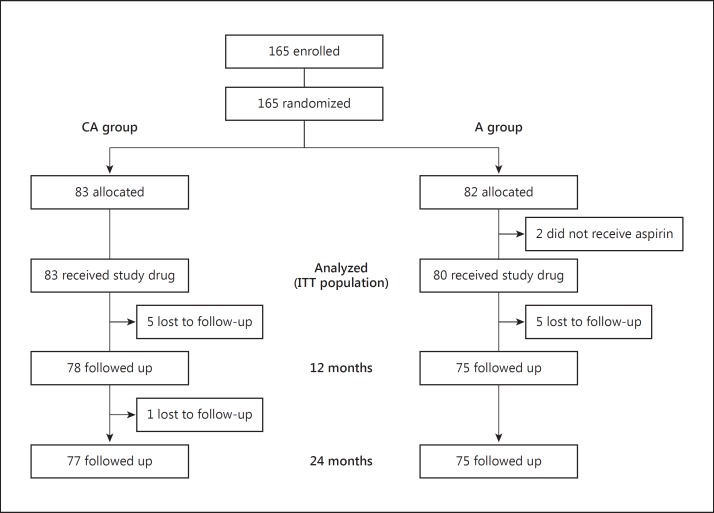
A total of 165 patients were randomized to the CA group (n = 83) or the A group (n = 82); 2 patients in the A group did not receive any study drug, giving 163 patients (83 in the CA group; 80 in the A group) in the ITT population (fig. 1). The mean duration of follow-up was 762 days. Patient characteristics are shown in table 1. The CA group included more males (77.1 vs. 53.8%) and more patients with hypertension (83.1 vs. 68.8%) and diabetes mellitus (48.2 vs. 25.0%) compared with the A group.
Fig. 1.
Flow diagram of patients studied.
Table 1.
Baseline characteristics of the CA and A groups
| CA group (n = 83) | A group (n = 80) | p value | |
|---|---|---|---|
| Age1, years | 68.3 (45–84) | 68.3 (50–82) | 0.88 |
| Male gender | 64 (77.1%) | 43 (53.8%) | <0.01 |
| Body mass index1 | 23.7 (15.0 – 33.3) | 23.9 (17.6–32.7) | 0.86 |
| Coronary artery disease | 4 (4.8%) | 4 (5.0%) | 1.00 |
| Hypertension | 69 (83.1%) | 55 (68.8%) | 0.04 |
| Diabetes mellitus | 40 (48.2%) | 20 (25.0%) | <0.01 |
| Dyslipidemia | 42 (50.6%) | 47 (58.8%) | 0.35 |
| Current smoker | 20 (24.1%) | 15 (18.8%) | 0.45 |
| Chronic kidney disease mRS score | 29 (34.9%) | 18 (22.5%) | 0.09 |
| 0 | 30 (36.1%) | 31 (38.8%) | |
| 1 | 28 (33.7%) | 35 (43.8%) | |
| 2 | 18 (21.7%) | 7 (8.8%) | 0.31 |
| 3 – 4 | 7 (8.4%) | 7 (8.8%) | |
| Size of infarction | |||
| Small | 51 (61.4%) | 42 (52.5%) | |
| Medium | 31 (37.3%) | 38 (47.5%) | 0.24 |
| Large | 1 (1.2%) | 0 | |
| Severity of stenosis | |||
| Moderate | 45 (54.2%) | 44 (55.0%) | |
| Severe | 38 (45.8%) | 36 (45.0%) | 1.00 |
| ICA | 10 (12.0%) | 8 (10.0%) | |
| Location of stenosis | |||
| M1 of MCA | 63 (75.9%) | 64 (80.0%) | <0.82 |
| BA | 10 (12.0%) | 8 (10.0%) |
MCA = Middle cerebral artery; BA = basilar artery.
Figures are medians with ranges in parentheses..
