Abstract
Studies have shown the cariostatic effect of Er,Cr:YSGG (2.78 μm) laser irradiation on human enamel and have suggested its use on caries prevention. However there are still no reports on the intrapulpal temperature increase during enamel irradiation using parameters for caries prevention. The aim of this in vitro study was to evaluate the temperature variation in the pulp chamber during human enamel irradiation with Er,Cr:YSGG laser at different energy densities. Fifteen enamel blocks obtained from third molars (3 x 3 x 3 mm) were randomly assigned to 3 groups (n=5): G1 – Er,Cr:YSGG laser 0.25 W, 20 Hz, 2.84 J/cm2, G2 – Er,Cr:YSGG laser 0.50 W, 20 Hz, 5.68 J/cm2, G3 – Er,Cr:YSGG laser 0.75 W, 20 Hz, 8.52 J/cm2. During enamel irradiation, two thermocouples were fixed in the inner surface of the specimens and a thermal conducting paste was used. One-way ANOVA did not show statistically significant difference among the experimental groups (α=0.05). There was intrapulpal temperature variation ≤0.1°C for all irradiation parameters. In conclusion, under the tested conditions, the use of Er,Cr:YSGG laser with parameters set for caries prevention lead to an acceptable temperature increase in the pulp chamber.
Keywords: Temperature, Dental enamel, Laser, Prevention
INTRODUCTION
The effectiveness of laser irradiation as a caries preventive treatment is closely related to the light interaction with some of the enamel compounds10,16,19,23. As enamel composition in 85% by volume carbonated hydroxyapatite with 12% water and 3% protein and lipid by volume14, the use of wavelengths that are highly absorbed by both water and hydroxyapatite is expected to generate thermal changes on enamel that may be able to chemically and/or morphologically alter its structure6,10–12,15,16.
Er,Cr:YSGG laser devices are relatively new and work at a wavelength of2.78 μm. Er,Cr:YSGG laser has been reported to ablate dental hard tissues with minimal injury to the pulp and surrounding tissues9; it can ablate enamel effectively due to its high absorption in water22 and because it is also strongly absorbed by the hydroxyl radicals present in the hydroxyapatite structure12,16,18. It is supposed that, for caries prevention, Er,Cr:YSGG laser does not ablate the surface, but rather change the enamel chemically by enhancing its surface temperature.
Enamel chemical alterations occur gradually with the increase of temperature12,15. When the surface temperature increases between 100 and 650°C, the main chemical changes that occur in the tooth are that the major CO3 component in the phosphate position decreases and the acid phosphate ions condense to form pyrophosphate ions. At 650-1,100°C, the main changes are thermal recrystallization and crystal size growth, and pyrophosphate react with apatite to form PO4 along with the formation of β-TCP. At temperatures >1,100°C, the main change is that the β-TCP is converted to a-TCP and when the temperature reaches 1,430°C this compound changes into a high-temperature polymorph. Alpha and beta tri-calcium-phosphate (TCP) are potentially soluble in acid environments12,15.
Although sub-ablative energy densities have been suggested for caries preventive treatment, it is recommended that the irradiation be performed without water cooling1,20,22. There is still some debate in the literature regarding the parameters of irradiation and there are no reports on the temperature increase in the pulp chamber during enamel irradiation. According to Zach and Cohen29 (1965), an intrapulpal temperature increase above 5.5°C can damage the pulpal tissue26,29. The aim of the present study was to evaluate the temperature variation during enamel irradiation with Er,Cr:YSGG laser using different parameters for caries prevention.
MATERIALS AND METHODS
Ethical Aspects
The teeth used in this investigation were collected in conformity with the guidelines of the Ethics in Research Committee of the Dental School of the University of São Paulo (Process 182/03).
