Abstract
This study analyzed the relationship between the degree of conversion (DC), solubility, and salivary sorption of a hybrid (Filtek P 60) and a nanofilled resin composite (Filtek Supreme), and evaluated the influence of the light-activation mode on these properties. Two light-activation modes were used: Conventional (C; 850 mW/cm2 for 20 s) and Soft-start (SS; 100-1,000 mW/cm2 for 10 s + 1,000 mW/cm2 for 10 s). The DC (%) was evaluated by FT-Raman spectroscopy. The solubility and salivary sorption were measured after immersion in artificial saliva for 7 days. Data were analyzed by ANOVA and Student-Newman-Keuls' test and linear regression analysis (α = 0.05). The DC varied from 50.52% (nanofilled composite) to 57.15% (hybrid composite), and was influenced by the light-activation mode: C > SS. The solubility (0.45 μg/mm3) and salivary sorption (8.04 μg/mm3) of the nanofilled composite were greater than those of the hybrid composite (0.40 μg/mm3/ 6.87 μg/mm3), and were influenced by the light-activation mode: SS > C. Correlation was found between DC and solubility (r = - 0.89, p<0.05), as well as between solubility and salivary sorption (r = 0.95). These findings suggest that nanofilled composites may present higher degradation in the oral environment than hybrid ones. Soft-start light-activation mode may increase the solubility of resin composites.
Keywords: Composite resins, Degree of conversion, Solubility, Salivary sorption, Light-activation mode
INTRODUCTION
During the last few years, resin composites have been classified according to their filler particle size, as hybrid, microhybrid and microfilled. More recently, however, with the introduction of nanotechnology in dentistry18, a new class of resin composites, the so-called nanocomposites, is available to clinicians. Filtek Supreme (3M ESPE) is a typical nanofilled composite that has a filler particle system that combines silica nanofillers with a primary particle size of 20 or 75 nm and zirconia-silica nanoclusters, 0.6-1.4 μm in diameter. Its filler load is 59.5 vol%, which is close to several hybrid and microhybrid composites.18 Several published studies have shown that this resin composite has good mechanical properties3,21. However, there is a lack of data about the sorption and solubility phenomena of this new class of restorative materials.
Resistance to degradation in the oral environment is essential to the longevity of resin composite restorations4. From this point of view, it is crucial to understand the solubility and salivary sorption phenomena, in order to predict the behavior of resin composite restorations. Water sorption by resin composites is a diffusion-controlled process that may cause chemical degradation of the material, leading to several drawbacks, such as filler-polymeric matrix debonding26 and residual monomer release. This process can decrease the mechanical properties of the material27 and reduce the longevity of resin composite restorations. The solubility of resin composites is reflected by the amount of leachable unreacted monomers. During polymerization, these unreacted monomers are either trapped in microgels between the polymer chains and adsorbed to the surrounding network, or trapped in nanopores. The monomers in the nanopores are more prone to leaching out from the material than those inside the microgels24.
Several factors, such as the polymeric matrix composition24, filler particle type and content27, and the DC reached after the polymerization reaction24 can influence the solubility and sorption behavior of resin composites. Some light-activation modes can be used to start the polymerization reaction of resin composites.20 Modes that use a high initial irradiance provide a higher DC. However, it has been shown that this activation mode may induce a high shrinkage stress in the material, thus increasing marginal gap formation at the cavity wall-resin composite interface.25 Soft-start light-activation modes have been introduced in attempt to overcome this drawback in composite polymerization. These polymerization modes, in which the composite is first submitted to a low irradiance followed by higher irradiance, allow shrinkage stress relief by polymer chain relaxation15,25. It has been demonstrated that these light-activation modes do not reduce the DC of dental composites or dental polymers2,15.
The solubility and sorption of resin composites and dimethacrylate-polymeric matrices have been extensively investigated5,11,12,17,24,26. Few of these studies, however, have analyzed the material's behavior when immersed in artificial saliva11,26, which is a more compatible model with the oral environment conditions and would therefore supply a more realistic knowledge of these phenomena. Indeed, the effect of saliva on composites can be more deleterious than that of water26.