Primary Endpoint
During the 2-year observation period, progression of IAS was observed in 9.6% of the CA group patients and in 5.6% of the A group patients, with no significant intergroup difference (p = 0.53) (table 2). Additionally, at year 1, progression of IAS was observed in 11.0 and 7.9% of patients in the CA and the A groups, respectively, the proportions of which appeared higher than at year 2, indicating a tendency toward mitigation of stenosis over time. Moreover, the severity of IAS was initially classified into moderate or severe, but at year 2, the lesion resolved in 7 patients [3 (3.6%) in the CA group and 4 (5.0%) in the A group].
Table 2.
Primary and secondary outcome in the CA and A groups
| CA group (n = 83) | A group (n = 80) | p value | |
|---|---|---|---|
| Primary endpoint | |||
| Progression of IAS | 7 (9.6%) | 4 (5.6%) | 0.53 |
| Secondary endpoint | |||
| New silent brain infarcts | 4 (4.8%) | 8 (10.0%) | 0.24 |
| Worsening of mRS score | 8 (10.1%)a | 14 (18.9%)a | 0.17 |
Missing data not included.
Secondary Endpoints
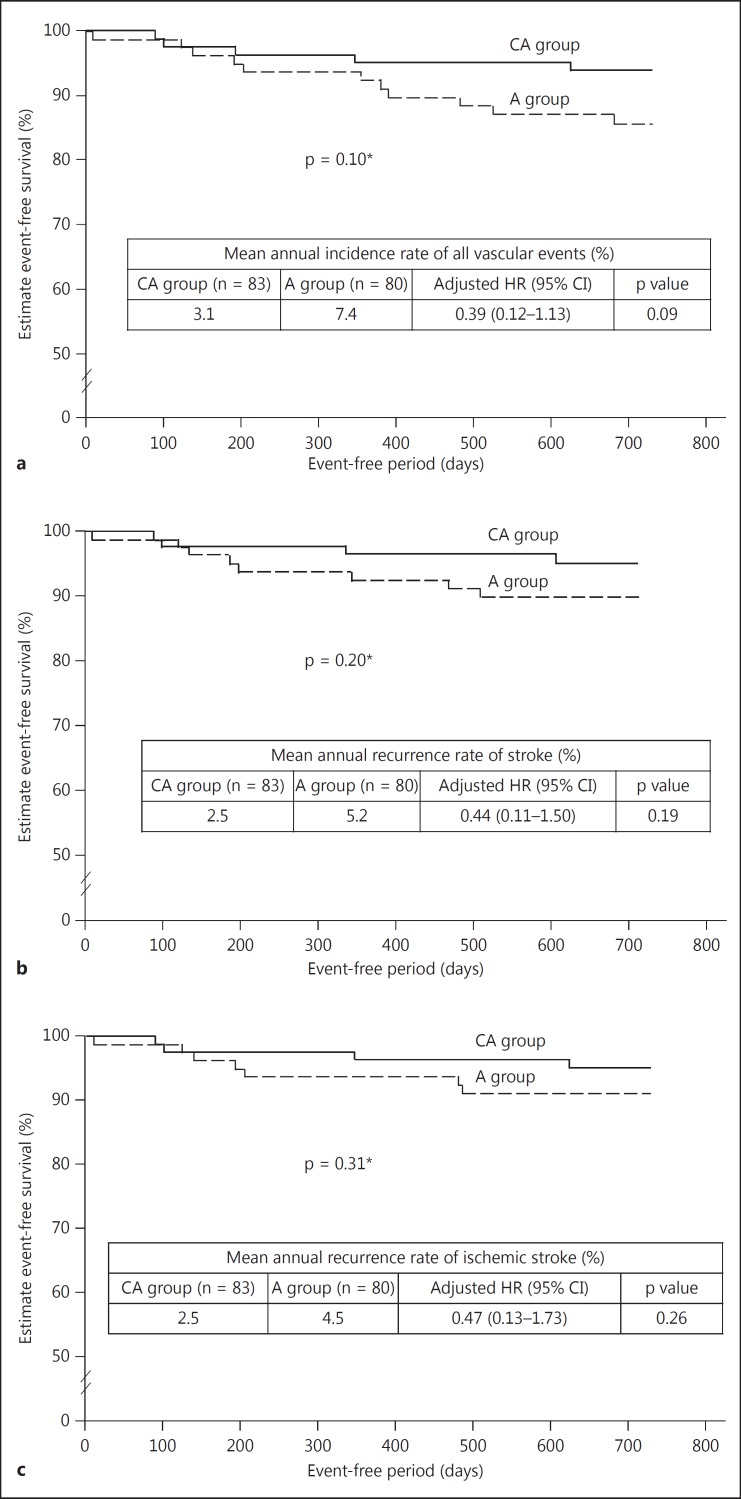
No difference was seen between the CA and the A group in the emergence of new silent brain infarcts (4.8 vs. 10.0%, p = 0.24) or worsening of the mRS score (10.1 vs. 18.9%, p = 0.17) (table 2). The numbers of brain infarcts (both symptomatic and asymptomatic) during the 2-year period were 4 and 7 in the responsible artery and 4 and 7 in the nonresponsible artery, in the CA group and the A group, respectively (table 3). The mean annual incidence of all vascular events, stroke, and ischemic stroke also tended to be lower in the CA group compared with the A group, although the difference was not significant (fig. 2). Details of 5 and 11 vascular events that occurred in the CA and A groups are shown in table 4.
Table 3.
Number of symptomatic/asymptomatic new brain infarcts by vascular territories during the 2-year follow-up
| Location | CA group (n = 83) |
A group (n = 80) |
||||
|---|---|---|---|---|---|---|
| symptomatic | asymptomatic | total | symptomatic | asymptomatic | total | |
| Responsible artery | 2 | 2 | 4 | 4 | 3 | 7 |