Study Design
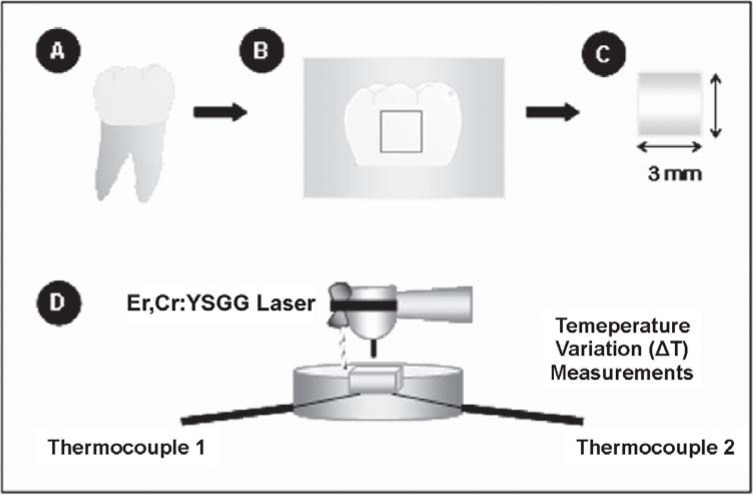
The surface treatment was performed under the following conditions: G1 (group 1) - Er,Cr:YSGG laser at 0.25 W, 20 Hz, 2.8 J/cm2; G2 (group 2) - Er,Cr:YSGG laser at 0.50 W, 20 Hz, 5.7 J/cm2; G3 (group 3) - Er,Cr:YSGG laser at 0.75 W, 20 Hz, 8.5 J/cm2. This study used 15 human enamel specimens (n=5) obtained from unerupted third molars. The temperature measurements (ΔT) were carried out for each specimen during the irradiation. Figure 1 illustrates the experimental design.
FIGURE 1. Schematic diagram of the study design A and B. Third human molars were collected and had their crowns removed close to the cementoenamel junction and sectioned longitudinally. C. Fifteen 15 enamel blocks (3 x 3 mm) were obtained and further fixed individually on an acrylic device; D. Laser irradiation was carried out on the specimen of each experimental group and temperature variation (ΔT) measurements were performed during laser irradiation.
Preparation of the enamel specimens
Fifteen freshly extracted, non-erupted third molars were collected and immediately stored in 0.1% thymol solution (pH 7.00) (Figure 1A). The crowns were removed close to the cementoenamel junction and were longitudinally sectioned with double-face diamond disks (KG Sorensen, Barueri, SP, Brazil) used at a low speed handpiece (Kavo, Joinville, SC, Brazil). Fifteen enamel specimens of 3 x 3 x 3 mm were obtained (Figures 1B and 1C). The total thickness of the specimens was 3 mm and the enamel and dentin thickness ranged from 0.6-0.9 mm and 2.1-2.4 mm, respectively. The specimens were examined under a stereomicroscope at x30 (EMZ-10, Meiji Techno, San Jose, CA, USA) and those with stains or cracks were discarded. After sectioning was completed, the enamel specimens were randomly assigned to the experimental groups (Table 1).
TABLE 1. Experimental groups.
| Group | Power (W) | Energy Density (J/cm2) | Repetition rate (Hz) |
|---|---|---|---|
| G1 (n=5) | 0.25 | 2.8 | 20 |
| G2 (n=5) | 0.50 | 5.7 | 20 |
| G3 (n=5) | 0.75 | 8.5 | 20 |
Laser Irradiation
The enamel specimens were irradiated with Er,Cr:YSGG laser (Waterlase Millennium™, Biolase Technologies Inc., San Clemente, CA, USA) emitting photons at a wavelength of 2.78 μm. The output power of this equipment ranges from 0.25 to 6.0 W and the repetition rate is fixed on 20 Hz. Pulse duration ranges from 140 to 200 μs. The beam diameter at the focal area for the handpiece used with the S75 tip is 0.75 mm. The tip was positioned 1 mm from the enamel surface (focused mode), as shown in Figure 1D. To ensure a consistent spot size, an endodontic file was fixed at the handpiece and the distance of 1 mm from the surface was kept during the irradiation. The energy density per pulse used for laser irradiation in each experimental group is shown in Table 1. The handpiece was positioned perpendicular to the enamel surface and the specimens were irradiated once in each direction, moving the handpiece slowly horizontally (10 s) and vertically (10 s), in order to promote homogeneous irradiation and to cover the entire specimen area. Irradiation was performed by hand, scanning the enamel surface with a uniform motion3 both with air spray (30%) and without water cooling1,20,22.