The purposes of this in vitro study were to analyze the correlation between the DC, solubility and salivary sorption of a hybrid and a nanofilled composite with similar polymeric matrices, and to evaluate the influence of the light-activation mode on these properties. The research hypotheses of the study were: (1) the light-activation mode does not influence the DC of resin composites, (2) nanofilled composite presents higher solubility and salivary sorption than the hybrid composite, and (3) there is a positive correlation between DC and solubility, as well as between DC and salivary sorption.
MATERIAL AND METHODS
Two commercially available resin composites with different types of filler particles were selected: a hybrid (Filtek P 60) and a nanofilled composite (Filtek Supreme – Su). Both materials have the same polymeric matrix (Bis-GMA, Bis-EMA, TEGDMA and UDMA). Their compositions are described in Table 1.
TABLE 1. Composition of the materials used in this study.
| Material | Composition | Manufacturer |
|---|---|---|
| Filtek P 60 (A3 shade) | Filler: 61 vol% silica/zirconia filler (mean particle size of 0.6 μm) Polymeric matrix: Bis-GMA, Bis-EMA, UDMA TEGDMA |
3M ESPE, St Paul, MN, USA |
| Filtek Supreme (A3B shade) | Filler: 59.5 vol% combination of aggregated zirconia/silica cluster filler with primary particles size of 5-20 nm, and nonagglomerated 20 nm silica filler. Polymeric matrix: Bis-GMA, Bis-EMA, UDMA TEGDMA |
3M ESPE, St Paul, MN, USA |
A quartz-tungsten-halogen unit (Optilux 501; Kerr, Danbury, CT, USA) was used to photoactivate the materials. Two light-activation modes were used: Conventional (C) – 850 mW/cm2 for 10 s (17 J/cm2); and Soft-start (SS) – from 100 to 1,000 mW/cm2 for 10 s and 1,000 for 20 s (≈17 J/cm2). The radiant exposure was calculated as the product of the irradiance of the curing unit, as measured with a curing radiometer (model 100; Demetron Inc. Danbury, CT, USA) and the time of irradiation. For the Soft-start mode, the radiant exposure was obtained by the sum of mean irradiance over the first 10 s multiplied by 10 s with 10 J/cm2, corresponding to the radiant exposure in the last 10 s of light exposure.
Degree of Conversion (DC%)
Raman spectra of the uncured and cured specimens of each resin composite were recorded by a Raman spectrometer (Nicolet FT-Raman 950, Thermo Nicolet Inc., Madison, WI, USA) operating with 120 scans at a resolution of 2 cm-1. Increments of each material were compressed between two polyester strips and two glass slides to produce a thin film (thickness of approximately 60 μm). Five films of each material were then photoactivated according to the light-activation modes, with the light tip in contact with the glass slide. Raman spectra were recorded after a 24-h dry storage at 37°C. The DC (%) was calculated from the ratio between the peak intensities of the aliphatic C=C bond (1638 cm-1) to the aromatic C=C bond (1608 cm-1), obtained from the cured and uncured specimens by the following equation:
| (1) |
where R = peak at 1638 cm-1 / peak at 1608 cm-1
Solubility and Salivary Sorption
