Abstract
Background:
A new fibrin adhesive made of buffalo plasma-derived fibrinogen and a thrombin-like snake venom enzyme, has been successfully used to immobilize free gingival grafts. This case series histologically compared sutured grafts (control group) with others immobilized by using the fibrin adhesive (experimental group).
Case Description:
The grafts were placed in the contralateral mandibular bicuspids of 15 patients, so that each subject received one treatment of each type. Five biopsies of each group were collected at 7, 14 and 45 days of healing, which were histologically and morphometrically analyzed as regards the relative volume density of the different connective tissue components.
Results:
The sites in the control group presented a higher inflammatory cell density at 7 days and a tendency towards a lower collagen density. In the experimental group, the grafts had an appearance of more advanced healing. Tissue maturity characteristics progressed until 14 and 45 days, but no difference between groups could be noted at these times.
Conclusions:
Within the limits of the present study, it may be suggested that the alternative fibrin adhesive tested could represent an alternative to sutures in gingival grafts procedures.
Keywords: Fibrin tissue adhesive, therapeutic use; Mouth mucosa; Grafts, transplantation
INTRODUCTION
The first event following tissue damage is impaired blood vessel hemorrhage. The subsequent coagulation process produces a fibrin network that fills the lesion space, acting as a natural adhesive that allows the edges of the wound to be kept together. The fibrin network may also act as an intermediate layer facilitating fibroblast adherence, migration and proliferation. Meanwhile, the fibrin degradation byproducts activate macrophages, which represent a fundamental cell population for the normal repair process, contributing with growth factors9.
The role of the exogenous fibrin used as a tissue adhesive is to mime and amplify the last physiologic steps of the coagulation stage. In 1989, while seeking a material with no human-derived products in its composition, a new fibrin adhesive was presented by a multidisciplinary group of researchers at the Center for Study of Venoms and Poisonous Animals (CEVAP) of the Sao Paulo State University, Brazil. It is an adhesive consisting of a thrombinlike enzyme isolated from the Crotalus durissus terrificus snake venom (rattlesnake), which replaces the thrombin. Human fibrinogen is replaced by another enzyme obtained by means of buffalo blood fractioning12. Stolf12 (1999) evaluated this new adhesive in human skin surgery. Cosmetic evaluation of the scar formation was excellent for the glued area and the patients did not show any local or systemic toxic effects. However, no studies have histologically analyzed this adhesive in humans until now.
With a view to evaluating the effect of using this new adhesive in periodontal surgery, the repair of gingival grafts immobilized with the adhesive was compared with those immobilized by sutures2. The present case series reports the histological findings of this evaluation.
CASE DESCRIPTION
A total of 15 patients, 10 women and 5 men, were selected for this study. Most of the selected patients were indicated for gingival grafts because of orthodontics and prosthetic reasons. The study protocol was approved by the Bauru Dental School Ethics Committee, and an informed written consent was obtained from each subject.
The surgical procedures were performed in 2 homologous contralateral sites, around the area of the mandibular bicuspids of each patient, according to the technique suggested by Sullivan and Atkins13. A receptor site was randomly selected for each patient as the test area, where the graft was immobilized with the fibrin adhesive. The contralateral site was thus used as a control, where the graft was sutured with polyglactin 910 5.0 suture*. All grafts were trimmed to a standardized size. It was opted to place the grafts on both sides in a single surgical procedure, in order to eliminate possible variables, such as the subject's systemic features in a certain period.
The adhesive used was donated by CEVAP, and consisted of three separate solutions: one of fibrinogen, another of calcium chloride and the third composed of a thrombin-like fraction. This material was stored at 4°C (thrombin-like fraction) and −20°C (other components) up to a few minutes before it was used, when it was kept at room temperature. Two 5-ml sterile insulin syringes † were used to apply the adhesive. One syringe was filled with fibrinogen and the other with calcium chloride and the thrombin-like enzyme. Afterwards, equal amounts from each syringe were concomitantly applied to the entire extent of the receptor bed and internal aspect of the graft, which was then positioned under uniform digital pressure for 5 minutes. A similar pressure was exerted on the graft in the control side. Surgical wounds were protected by surgical dressings ‡ during one week. Sutures were removed after seven days.
Biopsies - Biopsies were collected from each group (control and experimental) from each patient at a certain moment of the repair process, making use of a circular surgical blade measuring 4.0 mm in diameter. Each sample involved the apical mesial area of each graft. A total of five samples were obtained from each group at 7, 14 and 45 days after surgery.