Temperature Measurements
During laser irradiation, the variation on the intrapulpal temperature was measured for each specimen. For measurement of the temperature variation (ΔT), two thermocouples (MV100, Yokogawa Electric China Co., Ltd., Shanghai, China) were positioned under the enamel specimen, as shown in Figure 1. In order to maximize the temperature transference between the two thermocouples and the inner specimen surface (dentin), the acrylic device was filled with a thermal conducting paste (Implastec, Votorantin, Brazil). The temperature was captured after each 2 s.
Statistical Analysis
The mean values of temperature increase were taken for statistical analysis (α=0.05) and were submitted to ANOVA test using the SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
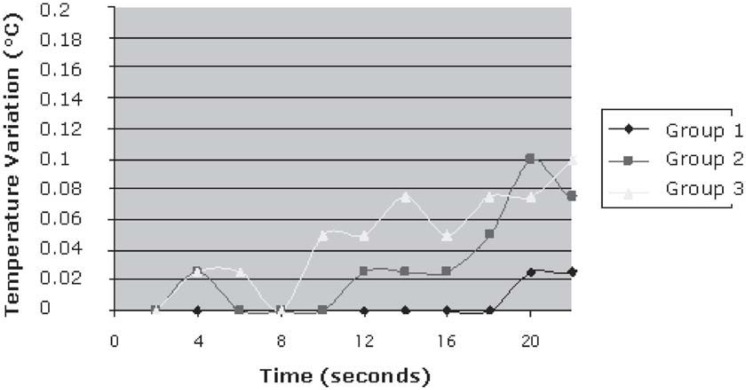
There was no statistically significant difference among the experimental groups (p=0.178). Temperature variation did not exceed 0.1°C for all tested parameter settings (Table 2 and Figure 2). The minimum and maximum temperatures measured were 0.025 and 0.1, respectively.
TABLE 2. Results of one-way analysis of variance (α=5%) for comparison of the mean values of temperature variation recorded in the experimental groups.
| Among the experimental groups Experimental Groups | F 1.799 Experimental Groups | Significance 0.178 Statistical Significance (p) |
|---|---|---|
| 1 | 2 | 0.211 |
| 3 | 0.275 | |
| 2 | 1 | 0.211 |
| 3 | 0.986 | |
| 3 | 1 | 0.275 |
| 2 | 0.986 |
FIGURE 2. Mean values for the temperature variation during enamel laser irradiation using caries preventive parameters.
DISCUSSION
The present study aimed to evaluate the temperature increase at the pulp chamber during enamel irradiation with Er,Cr:YSGG laser at sub-ablative parameters. These parameters have been reported in the literature to possibly induce enamel acid resistance20,27. Therefore, it is important to verify the temperature variation at the inner dentin in order to avoid pulp damage.
It is well known that enamel acid resistance produced by high intensity laser is obtained with the increase of surface temperature. According to Fowler and Kuroda15 (1986), the temperature necessary to achieve the photothermal effect and the enhancement of enamel acid resistance would be of approximately 100°C-650°C. Studies have shown that the energy density necessary to reach enamel acid resistance using Er,Cr:YSGG laser is approximately 8-13 J/cm2 16. These sub-ablative parameters2,3,12,16 are expected to decrease enamel solubility by promoting thermal decomposition of the more soluble carbonate hydroxyapatite into the less soluble hydroxyapatite with corresponding changes in the crystallinity16.