Disc-shaped specimens (1 mm thick and 6 mm in diameter) were fabricated using an aluminum mold. After filling the mold to excess, the material surface was covered with a polyester strip and a glass slide, and compressed with a device (500 g) for 20 s to avoid porosities. The discs were photoactivated from the top. Ten discs were prepared for each studied material. The discs were placed in a desiccator containing freshly dried silica gel and transferred to an oven at 37°C. After 24 h, the discs were repeatedly weighed (AX 220, Shimadzu, Tokyo, Japan) until a constant mass (m1) was obtained (i.e., disc mass variation was less than ± 0.2 mg). The discs were immersed in artificial saliva (KCl, NaCl, MgCl, CaCl, Nipagin, CNC, Sorbitol, and deionized water)11, neutral pH, at 37°C for 7 days, removed, washed in distilled water, dried at room temperature for 15 min and weighed (m2). Thereafter, the discs were reconditioned to a constant mass (m3), using the same protocol as for m1. The thickness and the diameter of the discs were measured at four points with a digital caliper (MPI/E-101, Mitutoyo, Tokyo, Japan), and the volume (V) was calculated in mm3. The values of solubility (SL) and salivary sorption (SS) were obtained in μg/mm3 using the following equations:
| (2) |
| (3) |
Statistical Analysis
Statistical analysis was performed using Statgraphics 5.1 Software (Manugistics, Rockville, MD, USA). After verifying normal distribution of errors and the homogeneity of variance using Shapiro-Wilk's test and Levene's test, respectively, each test variable (DC, solubility and salivary sorption) was analyzed separately by two-way analysis of variance and Student-Newman-Keuls' test for multiple comparisons. Data were also submitted to regression analysis, with salivary sorption and solubility as the dependent variables and the DC as the independent variable. All statistical analyses were performed at a significance level of α = 0.05
RESULTS
The results of the DC, solubility and salivary sorption are presented in Table 2. Regarding DC (%), two-way ANOVA detected a significant influence for resin composite independent factor (p = 0.0071). The DC of Filtek P60 (54.22 ± 3.64) was higher than that of Filtek Supreme (51.70 ± 1.95) (p<0.05). Light-activation mode factor was proved significant (p=0.0001), with C mode presenting the highest DC (55.01 ± 3.15). The double interaction (resin composite vs. light-activation mode) was not statistically significant (p>0.05).
TABLE 2. Results of degree of conversion (DC%), solubility and salivary sorption.
| Groups | DC% | Solubility (μg/mm3) | Salivary sorption (μg/mm3) |
|---|---|---|---|
| P60C | a57.15 ± 2.64 | a0.38 ± 0.02 | a6.70 ± 0.32 |
| P60SS | b51.28 ±1.11 | b0.43 ± 0.02 | b7.04 ± 0.19 |
| SuC | b52.88 ± 1.96 | b0.41 ± 0.03 | c7.35 ± 0.24 |
| SuSS | b50.52 ± 1.13 | c0.49 ± 0.02 | d8.74 ± 0.35 |
Within each column, values with same superscript letters are not statistically different (α = 0.05).
Regarding solubility, two-way ANOVA detected a significant influence for the two main factors: resin composite and light-activation mode (p<0.05). The solubility of P60 (0.40 ± 0.03 μg/mm3) was lower than that of Su (0.45 ± 0.05 μg/mm3). Furthermore, the solubility with C mode (0.39 ± 0.03 μg/mm3) was lower than that presented by SS mode (0.46 ± 0.04 μg/mm3), (p<0.05). The double interaction was also proved significant (p = 0.0138).
For salivary sorption, two-way ANOVA revealed statistically significant difference for both main factors, as well as for the double interaction (p<0.05). The salivary sorption of P60 (6.87 ± 0.31 μg/mm3) was lower than that presented by Su (8.04 ± 0.77 μg/mm3). Indeed, the sorption of specimens photoactivated with C mode (7.02 ± 0.43 μg/mm3) was lower than that of specimens activated with SS mode (7.89 ± 0.91 μg/mm3) (p<0.05).