| Nonresponsible artery | 2 | 2 | 4 | 2 | 5 | 7 |
Fig. 2.
Secondary endpoints, Kaplan-Meier plots for event-free survival for all vascular events (a), stroke (b), and ischemic stroke (c). The asterisk indicates the log rank test.
Table 4.
Vascular events during the 2-year follow-up period in the CA and A groups
| CA group (n = 83) | A group (n = 80) | ||
|---|---|---|---|
| Total events | 5 | Total events | 11 |
| Ischemic stroke | 4 | Ischemic stroke | 6 |
| Lacunar | 3 | Lacunar | 0 |
| Atherothrombotic | 1 | Atherothrombotic | 6 |
| Other | 1 | Cerebral hemorrhage | 2 |
| PTA for PAD | 1 | Other | 3 |
| EC-IC bypass surgery | 1 | ||
| Coronary stenting | 1 | ||
| Central retinal vein occlusion | 1 |
PTA = Percutaneous transluminal angioplasty; PAD = peripheral artery disease; EC-IC = extracranial-intracranial.
Logistic Regression Analysis
By exploratory logistic regression analysis adjusted for patient background characteristics, the risk for certain combinations of secondary endpoints was lower in the CA group than in the A group [all vascular events and silent brain infarcts: odds ratio (OR) 0.37, 95% CI 0.14-0.97, p = 0.04; stroke and silent brain infarcts: OR 0.34, 95% CI 0.12-0.96, p = 0.04; all vascular events, worsening of mRS scores and silent brain infracts: OR 0.41, 95% CI 0.18-0.92, p = 0.03] (fig. 3).
Fig. 3.
Logistic regression analysis adjusted by patient characteristics.
Laboratory Analyses
Mean systolic (SBP) and diastolic blood pressure (DBP) at baseline, year 1, and year 2 were 137.4/77.7, 134.0/75.4, and 131.1/74.4 mm Hg, respectively, in all patients. Blood pressure was fairly well controlled, and a significant reduction in SBP and DBP (p < 0.01) was observed at year 2 compared with baseline. Mean total cholesterol and high-density lipoprotein cholesterol levels at baseline, year 1 and year 2 were 195.4/49.5, 183.8/55.6, and 182.9/55.8 mg/dl; significant improvements in both parameters (p < 0.01) were observed both at years 1 and 2 compared with the baseline value.
Serious Adverse Events
Major hemorrhage occurred in 4 and 3 patients in the CA and A groups, respectively (table 5). No death was observed in either treatment group.