According to Fried, et al.16 (1996), Er:YSGG laser radiation, which is directly absorbed by the hydroxyl group of the carbonated hydroxyapatite mineral, will be more highly absorbed in the outer water-deficient layer of the enamel. This is based on the assumption that the outer few microns of the enamel contain a gradient in water content, with the water increasing with distance from the surface. Regarding the use of sub-ablative parameters, there will be no ejection of mineral (ablation) and the OH- present in the hydroxyapatite group will absorb the laser energy and change the chemical composition of the hard tissue. Moreover, Meister, et al.25 (2006) have investigated the effect of the water contained in dental hard tissues (enamel and dentin) on the efficiency of ablation. The authors reported that only in dentin, and then only with irradiation with the Er:YAG laser, is the water contained in the tissue found to have a significant influence on the ablated volume. The water content in enamel and dentin has no effect on the efficiency of laser ablation in Er,Cr:YSGG laser-treated groups. In contrast, the externally supplied water always has a significant influence on the effectiveness of the ablation process. The present study did not consider the use of water cooling during laser irradiation, as previously mentioned. Therefore, enamel ablation during caries preventive treatment was not expected.
Even though sub-ablative parameters are used for caries preventive treatment, it is recommended to perform irradiation without water cooling. Although water cooling seems to be important to avoid the increase of temperature28, the continued exposure of enamel to water during irradiation was reported to lead to enamel demineralization during an acid challenge21. This can be justified by the fact that water can absorb part of the energy delivered from the laser and lead to enamel ablation28. These areas can be more susceptible to demineralization after treatment. When performed with low energy densities and air cooling, as shown in this in vitro study, enamel irradiation can be performed safely without pulp tissue damage21.
Zach and Cohen (1965)29 have reported that temperature rises above 5.5°C are unacceptable because of the potential for loss of pulpal vitality. In the present study, temperature variation did not exceed 0.1°C in all tested irradiation parameters. One of the reasons for the low temperature increase is that Er,Cr:YSGG laser works at a wavelength of 2.78 μm, which is highly absorbed by the hydroxyl free radicals (OH-) present in hydroxiapatite14. Therefore, it can reach the enamel structure and be highly absorbed at the outer surface, increasing the temperature of enamel compounds and changing them chemically14 without causing side effects to the surrounding tissues. Additionally, Er,Cr:YSGG laser works at a pulse mode, which means that the temperature increase at the enamel surface using correct parameters of irradiation is expected not to lead to pulp damage. The third hypothesis for the maintenance of temperature can be attributed to the thickness of the specimens used in this study (3 mm), which could have influenced the thermal conductivity through enamel and dentin30, not allowing the energy to reach the pulp chamber.
Finally, it can be assumed that Er,Cr:YSGG laser irradiation at sub-ablative parameters can be considered for caries preventive treatment because it is not able to increase the intrapulpal temperature even without water cooling.
CONCLUSIONS
Within the limits of this in vitro study it can be concluded that Er,Cr:YSGG laser irradiation at sub-ablative parameters for caries prevention led to an acceptable temperature increase in the pulp chamber without exceeding 0.1°C.
ACKNOWLEGMENTS
The authors would like to thank the Special Laboratory of Lasers in Dentistry (LELO-FOUSP) and Dr. Eleni Kairalla for her contribution in this study. They also thank the State of São Paulo Research Foundation (FAPESP grants #03/10000-6, #98/14270-8 and Project CEPID-CEPOF, grant # 98/14270-8) for the financial support.
