Abstract
Objectives:
Production of acids such as lactic acid contributes to establish a cariogenic environment that leads to dental substrate demineralization. Fluoride plays an important role in this case and, as fluoride-releasing materials, glass-ionomer cements are expected to contribute to minimize deleterious reactions. This study evaluated interactions of glass-ionomer cements used in atraumatic restorative treatment (ART-GICs) with an aqueous lactic acid solution, testing the null hypotheses that no changes occur in the pH of the solution or on the surface roughness and mass of the ART-GICs when exposed to lactic acid solution over a 6-week period.
Material and Methods:
Ketac Molar, Fuji IX, Vitro Molar and Magic Glass were tested, and compared to Filtek Z250 and Ketac Fil Plus as control groups. Six specimens of each material were made according to manufacturers' instructions. The pH of the solution and roughness and mass changes of each specimen were determined over 6 weeks. Each specimen was individually stored in 2 mL of 0.02 M lactic acid solution for 1 week, renewing the solution every week. pH of solution and mass of the specimens were monitored weekly, and surface roughness of the specimens was assessed before and at the end of the 6-week acid challenge. pH and mass data were analyzed statistically by repeated measures using one-way ANOVA and Tukey's post-hoc tests for each material. Paired t-tests were used for roughness analysis. Tukey's post-hoc tests were applied to verify differences of final roughness among the materials. Significance level was set at 5%.
Results:
The null hypotheses were partially rejected. All materials were able to increase the pH of the lactic acid solution and presented rougher surfaces after immersion, while mass change was minimal and generally not statistically significant.
Conclusions:
These findings can be helpful to predict the performance of these materials under clinical conditions. A protective action against the carious process with significant surface damage due to erosion may be expected.
Keywords: Glass-ionomer cements, Atraumatic restorative treatment, Lactic acid, pH
INTRODUCTION
Atraumatic restorative treatment (ART) is a dental approach based on the removal of carious tissue with hand instruments followed by placement of an adhesive restoration2,7,24,29. It is recommended by the World Health Organization (WHO), mainly in communities with poor financial resources and for people who have physical disabilities or are fearful2,7,24,29. As ART is usually applied to unprivileged people, poor oral hygiene is common, with consequent unbalance of the demineralization-remineralization cycles, is common.
Glass-ionomer cement (GIC) is usually the adhesive material of choice to treat high-caries risk patients, and it is feasible to assume that the GIC will be in direct contact with acid substances9,10,30. Variation in acid profile and concentration may relate to caries progression in hard dental tissues12,13. When sugar is available in the oral environment, microorganisms produce organic acids such as lactate and acetate23. Another relevant factor involved in caries progression is related to the low pH generated from carbohydrate metabolism that selects cariogenic species3,23. Hojo, et al. 12 (1994) observed that a low pH environment characterizes this condition with a lactate dominant acid profile in active lesions. Thus, the interaction between lactic acid and restorative materials should also be considered.
ART requires a material with improved mechanical properties. Since conventional GICs are not indicated for restoring stress-bearing contact areas5,14,22,25,26,30, some types of GICs have been specifically formulated for ART restorations. In vitro studies have compared the performance of conventional GICs to high density GICs5,14,22,25,26,30. However, some properties of ART-GICs still need to be further investigated. Previous studies have stated the negative influence of acids on restorative materials11,19,20,23. Resistance to biodegradation is a highly desirable property of dental materials to allow for satisfactory clinical performance.
The aim of this study was to evaluate interactions of ART-GICs with an aqueous lactic acid solution. The null hypotheses tested are that no significant changes occur in the pH of the solution or on the surface roughness and mass of the ART-GICs when the materials are exposed to an aqueous lactic acid storage solution over a 6-week period.