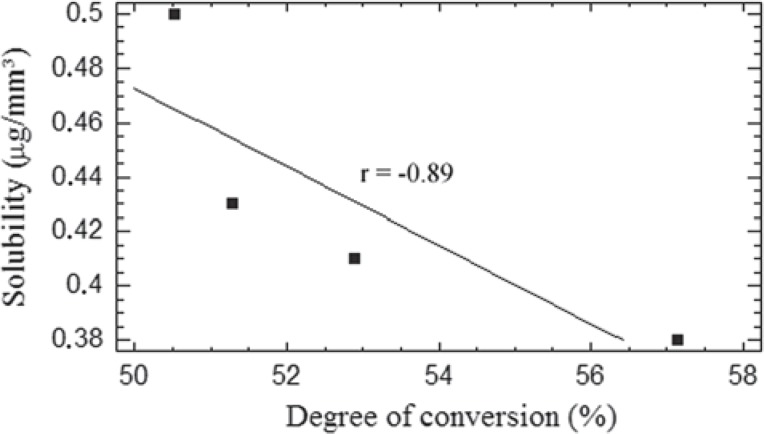
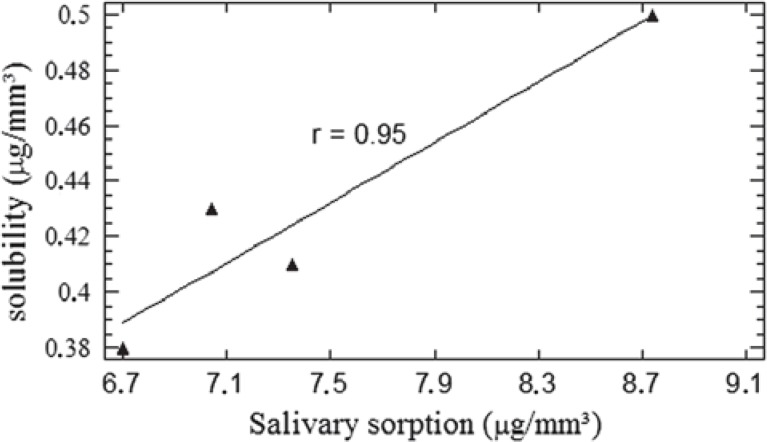
Correlation was found between solubility and DC, r = - 0.89 (Figure 1), as well as between solubility and salivary sorption, r = 95 (Figure 2). No correlation was found between DC and salivary sorption.
FIGURE 1. Regression line (linear model) of solubility plotted against the degree of conversion (r = -0.89, p < 0.05).
FIGURE 2. Regression line (linear model) of solubility plotted against the salivary sorption (r = 0.95, p < 0.05).
DISCUSSION
As in the present study the DC obtained with C light-activation mode was higher than that obtained with SS mode, the first research hypothesis of the present study was rejected. On the other hand, as the solubility and salivary sorption of the nanofilled composite was higher than that of the hybrid one, the second research hypothesis was supported. Finally, as only the correlation between the DC and solubility was proved, the third research hypothesis was partially accepted.
The properties of resin composites as well as their DCs are influenced by both phases of their composition, i.e. polymeric matrix and filler particle system.2,21. Both resin composites analyzed in the present study are representative of Filtek series (3M ESPE, St Paul, MN, USA). According to this manufacturer, all composites from this series have the same composition regarding polymeric matrix. Based on this, all findings about the properties assessed in the present study were discussed taking into account only the influence of the filler particle systems.
The physical and mechanical properties of resin composites are strongly influenced by the degree of conversion, defined as the percentage of reacted aliphatic C=C bonds from the dimethacrylate monomers present in their polymeric matrices9. Thus, the extent of this response plays a crucial role in the clinical performance of resin composite restorations. In the present study, the values of DC ranged from 50.52% (nanofilled composite) to 57.15% (hybrid composite). These values are in agreement with those of other studies that have used Raman spectroscopy to evaluate the degree of conversion of resin composites19. The DC obtained with C light-activation mode was higher than that obtained with SS mode. Given that the radiant exposures generated by both light-activation modes were close to the same value, this result was unexpected. Several published studies have shown that light-activation modes that used similar radiant exposures produced statistically similar DC6,7. However, the data in Table 2 show that this result was strongly influenced by the behavior of hybrid resin composite. The only reasonable explanation for this result could be based on light-scattering phenomenon. A previous published study1 showed that at a wavelength of 470 nm a resin composite with mean filler particle size in the nanoscale, i.e. Silux Plus (3M, St. Paul, MN, USA), showed a significantly lower light transmittance than a hybrid composite. According to the authors1, the light that passed through the resin composite was scattered by the small filler particles and light transmittance was reduced. It is possible that in the presented study the non-agglomerated silica nanoparticles with mean size of 20 nm may have caused a light-scattering effect in nanofilled composite. Thus, the light intensity might have been attenuated and the DC decreased. On the other hand, in the hybrid composite, light transmittance was probably higher, and so was its DC.