Table 5.
Serious hemorrhage reported during the 2-year follow-up period in the CA and A groups
| CA group (n = 83) | A group (n = 80) | ||
|---|---|---|---|
| Total events | 4 | Total events | 3 |
| Gastrointestinal bleeding | 2 | Subarachnoid hemorrhage | 1 |
| Vitreous hemorrhage | 1 | Cerebral hemorrhage | 1 |
| Hematuria | 1 | Gastrointestinal bleeding | 1 |
Discussion
Bypass surgery has not been proven effective in preventing recurrence of stroke in patients with symptomatic IAS; intravascular intervention, such as vascular percutaneous transluminal angioplasty and stent placement, requires careful consideration and planning from both safety and technical perspectives [8]. Thus, the development and establishment of new strategies for stroke prevention is an urgent medical need in patients with symptomatic IAS.
The CATHARSIS study was designed to examine IAS progression in patients on aspirin with or without cilostazol over a 2-year period. Although relative 2-year rates of progression were initially assumed to be 35 and 60% based on a previous study [4], observed percentages of IAS progression were much lower than expected: 9.6% for cilostazol with aspirin and 5.6% for aspirin alone. This unexpectedly lower rate of IAS progression can be partly attributable to the improvement in risk factor management in the past decade. In the CATHARSIS study, blood pressure and lipid levels were adequately controlled in the majority of patients, achieving each therapeutic target. This was different from the WASID study, in which, despite similar baseline SBP/DBP levels to this study (137.4/77.7 mm Hg in the CATHARSIS study vs. 139.8/76.8 mm Hg in the WASID study), blood pressure was not virtually reduced during the study period (139.9 mm Hg at year 1 and 137.3 mm Hg at year 2). Also, total cholesterol was suitably managed with the percentage of patients with total cholesterol levels ≥200 mg/dl significantly decreased from 50 to 21% at year 2; no significant difference was seen in the percentage of patients with high-density lipoprotein cholesterol levels <40 mg/dl between baseline and year 2 in the WASID study [11], while both total cholesterol and high-density lipoprotein cholesterol levels had shown significant improvement in the CATHARSIS study. The results of recent interventional and observational studies [12,13] have suggested that the refined risk factor management contributed to the lower rates of IAS progression, which is compatible with the findings of this study. Also, Wang et al. [14] have recently reported that the majority (71.8%) of 39 patients with symptomatic IAS (>70%) underwent regression of stenosis by intensive control of cardiovascular risks (smoking cessation, low-density lipoprotein level <70 mg/dl, hemoglobin A1c <6.5%, and SBP <140 mm Hg during 1-year follow-up), which is in line with the disappearance of IAS as observed in this study.
Compared to the TOSS study, less IAS progression was found in this study that can potentially be explicable on the basis of a difference in disease stage of the target patients. In the TOSS study, ischemic stroke patients within 2 weeks of onset were enrolled, while such patients were excluded in this study. In patients at the acute stage of stroke, thrombus formation or regression can dynamically occur, leading to the progression or mitigation of IAS, compared with patients at the chronic stage. However, on the basis of vascular imaging, differentiation between atherosclerotic stenosis and thrombotic stenosis is difficult, although the latter would be more likely to occur during the first 2 weeks after stroke onset. Another issue to be addressed is that new infarcts equivalently emerged in the territory of both responsible and nonresponsible arteries, suggesting that attention should be paid to de novo infarcts in the territories not only of responsible arteries but also of nonresponsible arteries for secondary stroke prevention.
Although a greater number of patients with vascular risk factors, such as male gender, hypertension and diabetes mellitus, were included in the CA group than in the A group, a possible benefit of the cilostazol and aspirin combination over aspirin alone was suggested by the tendency towards a lower incidence of all vascular events (ischemic stroke, myocardial infarct, and other vascular events), new silent brain infarcts, and worsening of the mRS score. By exploratory logistic regression analysis adjusted for patient background characteristics, the risk for certain combinations of secondary endpoints was lower in the CA group than in the A group (fig. 3), suggesting stronger cardiovascular protective effects of cilostazol and aspirin combination compared with aspirin alone. Larger prospective studies with adequately defined endpoints are necessary to further examine such effects.
