Abstract
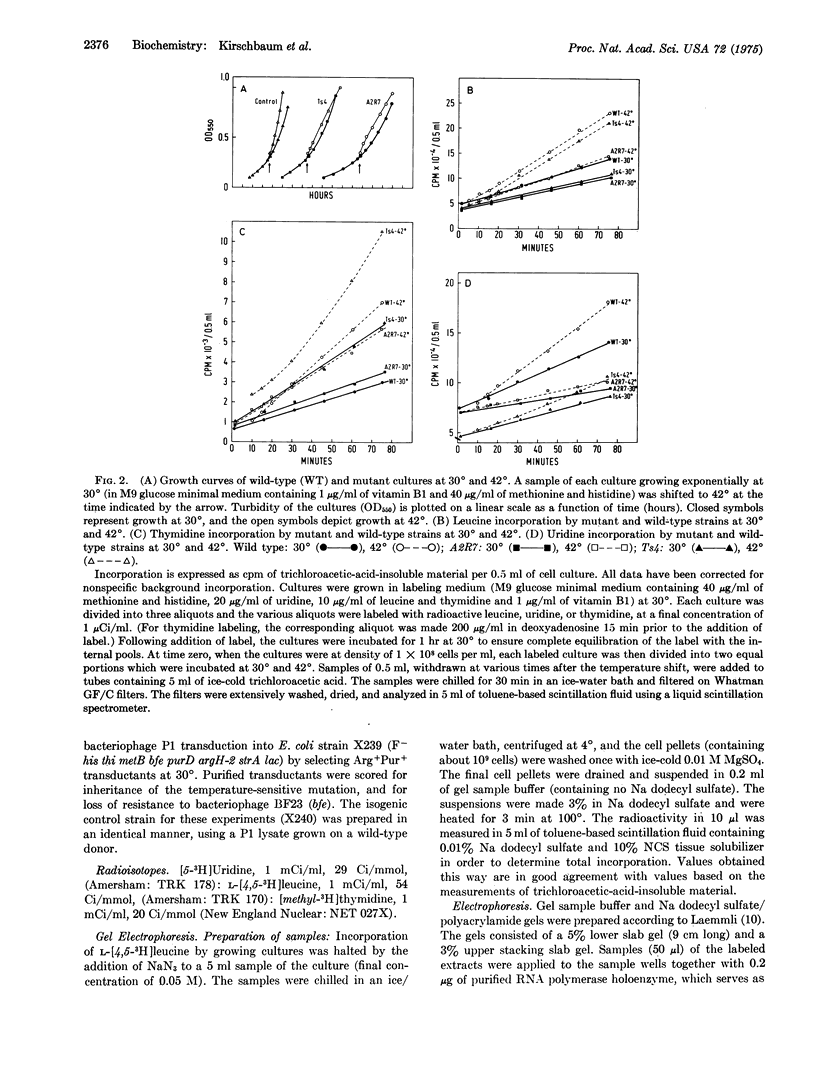
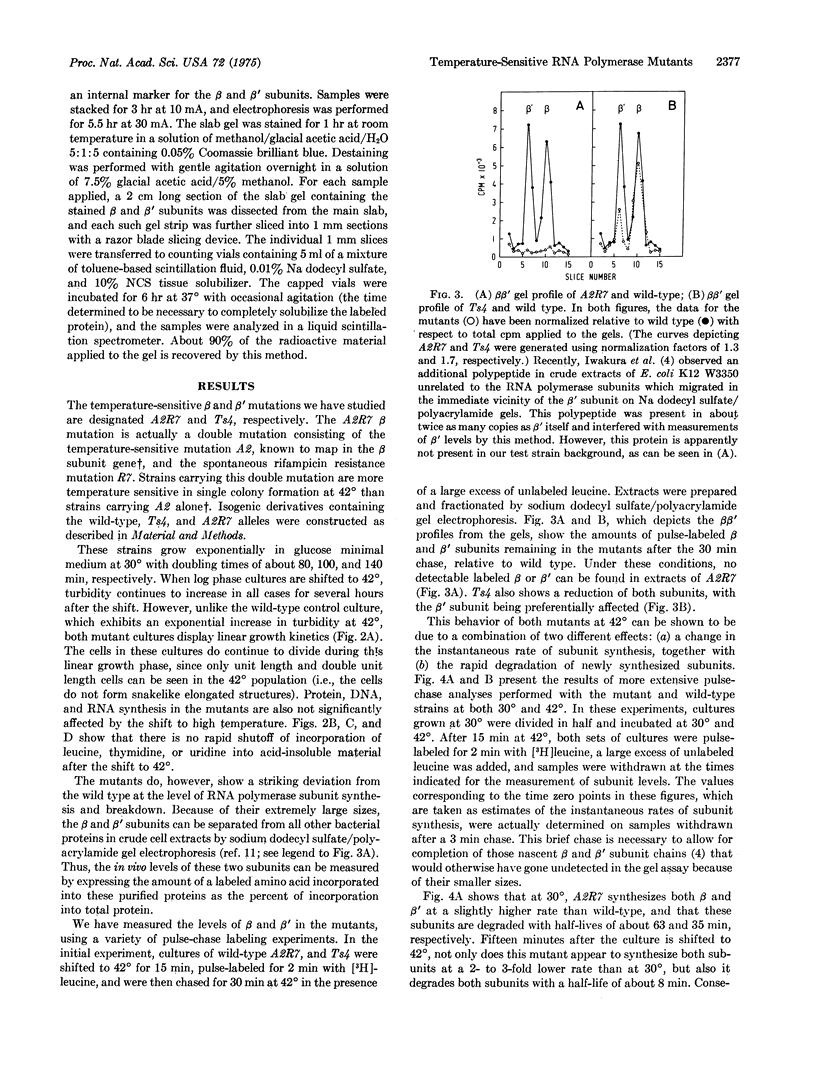
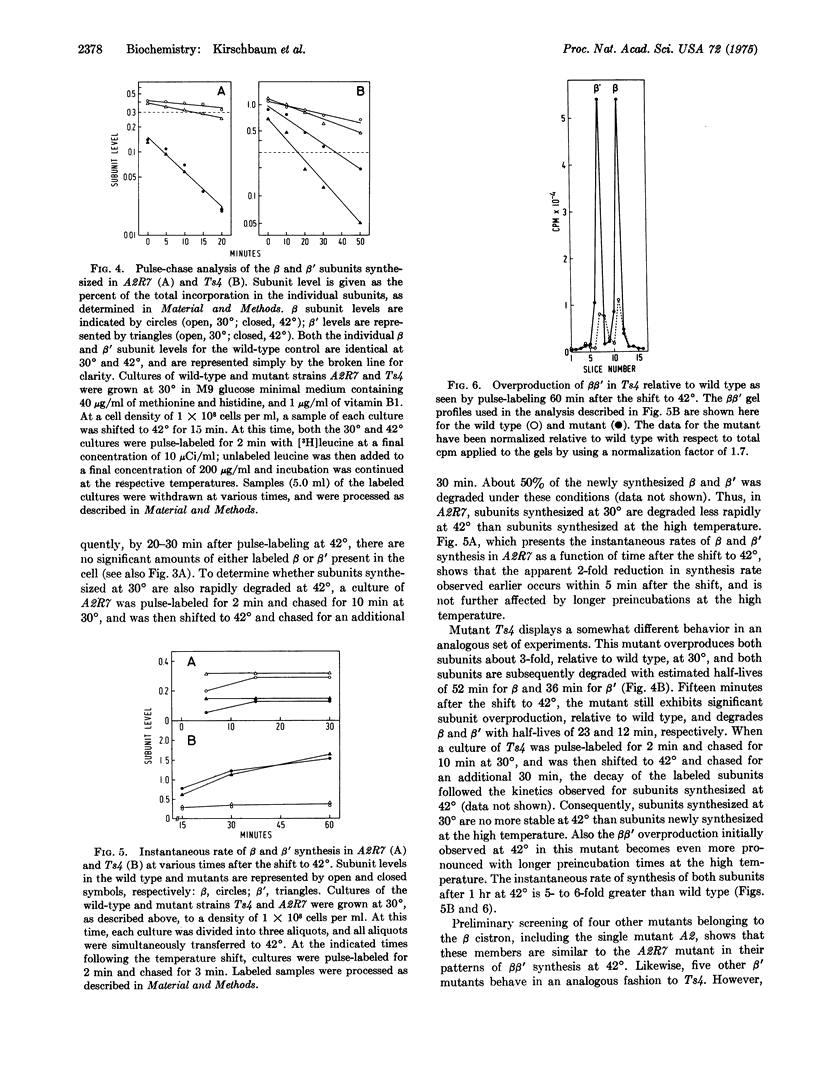
A temperature-sensitive mutant having a lethal mutation in the gene for the beta subunit of RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) exhibits an apparent 2- to 3-fold decrease in the rates of both beta and beta' subunit synthesis at the non-permissive temperature, relative to total protein. In contrast, a temperature-sensitive mutant with a lethal mutation in the gene encoding beta' has a 5- to 6-fold increase in the rates of beta and beta' synthesis at 42 degrees. These beta and beta' mutants also exhibit rapid degradation of both subunits at the high temperature.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D., Chamberlin M. Physical studies on ribonucleic acid polymerase from Escherichia coli B. Biochemistry. 1970 Dec 22;9(26):5055–5064. doi: 10.1021/bi00828a003. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Hayward R. S., Austin S. J., Scaife J. G. The effect of gene dosage on the synthesis and stability of RNA polymerase subunits in Escherichia coli. Mol Gen Genet. 1974;131(2):173–180. doi: 10.1007/BF00266152. [DOI] [PubMed] [Google Scholar]
- Hayward R. S., Tittawella I. P., Scaife J. G. Evidence for specific control of RNA polymerase synthesis in Escherichia coli. Nat New Biol. 1973 May 2;243(122):6–9. [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Khesin R. B., Gorlenko Z. M., Shemyakin M. F., Stvolinsky S. L., Mindlin S. Z., Ilyina T. S. Studies on the functions of the RNA polymerase components by means of mutations. Mol Gen Genet. 1969;105(3):243–261. doi: 10.1007/BF00337475. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B. Regulation of subunit synthesis of Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2651–2655. doi: 10.1073/pnas.70.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Scaife J. Evidence for a lambda transducing phage carrying the genes for the beta and beta' subunits of Escherichia coli RNA polymerase. Mol Gen Genet. 1974;132(3):193–201. doi: 10.1007/BF00269392. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matzura H., Molin S., Maaloe O. Sequential biosynthesis of the and ' subunits of the DNA-dependent RNA polymerase from Escherichia coli. J Mol Biol. 1971 Jul 14;59(1):17–25. doi: 10.1016/0022-2836(71)90410-4. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. Regulation of RNA polymerase synthesis in Escherichia coli: a mutant unable to synthesize the enzyme at 43 degrees. Proc Natl Acad Sci U S A. 1975 Mar;72(3):911–915. doi: 10.1073/pnas.72.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panny S. R., Heil A., Mazus B., Palm P., Zillig W., Mindlin S. Z., Ilyina T. S., Khesin R. B. A temperature sensitive mutation of the beta'-subunit of DNA-dependent RNA polymerase from E. coli T 16. FEBS Lett. 1974 Nov 15;48(2):241–245. doi: 10.1016/0014-5793(74)80477-1. [DOI] [PubMed] [Google Scholar]