Previous studies have shown that soft-start light-activation modes improved the cavity wall-resin composite interface sealing25,30. As in the present study the nanofilled composite presented similar DCs with either of the light-activation modes, it is reasonable to consider SS light-activation mode more advantageous for this type of resin composite.
The mean solubility values presented by the tested resin composites varied from 0.38 to 0.49 μg/mm3. This range is in agreement with the findings of a previous study29. As expected, the nanofilled composite presented a higher solubility than the hybrid one. This result may be attributed to the differences in the filler particle systems of the two resin composites. Although both materials have similar volumetric filler content, i.e. 59.5 vol% for nanofilled and 61 vol% for hybrid, the filler particles of nanofilled composite will theoretically present a greater total surface area, due to nonagglomerated 20 nm silica filler. Two factors derived from this fact can increase the solubility. Firstly, a great amount of ions will be released from the surfaces of filler particles10. Secondly, coupling agents associated with the filler particles, e.g. g-methacryloxypropyltrimethoxysilane, are prone to hydrolysis via ester linkages within the molecules or siloxane links that are formed with the filler particles22. Water in contact with silica filler surfaces breaks siloxane bonds to form silanol groups and facilitate particles debonding. Based on this, it is reasonable to speculate that more filler particles were eluted from the nanofilled composite, thus increasing its solubility26,28. To emphasize this discussion, Hofmann, et al.12 (2002) analyzing the leachable components from several resin composites showed that Silux Plus (a microfill composite with silica filler particles similar to those present in Supreme) had a higher solubility than Z250 (a resin composite similar to P60). Obviously, among other aspects, this behavior could also be associated with differences in filler particles between these resin composites. As shown in Figure 1, correlation was found between DC and SL. This outcome is supported by the findings of previous studies24,27.
With regard to salivary sorption, differences were also found between resin composites, with the nanofilled (8.04 ± 0.77 μg/cm3) presenting a worse behavior than hybrid composite (6.87 ± 0.31 μg/cm3). The sorption phenomenon in polymer-based restorative materials, such as resin composites, is mainly dependent on the hydrophilicity of their polymeric matrices24. The chemistry of the monomers present in the matrix is the key to the hydrophilic nature of the polymer16. The monomers present in the polymeric matrices of the two resin composites analyzed in the present study (Bis-GMA, Bis-EMA, TEGDMA, UDMA) have hydrophilic groups in their backbones, i.e., -OH-, >C=O, -O-, -NH-,22 which probably make them more prone to salivary sorption. In addition to the polymeric matrix, the filler particle system may also influence the sorption phenomenon in resin composites. Analyzing several commercial resin composites and experimental dimethacrylate models, Kalachandra and Wilson14 (1992) found that Silux Plus, i.e., a microfill resin composite with a 0.04 μm silica filler, displayed a more drastic reduction in elastic modulus after storage in water at 37°C. This result was related to the greater amount of water accumulated at the matrix-filler interface. In conclusion, these authors claimed that the interface between the inorganic filler particle and the polymeric matrix is the most probable site for accommodation of additional water in resin composites.
It seems obvious that the large total surface area of silica filler in Silux Plus resin composite contributed to the results of the above-mentioned study. The same reasoning could be used to justify the differences in salivary sorption between the materials studied in the present study. It is reasonable to assume that the theoretically larger total surface area of nanofilled composite particles, derived from the nonagglomerated 20 nm silica filler, allowed more water to accumulate at the filler particle-polymeric matrix interfaces, thus increasing its salivary sorption. Another possibility that could explain the greater salivary sorption in the nanofilled composite is that the water accumulated at the aggregated zirconia/silica cluster filler-organic matrix interface could have created paths for water diffusion towards the inside of aggregates, where the presence of microvoids is quite probable, due to lack of 5-20 nm-sized primary particles being impregnated in the polymeric matrix23.