REFERENCES
- 1.Ana PA, Zezzel DM, Blay CC, Blay A, Eduardo CP, Miyakawa W. Thermal analysis of dental enamel following Er,Cr:YSGG laser irradiation at low fluencies. Lasers Surg Med. 2004;16:53–63. [Google Scholar]
- 2.Apel C, Birker L, Meister J, Gutknecht N. The caries-preventive potential of subablative Er:YAG and Er:YSGG laser radiation in a intraoral model: a pilot study. Photomed Laser Surg. 2004;22:312–317. doi: 10.1089/pho.2004.22.312. [DOI] [PubMed] [Google Scholar]
- 3.Apel C, Meister J, Gotz H, Duschner H, Gutknecht N. Structural changes in human dental enamel after subablative erbium laser irradiation and its potential use for caries prevention. Caries Res. 2005;39:65–70. doi: 10.1159/000081659. [DOI] [PubMed] [Google Scholar]
- 4.Apel C, Meister J, Schmitt N, Gräber HG, Gutknecht N. Calcium solubility of dental enamel following sub-ablative Er:YAG and Er,Cr:YSGG laser irradiation in vitro. Lasers Surg Med. 2002;30:337–341. doi: 10.1002/lsm.10058. [DOI] [PubMed] [Google Scholar]
- 5.Apel C, Schäfer C, Gutknecht N. Demineralization of Er:YAG and Er, Cr:YSGG laser-prepared enamel cavities in vitro. Caries Res. 2003;37:34–37. doi: 10.1159/000068228. [DOI] [PubMed] [Google Scholar]
- 6.Apfelbaum F, Mayer I, Featherstone JBD. The role of HPO42- and CO32- ions in the transformation of synthetic apatites to â- Ca5(PO4)2. J Inorg Biochem. 1990;38:1–8. [Google Scholar]
- 7.Clarkson BH, Rafter ME. Emerging methods used in the prevention and repair of carious tissues. J Dent Educ. 2001;65:1114–1120. [PubMed] [Google Scholar]
- 8.Delbem AC, Cury JA, Nakassima CK, Gouveia VG, Theodoro LH. Effect of Er:YAG laser on CaF2 formation and its anti-cariogenic action on human enamel: an in vitro study. J Clin Laser Med Surg. 2003;21:197–201. doi: 10.1089/104454703768247765. [DOI] [PubMed] [Google Scholar]
- 9.Eversole LR, Rizoiu I, Kimmel AI. Pulpal response to cavity preparation by an erbium,chromium:YSGG laser-powered hydrokinetic system. J Am Dent Assoc. 1997;128:1099–1106. doi: 10.14219/jada.archive.1997.0367. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone JD, Barrett-Vespone NA, Fried D, Kantorowitz Z, Seka W. CO2 laser inhibitor of artificial caries-like lesion progression in dental. J Dent Res. 1998;77:1397–1403. doi: 10.1177/00220345980770060401. [DOI] [PubMed] [Google Scholar]
- 11.Featherstone JDB, Fried D, Bitten ER. Mechanism of laser induced solubility reduction of dental enamel; Lasers in Dentistry. Proceedings of the SPIE Meeting; San Jose. Washington: Bellingham; 1997. Jan 28-29, 1996. pp. 112–116. [Google Scholar]
- 12.Featherstone JDB, Fried D. Fundamental interactions of lasers with dental hard tissues. Med Laser Appl. 2001;16:181–194. [Google Scholar]
- 13.Featherstone JDB. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 14.Fejerskov O, Thylstrup A. Dental enamel. In: Mjor I, Fejerskov O, editors. Human oral embryology. Copenhagen: Blackwell Munksgaard; 1986. pp. 50–89. [Google Scholar]
- 15.Fowler BO, Kuroda S. Changes in heated and in laser-irradiated human tooth enamel and their probable effects on solubility. Calcif Tissue Int. 1986;38:197–208. doi: 10.1007/BF02556711. [DOI] [PubMed] [Google Scholar]
- 16.Fried D, Featherstone JDB, Visuri SR, Seka W, Walsh JT. The caries inhibition potential of Er:YAG and Er:YSGG laser radiation; Lasers in dentistry. Proceedings of the SPIE Meeting; San Jose. Washington: Bellingham; 1996. Jan. 1996. pp. 73–78. [Google Scholar]