CA combination therapy did not increase hemorrhagic events compared with aspirin monotherapy. Previous large clinical trials [15,16] demonstrated that combination therapy with clopidogrel plus aspirin did not show additional reduction in the risk of vascular events and resulted in increased risk of hemorrhagic events compared with either monotherapy. This suggests that treatments targeting platelets alone to prevent stroke or other vascular events are of limited clinical efficacy and may result in an increased risk of hemorrhage. In the CSPS2, cilostazol significantly reduced recurrent stroke, with significantly fewer hemorrhagic events than aspirin, which was considered attributable to both antiplatelet and vascular protective effects of cilostazol. These effects include improvement in endothelial function and dilation of blood vessels by increased production of nitric oxide and endogenous vasodilating factor, and a reduction in intracellular ionized calcium concentrations [17]. Cilostazol also inhibits smooth muscle cell proliferation [18] and inflammation [19,20] in various vascular beds. These actions of cilostazol may contribute to the reduced risk of subsequent vascular events and lesions without an increase in hemorrhagic events even in patients at high risk for vascular events such as those with symptomatic IAS.
Limitation
Although the sample size of this study was calculated referring to the TOSS study, the number of patients that reached defined endpoints was not large enough for analyses. However, by exploratory logistic regression analyses, a benefit of the combination therapy with cilostazol and aspirin over aspirin monotherapy was suggested for the prevention of overall vascular events. A larger study (CSPS.com, UMIN 000012180) is currently conducted in 4,000 patients at high risk for ischemic stroke including IAS, which may provide further evidence.
Conclusions
Progression of IAS appears to be less frequent than previously reported in chronic stroke patients on antiplatelet agents, which could be due to the good control of risk factors. The results of the CATHARSIS study suggest a potential utility of pharmacotherapies for the management of symptomatic IAS. However, larger studies with higher statistical power are required to obtain conclusive results.
Disclosure Statement
Dr. Uchiyama reports having received consulting fees, lecture fees, and research grants from Otsuka Pharmaceutical, Sanofi Aventis, Boehringer Ingelheim, Daiichi-Sankyo, and Bayer Healthcare. Dr. Sakai reports having received consulting fees, lecture fees, and research grants from Otsuka Pharmaceutical, Sanofi Aventis, and Daiichi-Sankyo. Dr. Toi reports no conflict of interest. Dr. Ezura reports having received no consulting fees, lecture fees, and research grants. Dr. Okada reports having received consulting fees and lecture fees from Otsuka Pharmaceutical, Sanofi Aventis, Boehringer Ingelheim, Daiichi-Sankyo, Pfizer, Bristol-Myers, and Bayer Healthcare. Dr. Takagi reports having received lecture fees from Sanofi Aventis. Dr. Nagai reports having received no consulting or lecture fees, nor research grants from any domestic or foreign pharmaceutical companies. Dr. Matsubara reports no conflict of interest. Dr. Minematsu reports having received consulting fees and lecture fees from Sanofi-Aventis, Otsuka Pharmaceuticals, Bayer Healthcare, Daiichi-Sankyo, Sawai, and AstraZeneca. Dr. Suzuki reports having received consulting fees, lecture fees, and research grants from Otsuka Pharmaceutical, Sanofi Aventis, and Astellas. Dr. Tanahashi reports having received consulting fees, lecture fees, and research grants from Sanofi Aventis, AstraZeneca, Takeda, Tanabe-Mitsubishi, and Bayer Healthcare. Dr. Taki reports no conflict of interest. Dr. Nagata reports having received consulting fees, lecture fees, and research grants from Otsuka Pharmaceutical, Sanofi Aventis, Daiichi-Sankyo, and Bayer Healthcare. Dr. Matsumoto reports having received consulting fees, lecture fees, and research grants from Otsuka Pharmaceutical, Sanofi Aventis, Boehringer Ingelheim, Daiichi-Sankyo, and Bayer Healthcare.