Histological processing
Tissue samples were fixed in 10%-formaldehyde and then processed for posterior embedment in liquefied resin. Semiserial cuts, 5-^m thick were made perpendicular to the epithelial surface. They were stained with hematoxylin-eosin (HE).
Morphometric examination
The cellular volume density was quantified by the method proposed by Chalkley5, in accordance with the directions of Weibel and Gomes14, for blood vessels, the inflammatory cell population and fibroblasts. With regard to the latter, only the volume of the nuclei was taken into account, due to the impossibility of observing cytoplasm by the histological method used. Estimates of connective tissue components, other than vessels, fibroblasts and inflammatory cells, include collagen fibers.
Statistical Analysis
Means and standard errors were obtained and analyzed using a statistical software program § . Differences between different time points for each group and between different groups were sought using the repeated mesures of variance for 2 criteria. Differences with a P value <0.05 at a confidence level of 95% were considered significant.
RESULTS
Clinical findings
The decrease in the vertical dimension of the grafts was significant during the first 30 days and more dramatic for the control group (15.66% and 22.33% for test and control groups, respectively). Probing depth and attachment level at probing presented a significant decrease for both groups. The grafts of the experimental group presented better appearance during the first 14 post-operative days2.
Histological analysis
7 days
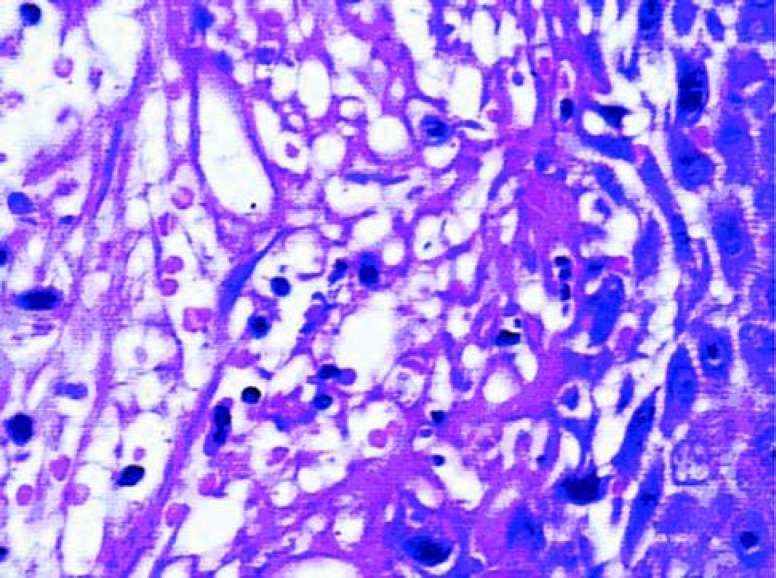
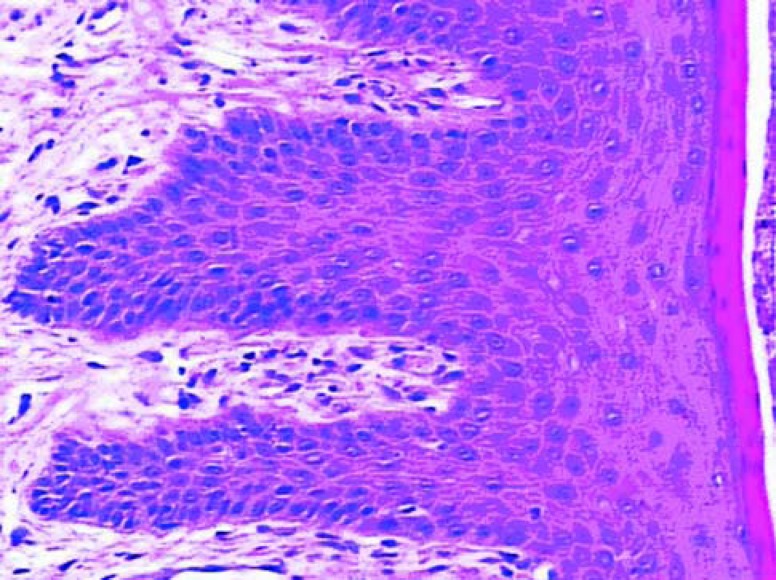
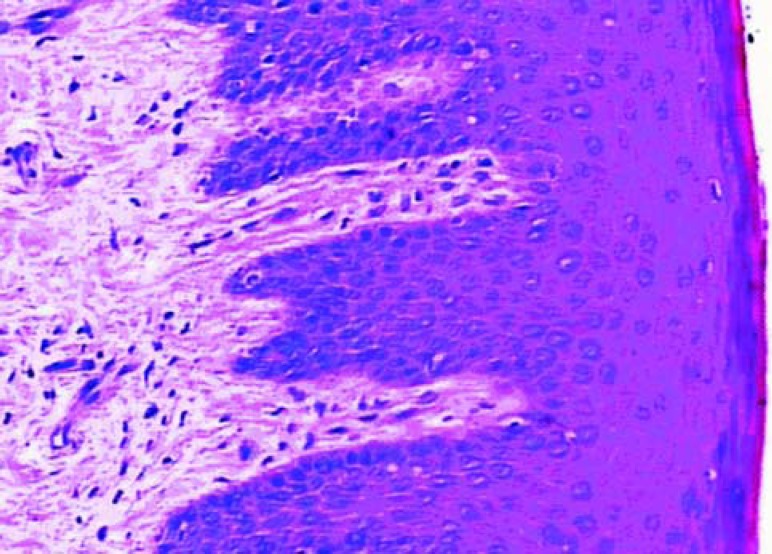