- 17.Fried D, Zuerlein MJ, Le CQ, Featherstone JDB. Thermal and mechanical modification of dentin by 9-11 micron CO2 laser pulses of 5-100 micros duration. Lasers Surg Med. 2002;31:275–282. doi: 10.1002/lsm.10100. [DOI] [PubMed] [Google Scholar]
- 18.Hadley J, Young DA, Eversole LR, Gornbein JA. A laser-powered hydrokinetic system for caries removal and cavities preparation. J Am Dent Assoc. 2000;131:777–785. doi: 10.14219/jada.archive.2000.0277. [DOI] [PubMed] [Google Scholar]
- 19.Hibst R, Keller V. Experimental studies of the application of the Er:YAG laser on the dental hard substances I: measurement of the ablation rate. Lasers Surg Med. 1989;9:338–344. doi: 10.1002/lsm.1900090405. [DOI] [PubMed] [Google Scholar]
- 20.Hossain M, Kimura Y, Yamada Y, Nakamura Y, Yamada Y, Kinoshita JI, et al. A study on acquired acid resistance of enamel and dentin irradiated by Er,Cr:YSGG laser. J Clin Laser Med Surg. 2001;19:153–159. doi: 10.1089/10445470152927991. [DOI] [PubMed] [Google Scholar]
- 21.Hossain M, Nakamura Y, Kimura Y, Yamada Y, Ito M, Matsumoto K. Caries-preventive effect of Er:YAG laser irradiation with or without water mist. J Clin Laser Med Surg. 2000;18:61–65. doi: 10.1089/clm.2000.18.61. [DOI] [PubMed] [Google Scholar]
- 22.Hossain M, Nakamura Y, Yamada Y, Kimura Y, Matsumoto N, Matsumoto K. Effects of Er,Cr:YSGG laser irradiation in human enamel and dentin: ablation and morphological studies. J Clin Laser Med Surg. 1999;17:155–159. doi: 10.1089/clm.1999.17.155. [DOI] [PubMed] [Google Scholar]
- 23.Keller U, Hibst R. Experimental studies of the application of the Er:YAG laser on dental hard substances: II. Light microscopic and SEM investigations. Lasers Surg Med. 1989;9:345–351. doi: 10.1002/lsm.1900090406. [DOI] [PubMed] [Google Scholar]
- 24.Mascarenhas AK. Risk factors for dental fluorosis: a re-view of the recent literature. Pediatr Dent. 2000;22:269–277. [PubMed] [Google Scholar]
- 25.Meister J, Franzen R, Forner K, Grebe H, Stanzel S, Lampert F, et al. Influence of the water content in dental enamel and dentin on ablation with erbium YAG and erbium YSGG lasers. J Biomed Opt. 2006;11:34030–34030. doi: 10.1117/1.2204028. [DOI] [PubMed] [Google Scholar]
- 26.Powell L, Morton TH, Whitsenant BK. Argon laser oral safety parameters. Lasers Surg Med. 1993;13:548–555. doi: 10.1002/lsm.1900130509. [DOI] [PubMed] [Google Scholar]
- 27.Qiao LY, Yu JT, Jia XY. A study on acquired acid resistance of enamel and dentine irradiated by Er,Cr:YSGG laser in vitro. Zhonghua Kou Qiang Yi Xue Za Zhi. 2005;40:34–37. [PubMed] [Google Scholar]
- 28.Visuri SR, Walsh JT, Jr, Wigdor HA. Erbium laser ablation of dental hard tissue: effect of water cooling. Lasers Surg Med. 1996;18:294–300. doi: 10.1002/(SICI)1096-9101(1996)18:3<294::AID-LSM11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Zach L, Cohen G. Pulp response to externally apllied heat. Oral Surg Oral Med Oral Pathol. 1965;19:515–530. doi: 10.1016/0030-4220(65)90015-0. [DOI] [PubMed] [Google Scholar]
- 30.Spierings TA, Vree JH, Peters MC, Plasschaert AJ. The influence of restorative dental materials on heat transmission in human teeth. J Dent Res. 1985;63:1096–1100. doi: 10.1177/00220345840630082001. [DOI] [PubMed] [Google Scholar]