Acknowledgements
The CATHARSIS study was conducted with operational and technical support by the Translational Research Informatics Center/Foundation for Biomedical Research and Innovation (FBRI), Kobe, Japan. The FBRI is a public interest incorporated foundation, receiving financial resources from the Japanese government and pharmaceutical companies, including Otsuka Pharmaceutical Co., Ltd., which commercializes cilostazol. However, the aforementioned company does not have any role in the data collection, data analysis, data interpretation, or writing of the report.
Principal Investigator: Shinichiro Uchiyama, Tokyo Women's Medical University (present affiliation: Clinical Research Center for Medicine, International University of Health and Welfare, Center for Brain and Cerebral Vessels, Sanno Hospital and Sanno Medical Center).
Co-Principal Investigator: Nobuyuki Sakai, Kobe City Medical Center General Hospital.
Collaborating Clinical Centers and Investigators: Kohnan Hospital (Eisuke Furui); Hiroshima University Hospital (Toshiho Ohtsuki); Kobe City Medical Center General Hospital (Nobuyuki Sakai); National Cerebral and Cardiovascular Center (Kazuo Minematsu); Toho University Omori Medical Center (Yasuo Iwasaki); Hatsudai Rehabilitation Hospital (Sakoh Masaharu); Japanese Red Cross Kyoto Daini Hospital (Yasumasa Yamamoto); Kagoshima City Hospital (Kazuho Hirahara); Kajikawa Hospital (Hiromitsu Naka); Nippon Medical School Hospital (Seiji Okubo); Shinsapporo Neurosurgical Hospital (Masahito Fujishige); Tokyo Women's Medical University (Shinichiro Uchiyama); Yamagata City Hospital Saiseikan (Shinjiro Saito); Dokkyo Medical University (Koichi Hirata); Hakodate Neurosurgical Hospital (Tsukasa Kubota); Kagoshima Medical Center (Naoaki Kanda); Mito Medical Center (Makoto Sonobe); Miyagi National Hospital (Tadashi Ando); Osaka Neurosurgical Hospital (Hideo Ohyama); St. Marianna University School of Medicine (Yasuhiro Hasegawa); Yamaguchi University Hospital (Michiyasu Suzuki); Yokohama Rosai Hospital (Ichiro Imafuku); Hiratsuka City Hospital (Hiromichi Miyazaki); Iwate Medical University (Yasuo Terayama); Juntendo University Hospital (Takao Urabe); Kameda Medical Center (Hidehiro Shibayama); Keio University (Haruhiko Hoshino); Kyoto Medical Center (Michikazu Nakamura); Kyushu Medical Center (Yasushi Okada); Mie University Hospital (Waro Taki); Nagahama City Hospital (Kazuo Yamamoto); Nagasaki University Graduate School of Biomedical Sciences (Izumi Nagata); Nakamura Memorial Hospital (George Nakagawara); Osaka University Hospital (Kazuo Kitagawa); Rinku General Medical Center (Takao Soda); Saitama Red Cross Hospital (Kenji Yamamoto); Sendai Medical Center (Masayuki Ezura); Tokai University Hospital (Shunya Takizawa); Tokushima Prefecture Naruto Hospital (Masahito Agawa); Aichi Medical University (Manabu Doyu); Atsugi City Hospital (Takuya Ishii); Hiroshima City Hospital (Nobuyuki Hirotsune); Hoshigaoka Medical Center (Ryuzo Fukunaga); Isesaki-Sawa Medical Association Hospital (Koji Arai); Jikei University School of Medicine (Soichiro Mochio); Juntendo University Urayasu Hospital (Shigeki Tanaka); Kinki University Hospital (Fumiharu Akai); Kishiwada City Hospital (Kohsuke Yamashita); Kyorin University Hospital (Hiroki Kurita); Nagoya Medical Center (Satoshi Okuda); Nanpuh Hospital (Shunichi Yokoyama); Nara Prefecture General Medical Center (Shoichiro Kawaguchi); National Center for Global Health and Medicine (Sousuke Takeuchi); Osaka General Hospital of West Japan Railway Company (Kazuhide Iwamoto); Sanin Rosai Hospital (Yasuaki Tanaka); Sapporo Medical University Hospital (Kiyohiro Houkin); Tokai University Hachioji Hospital (Yasuhisa Kitagawa); Tokyo Dental College Ichikawa General Hospital (Shigeru Nogawa); Tokyo Women's Medical University Yachiyo Medical Center (Takashi Ohashi); Yamagata Saisei Hospital (Hideo Kuchiki).