The epithelial growth was observed from the edge to the center of the grafts. The epithelium was still immature, thin and without epithelial crests, particularly in the center. In the graft portion closer to the alveolar mucosa, the epithelium was thicker and some irregular epithelial projections could be observed, forming structures that in the future could probably be defined as epithelial crests. Test samples (Figure 2) presented a tendency to more organized epithelium than the control samples (Figure 1). In the lamina itself, the subepithelial area was less dense, remarkably cellularized, rich in capillary vessels and inflammatory cells. There were many fibroblasts. The inflammatory infiltrate was diffuse in this area. There was less significant presence of polymorphonuclear leukocytes than of mononuclear leukocytes. The vessels were wide, frequently congested and endothelial swelling was generally remarkable. The inflammatory characteristics were more pronounced in the control group.
FIGURE 2. Histologic section of the test group after 7 days of healing (original magnification x 40).
FIGURE 1. Histologic section of the control group after 7 days of healing (original magnification x40).
14 days
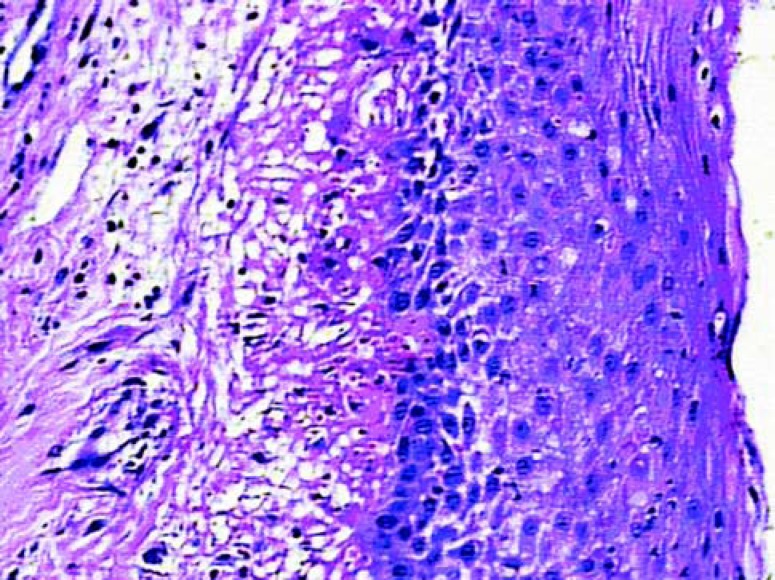
At this healing phase the samples were characterized by young connective tissue and by a remarkable reduction in inflammatory events. Higher density of fibroblast and collagen fibers could be observed than that present in the 7 day samples. In both the test (Figure 4) and in control (Figure 3) sites, the epithelial tissue clearly presented spinous characteristics and keratin production was evident in the analyzed samples. However, the test samples tended to be more mature.
FIGURE 4. Histologic section of the test group after 14 days of healing (original magnification x 10).
FIGURE 3. Histologic section of the control group after 14 days of healing (original magnification x 10).