The SL and SS were influenced by the light-activation mode. The mean values for SS mode (0.46 ± 0.04 / 7.89 ± 0.91 mg/mm3) were higher than those for C mode (0.39 ± 0.03 / 7.2 ± 0.43 mg/mm3). This result could be explained by the free volume theory24. In glassy polymers, such as dimethacrylate-based composites, part of the absorbed water diffuses through the network and is trapped in polymer nanovoids without establishing any interactions with polymer polar sites. The amount of absorbed water would be dependent on the total hole-free volume in the polymer network, which is dependent on the macromolecular packing density. Thus, the structure of the network will influence the amount of absorbed water. It has been suggested that Soft-start light-activation modes may produce polymers with linear chains and, consequently, low crosslink density2. Obviously, this feature will decrease the macromolecular packing density of the network. Based on the discussion above, it is reasonable to consider that SS light-activation mode produced composites with low polymeric matrix macromolecular packing density and greater hole-free volume, thus producing a greater SS.
At sufficiently high water sorption, macromolecular polymer chains undergo a relaxation process as they swell to absorb the water. Initially, the presence of water softens the polymer by swelling the network and reducing the frictional forces between the polymer chains8. After the relaxation process, unreacted monomers trapped in the polymer network are released to the surroundings at a rate that is controlled by the polymer's swelling and relaxation capacity. Taking into account that linear polymer chains have a great relaxation capacity, this explanation could justify the greater SL obtained with the SS light-activation mode. Figure 2 shows the correlation between SL and SS. It is clear that the greater the amount of saliva absorbed, the greater the amount of components that could leach out from resin composites.
CONCLUSIONS
In conclusion, the results of the present study showed that the SS light-activation mode produced a lower DC, indicating that, instead of the final radiant exposure, the initial low irradiance may affect the DC of resin composites. The nanofilled composite presented higher solubility and salivary sorption than the hybrid composite. This suggests that the total surface area of the filler particle systems plays a key role in the properties of these resin composites. Therefore, light-activation modes that lead to a higher DC may diminish the solubility of resin composites. From a clinical standpoint, these results suggest that nanofilled composites may present higher degradation in the oral environment than hybrid ones. Moreover, soft-start light-activation mode may increase the solubility of resin composites.
REFERENCES
- 1.Arikawa H, Fujii K, Kanie T, Inoue K. Light transmittance characteristics of light-cured composite resins. Dent Mater. 1998;14:405–411. doi: 10.1016/s0300-5712(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 2.Asmussen E, Peutzfeldt A. Two-step curing: influence on conversion and softening of a dental polymer. Dent Mater. 2003;19:466–470. doi: 10.1016/s0109-5641(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 3.Beun S, Glorieux T, Devaux J, Vreven J, Leloup G. Characterization of nanofilled compared to universal and microfilled composites. Dent Mater. 2007;23:51–59. doi: 10.1016/j.dental.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Christensen GJ. Longevity of posterior tooth dental restorations. J Am Dent Assoc. 2005;136:201–203. doi: 10.14219/jada.archive.2005.0142. [DOI] [PubMed] [Google Scholar]
- 5.Chung CM, Kim JG, Kim MS, Kim KM, Kim KN. Development of a new photocurable composite resin with reduced curing shrinkage. Dent Mater. 2002;18:174–178. doi: 10.1016/s0109-5641(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 6.Emami N, Söderholm KJM, Berglund LA. Effect of light power density variations on bulk curing properties of dental composites. J Dent. 2003;31:189–196. doi: 10.1016/s0300-5712(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 7.Emami N, Söderholm KJM. How light irradiance and curing time affect monomer conversion in light-cured resin composites. Eur J Oral Sci. 2003;111:536–542. doi: 10.1111/j.0909-8836.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferracane JL, Berge XH, Condon JR. In vitro aging of dental composites in water-effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Ferracane JL. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorative resins. Dent Mater. 1985;1:11–14. doi: 10.1016/S0109-5641(85)80058-0. [DOI] [PubMed] [Google Scholar]