References
- 1.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Romano JG. Current diagnosis and management of symptomatic intracranial atherosclerotic disease. Curr Opin Neurol. 2012;25:18–26. doi: 10.1097/WCO.0b013e32834ec16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor RA, Weigele JB, Kasner SE. Current management of symptomatic intracranial stenosis. Curr Atheroscler Rep. 2011;13:321–329. doi: 10.1007/s11883-011-0183-2. [DOI] [PubMed] [Google Scholar]
- 4.Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, Lee JH, Kim JS. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–786. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Lynn MJ, Howlett-Dmith H, Stern BJ, Herzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial artery stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 6.Levy EI, Horowitz MB, Koebbe CJ, Jungreis CC, Pride GL, Dutton K, Purdy PD. Transluminal stent-assisted angiplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results. Neurosurgery. 2001;48:1215–1221. doi: 10.1097/00006123-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Higashida RT, Tsai FY, Halbach VV, Dowd CF, Hieshima GB. Cerebral percutaneous transluminal angioplasty. Heart Dis Stroke. 1993;2:497–502. [PubMed] [Google Scholar]
- 8.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu C, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ. SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kitagawa Y, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C. CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 10.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, Frankel M, Chimowitz MI. WASID Study Group. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–2068. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 12.Tamura A, Yamamoto Y, Nagakane Y, Takezawa H, Koizumi T, Makita N, Makino M. The relationship between neurological worsening and lesion patterns in patients with acute middle cerebral artery stenosis. Cerebrovasc Dis. 2013;35:268–275. doi: 10.1159/000348313. [DOI] [PubMed] [Google Scholar]
- 13.Hallevi H, Chernyshev OY, EI Khoury R, Soileau MJ, Walker KC, Grotta JC, Savitz SI. Intracranial atherosclerosis is associated with progression of neurological deficit in subcortical stroke. Cerebrovasc Dis. 2012;33:64–68. doi: 10.1159/000333388. [DOI] [PubMed] [Google Scholar]
- 14.Wang LL, Yu SCH, Soo YOY, Wong KSL. Regression of intracranial stenosis under intensive risk factor control. Asia Oceanian Congress of Neurology, FP-6-Ab0154, Macao. 2014 March 5 [Google Scholar]
- 15.Diener H-C, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ. MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ. CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 17.Chi YW, Lavie CJ, Milani RV, White CJ. Safety and efficacy of cilostazol in the management of intermittent claudication. Vasc Health Risk Manag. 2008;4:1197–1203. doi: 10.2147/vhrm.s3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi S, Oida K, Fujiwara R, Maeda H, Hayashi S, Takai H, Tamai T, Nakai T, Miyabo S. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992;20:900–906. doi: 10.1097/00005344-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Otsuki M, Saito H, Xu X, Sumitani S, Kouhara H, Kurabayashi M, Kasayama S. Cilostazol represses vascular cell adhesion molecule-1 gene transcription via inhibiting NF-κB binding to its recognition sequence. Atherosclerosis. 2001;158:121–128. doi: 10.1016/s0021-9150(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 20.Nishio Y, Kashiwagi A, Takahara N, Hidaka H, Kikkawa R. Cilostazol, a cAMP phosphodiesterase inhibitor, attenuates the production of monocyte chemoattractant protein-1 in response to tumor necrosis factor-alpha in vascular endothelial cells. Horm Metab Res. 1997;29:491–495. doi: 10.1055/s-2007-979086. [DOI] [PubMed] [Google Scholar]