45 days
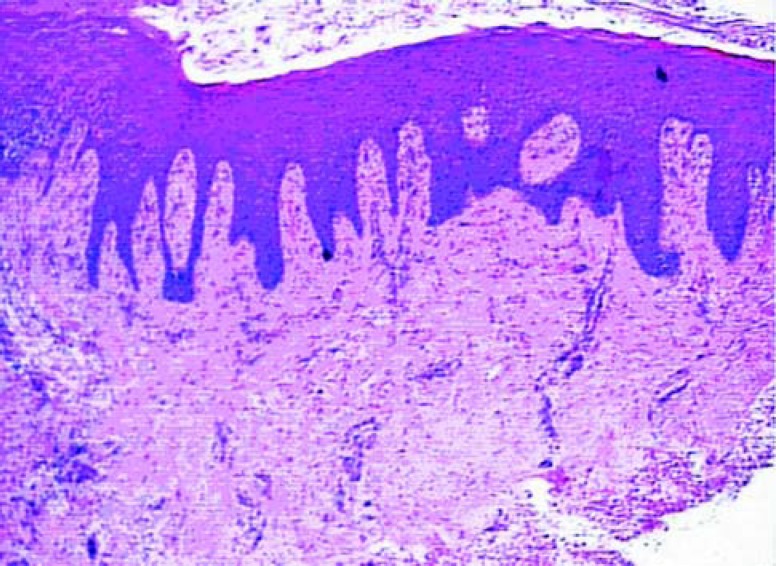
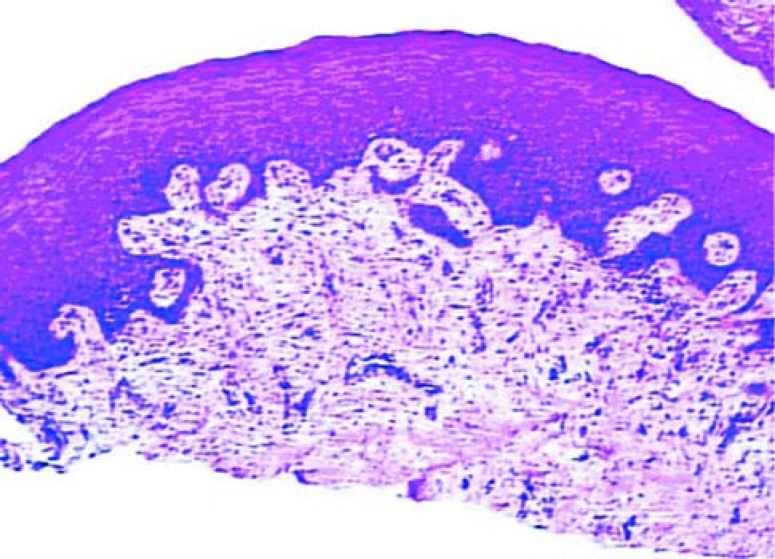
Differences from the 14 day samples characterized tissue maturation. In the connective tissue, the more evident characteristics were the decrease in cellularity and vascularity, and the increase in collagen fibers. The epithelium was mature and no difference could be noted between groups (Figures 5 and 6).
FIGURE 5. Histologic section of the control group after 45 days of healing (original magnification x 40).
FIGURE 6. Histologic section of the test group after 45 days of healing (original magnification x 40).
Morphometric analysis
The mean density of the volume of fibroblast nuclei (Table 1) was higher in the test group than in the control group at 7 days post-operatively, yet this difference was not statistically significant. At 14 days, the mean values were closer between the test and control groups and were even smaller at 45 days. This gradual reduction in the values of each group was statistically significant (p<0.0001). The mean relative volume density of the inflammatory cells (Table 2) was higher for the control group in comparison with the test group both at 7 and at 14 days. This difference between the groups was statistically significant at 7 days (p<0.0001). The reduction in density in both groups was significant between 7 and 14 days (p<0.0001). At 45 days, the mean values of the two groups were similar. The means of the relative volume density of blood vessels (Table 3) for the control and test groups gradually decreased from 7 days to 14 days and to 45 days. This reduction was significant between 7 and 14 days (p<0.0001). There was no statistically significant difference between the groups. The mean of the relative volume density of other components in the connective tissue (Table 4) in the test and control groups increased more remarkably between 7 and 14 days. At 45 days, these means reached the highest values. The differences, according to the period of repair, were statistically significant (p<0.0001). There was no significant difference between the groups.