- 10.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves L, Noronha JD, Filho, Guimarães JGA, Poskus LT, Silva EM. Solubility, salivary sorption and degree of conversion of dimethacrylate-based polymeric matrices. J Biomed Mater Res B Appl Biomater. 2007 doi: 10.1002/jbm.b.30949. In press. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann N, Renner J, Hugo B, Klaiber B. Elution of leachable components from resin composite after plasma arc vs. standard or soft-start halogen light irradiation. J Dent. 2002;30:223–232. doi: 10.1016/s0300-5712(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Dent Mater. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Kalachandra S, Wilson TW. Water sorption and mechanical properties of light-cured proprietary composite tooth restorative materials. Biomaterials. 1992;13:105–109. doi: 10.1016/0142-9612(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 15.Lim BS, Ferracane JL, Sakagushi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent. Mater. 2002;18:436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 16.Malacarne J, Carvalho RM, Goes MF, Svizero N, Pashley DH, Tay FR, et al. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Martin N, Jedynakiewicz NM, Fisher AC. Hygroscopic expansion and solubility of composite restoratives. Dent Mater. 2003;19:77–76. doi: 10.1016/s0109-5641(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 18.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 19.Pianelli C, Devaux J, Bebelman S, Leloup G. The micro-Raman spectroscopy, a useful tool to determine the degree of conversion of light-activated composite resins. J Biomed. Mater Res. 1999;48:675–681. doi: 10.1002/(sici)1097-4636(1999)48:5<675::aid-jbm11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Rahiotis C, Kakaboura A, Loukidis M, Vougiouklakis G. Curing efficiency of various types of light-curing units. Eur J Oral Sci. 2004;112:89–94. doi: 10.1111/j.0909-8836.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues SAJ, Zanchi CH, Carvalho RV, Demarco FF. Flexural strength and modulus of elasticity of different types of resin-based composites. Braz Oral Res. 2007;21:16–21. doi: 10.1590/s1806-83242007000100003. [DOI] [PubMed] [Google Scholar]
- 22.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–151. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 23.Santos C, Clarke RL, Braden M, Guitian F, Davy KWM. Water absorption characteristics of dental composites incorporating hydroxyapatite filler. Biomaterials. 2002;23:1897–1904. doi: 10.1016/s0142-9612(01)00331-3. [DOI] [PubMed] [Google Scholar]
- 24.Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylatebased dental resins. Biomaterials. 2003;24:655–665. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 25.Silva EM, Santos GO, Guimarães JGA, Barcellos AAL, Sampaio EM. The Influence of C-factor, Flexural Modulus and Viscous Flow on Gap Formation in Resin Composite Restorations. Oper Dent. 2007;32:358–364. doi: 10.2341/06-104. [DOI] [PubMed] [Google Scholar]
- 26.Söderholm KJ, Mukherjee R, Longmate J. Filler leachability of composites stored in distilled water or artificial saliva. J Dent Res. 1996;75:1692–1699. doi: 10.1177/00220345960750091201. [DOI] [PubMed] [Google Scholar]
- 27.Söderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248–1254. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- 28.Söderholm KJ. Leaking of fillers in dental composites. J Dent Res. 1983;62:126–130. doi: 10.1177/00220345830620020801. [DOI] [PubMed] [Google Scholar]
- 29.Toledano M, Osorio R, Osorio E, Fuentes V, Prati C, Garcia-Godoy F. Sorption and solubility of resin-based restorative dental materials. J Dent. 2003;31:43–50. doi: 10.1016/s0300-5712(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa T, Burrow MF, Tagami J. A light curing method for improving marginal sealing and cavity wall adaptation of resin composite restorations. Dent Mater. 2001;17:359–366. doi: 10.1016/s0109-5641(00)00095-6. [DOI] [PubMed] [Google Scholar]