TABLE 1. Mean Density of the Relative Volume of the Fibroblasts' Nuclei.
| Group | ||
|---|---|---|
| Day | Control | Test |
| 7 | 8.87 ± 1.394 |
10.81 ± 1.834 |
| 14 | 7.26 ± 0.513 |
7.41 ± 0.775 |
| 45 | 5.70 ± 0.606 |
4.78 ± 0.667 |
Mean ± standard error (mm)
TABLE 2. Mean Density of the Relative Volume of the Inflammatory Cells' Nuclei.
| Group | ||
|---|---|---|
| Day | Control | Test |
| 7 | 1.53 ± 0.222 |
0.84 ± 0.191 |
| 14 | 0.61 ± 0.231 |
0.36 ± 0.094 |
| 45 | 0.37 ± 0.131 |
0.37 ± 0.087 |
Mean ± standard error (mm)
TABLE 3. Mean Density of the Relative Volume of Blood Vessels.
| Group | ||
|---|---|---|
| Day | Control | Test |
| 7 | 12.25 ± 2.544 |
11.71 ± 2.971 |
| 14 | 2.18 ± 0.623 |
2.74 ± 1.194 |
| 45 | 1.69 ± 0.544 |
1.56 ± 1.136 |
Mean ± standard error (mm)
TABLE 4. Mean Density of the Relative Volume of Other Components in the Connective tissue.
| Group | ||
|---|---|---|
| Day | Control | Test |
| 7 | 77.3 ± 3.369 |
76.6 ± 4.240 |
| 14 | 89.9 ± 0.921 |
89.5 ± 0.759 |
| 45 | 92.2 ± 0.728 |
93.3 ± 0.652 |
Mean ± standard error (mm)
DISCUSSION
At 7 days post-operatively, a well-constituted fine stratified pavement epithelium was observed, with no crest projections, in control and test groups. The lamina itself was characterized by a dense connective tissue, with some areas of edema, and a remarkable presence of congested blood vessels and inflammatory cells, constituting a predominantly lymphoplasmacytoid infiltrate, especially within the subepithelial area. These findings are in agreement with other studies conducted with conventional sutured grafts3,11.
The predominant type of cell was the fibroblast that showed the highest relative volume density for control and test groups. Mean fibroblast density was higher in the test than in the control group, but without statistical significance. Inflammatory cells presented a significantly higher relative volume concentration in the control areas than at the test sites. One could suppose that these findings resulted from the presence of suture strands in the control group sites, which may act as stimulus for the accumulation and perpetuation of the inflammatory infiltrate10. However, these findings are common near the suture, so it is questionable whether this could explain the findings in control biopsies collected from more distant areas. It is also likely that by stimulating larger fibrin deposition, the tissue adhesive may have positively influenced fibroblast migration4,8,9, macrophage chemotaxis1 and wound vascularization6, stimulating the repair at the test group sites. This may explain the higher concentration of fibroblasts and lower concentration of inflammatory cells.
Nevertheless, the smaller concentration of inflammatory cells in the test group was possibly related to the patients' faster recovery, and a lower degree of morbidity, since the patients chose the test side as the area that healed the best2.
The comparison between the repair process of the test grafts and the control grafts revealed that the sites where the fibrin adhesive was applied presented a more notable decrease in the volume density of fibroblast nuclei at 45 days. Moreover, the test areas also presented a tendency towards a higher volume density of the other connective tissue components at 14 days, when the edema and endothelial tumefaction had almost completely disappeared. This suggests a higher accumulation of collagen fibers. Although there were no statistically significant differences between the groups studied, higher volume density values for the other connective tissue components were found for all subjects in the test area.
Considering all these findings, it was possible to infer that there was a relationship between them, that is, the remarkable reduction in fibroblasts concomitantly with the increase in the connective tissue density would be related to the maturation of the epithelium. In the present study, the areas treated with the fibrin adhesive presented a tendency to stimulate the repair process during the first post-operative days. The adhesive could be well identified histologically in the tissue as an amorphic eosinophilic substance up to day 3 and nearly disappeared by day 71. This would further support the results of the histological study conducted in dogs by Pini Prato, et al.7. In this study, the authors compared mucogingival flaps immobilized with silk sutures in comparison with those immobilized by using a fibrin adhesive of human plasma, and observed that seven days after surgery, analysis of the areas where the adhesive had been applied revealed a higher number of fibroblasts. At the 14th day, the tissues showed a similar aspect.
The histological tendency for faster maturation of the test group may be related to the clinical finding of better maturation of the test grafts. The difference in the clinical and histological characteristics of healing between the control and test groups in the 7-day follow-up was remarkable, being less noticeable in the 14-day follow-up 2.
The fibrin adhesive tested in this study seemed to promote gingival graft maturation during the first week, in comparison with the use of sutures. On the other hand, the limited number of samples studied limits the significance of the results? and the statistical findings. The histological results are elucidative but not sufficient to draw conclusions. Nevertheless, well-controlled clinical investigations should be conducted to morphometrically evaluate the repair characteristics of the material for different surgical techniques.
CONCLUSIONS
Therefore, within the limits of the present study, it may be suggested that the fibrin adhesive tested could represent an alternative to sutures in periodontal surgery.
ACKNOWLEDGMENTS
The authors thank the The Center for the Study of Venoms and Venomous Animals (CEVAP) – São Paulo State University (UNESP) Brazil. for their cooperation. This study was supported by the São Paulo Research Support Foundation.
Footnotes
Vicryl, Ethicon, Johnson & Johnson, São José dos Campos, Brazil
B-D Bard-Parker Co. Inc., USA
Coe Pack No Eugenol, Coe Laboratories Inc. – Chicago, Illinois, USA
Sigma Stat for Windows, Systat Software Inc. California, USA
REFERENCES
- 1.Bahar I, Weinberger D, Lusky M, Avisar R, Robinson A, Gaton D. Fibrin glue as a suture substitute: histological evaluation of trabeculectomy in rabbit eyes. Curr Eye Res. 2006;31(1):31–36. doi: 10.1080/02713680500477354. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa MDS, Greghi SLA, Passanezi E. Fibrin adhesive derived from snake venom in periodontal surgery. J Periodontol. 2007;78(10):2026–2031. doi: 10.1902/jop.2007.070005. [DOI] [PubMed] [Google Scholar]
- 3.Brackett RC, Gargiulo AW. Free gingival grafts in humans. J Periodontol. 1970;41:581–586. doi: 10.1902/jop.1970.41.10.581. [DOI] [PubMed] [Google Scholar]
- 4.Brown LF, Lanir N, McDonagh J, Tognazzi KI, Dvorak AM, Dvorak HF. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993;142(1):273–283. [PMC free article] [PubMed] [Google Scholar]
- 5.Chalkley HW. Method for the quantitative morphologic analyses of tissues. J Nat Cancer Invest. 1943:47–53. [Google Scholar]
- 6.Hashimoto J, Kurosaka M, Yoshiya S, Hirohata K. Meniscal repair using fibrin sealant and endothelial cell growth factor: an experimental study in dogs. Am J Sports Medicine. 1992;20(5):537–541. doi: 10.1177/036354659202000509. [DOI] [PubMed] [Google Scholar]
- 7.Pini Prato GP, De Paoli S, Cortellini P, Zerosi C, Clauser C. On the use of a biologic sealing system (tissucolâ) in periodontal therapy. II histological evaluation. Int J Periodontics Restorative Dent. 1985;5(3):33–41. [PubMed] [Google Scholar]
- 8.Romanos GE, Strub JR. Effect of tissucol on connective tissue matrix during wound healing: an immunohistochemical study in rat skin. J Biomed Mater Res. 1998;39(3):462–468. doi: 10.1002/(sici)1097-4636(19980305)39:3<462::aid-jbm17>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Schlag G, Redl H. Fibrin sealing in surgical and nonsurgical fields. 1ed. Berlin: Heidelberg; 1994. [Google Scholar]
- 10.Selvig KA, Biagiotti GR, Leknes KN, Wikesj UM. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18(5):475–487. [PubMed] [Google Scholar]
- 11.Staffileno H, Levy S. Histologic and clinical study of mucosal (gingival) transplants in dogs. J Periodontol. 1969;40:311–319. doi: 10.1902/jop.1969.40.6.311. [DOI] [PubMed] [Google Scholar]
- 12.Stolf HO. The use of fibrin adhesive derived from snake venom and the evaluation of skin graft from patients nasolabial fold. J Venom Anim Toxins. 1999;5(2):227–227. [Google Scholar]
- 13.Sullivan SM, Atkins JH. Free autogenous gingival grafts. I Principles of successful graftings. Periodontics. 1968;6(4):5–13. [PubMed] [Google Scholar]
- 14.Weibel ER, Gomes DM. A principe for counting tissue structures on random sections. J App Physiol. 1962;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]