MATERIAL AND METHODS
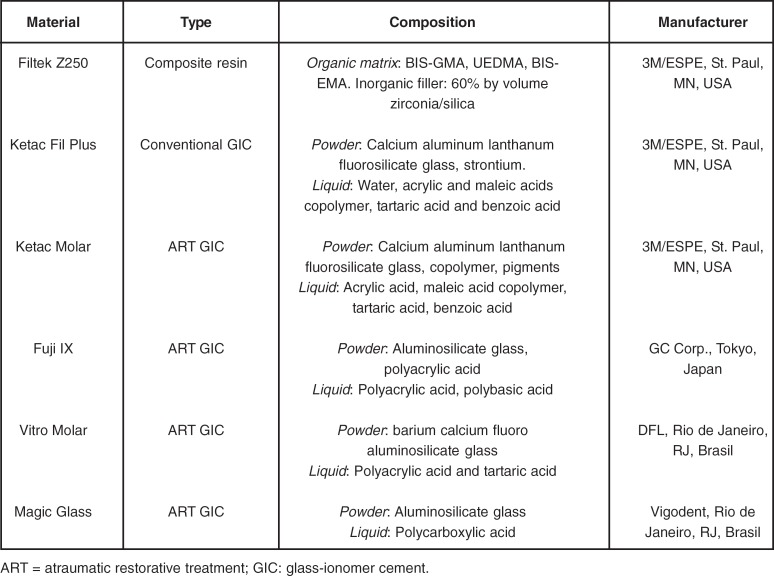
The materials under investigation with their compositions and manufacturers are presented in Figure 1.
FIGURE 1. Materials used in the study.
Six specimens of each material (6 mm diameter x 3 mm in height) were prepared. Conventional (positive control) and ART-GICs were weighed according to powder-to-liquid mixing ratio indicated by the manufacturers. Materials were inserted into a previously lubricated cylindrical polytetrafluoroethylene molds with a Centrix syringe (Centrix, Shelton, CT, USA) between two polyester strips (TDV Dental, Pomerode, SC, Brazil) and pressed with glass slabs under a constant load of 500 g. After 30 min, the specimens were removed from the molds and excess was cut off with a #12 Bard-Parker scalpel blade. Filtek Z250 specimens (negative control) were inserted into the molds in a single increment, which was light-cured for 20 s on both sides using a halogen lamp (Optilight Plus; Gnatus, Ribeirão Preto, SP, Brazil) with irradiance of400 mW/cm2, as measured with a curing radiometer (Model 100P/N-150503; Demetron Research Corp., Danbury, CT, USA).
Immediately after preparation, mass and surface roughness of the specimens were determined. The mass was assessed in an analytical balance (Bel Engineering SRL, Mark 205A, Monza, MI, Italy) accurate to 0.0001 g. Surface roughness was analyzed by a Hommel Tester T 1000 (Hommelwerke GmbH, Schwenningen, Germany) accurate to 0.01 mm and was expressed in mm as a Ra value. To record roughness measurements of the surfaces a device containing a diamond needle (Hommelwerke GmbH) was used. The average of three randomized readings was established as the baseline roughness value. Ra range was previously adjusted at 0.01 to 0.8 mm at a cut-off of 0.25 mm. Readings were obtained from 1.5 mm-long measurements.
A 0.02 M aqueous lactic acid solution was prepared 18 h before use to allow hydrolysis of lactone to occur, according to ISO standard 991715. This reagent was always freshly prepared for each set of tests. The specimens were individually stored for 6 weeks in glass vials containing 2 mL of the solution, which was weekly renewed. A vial containing lactic acid aqueous solution at pH 2.7 ± 0.02 with no specimen was used to monitor the pH of the solution over the week. The vials were maintained at 23 ± 1°C without agitation. After 1 week, the pH of solution in each vial was measured with a pHmeter (Tecnal pH meter Tec-2, Piracicaba, SP, Brazil). Mass change of each specimen was also recorded and the specimens were transferred to individual vials with fresh solution. After the end of the 6-week period, new surface roughness readings were performed.
The assumptions of equality of variances and normal distribution of errors were checked for the tested response variables. Since the assumptions were satisfied, data were subjected to one-way ANOVA using matched repeated measures and Tukey's post-hoc tests for pH and mass analysis. Paired t-tests were used for roughness analysis. Tukey's post-hoc tests were applied to verify differences of final roughness among the materials (p<0.05).
RESULTS
The pH increasing ability was material- and time-dependent as was the interaction between these variables (p<0.05). All materials increased the pH of the lactic acid storage solution at all evaluation periods (p<0.01). The pH changes of the aqueous lactic acid solution recorded over a 6-week period of storage of the materials are presented in Table 1. Greater increase in lactic acid pH was observed in the first week for all materials followed by a clear decrease over time. However, for all materials, the final pH of the acid solution was always higher than the initial pH (p<0.05). All GICs showed more increasing pH potential than the composite resin.
TABLE 1. Means (standard deviations) of pH values of lactic acid solutions over 6 weeks storage.
| Material | Initial | 1 week | 2 week | 3 week | 4 week | 5 week | 6 week |
|---|---|---|---|---|---|---|---|
| Filtek Z250 | 2.7a(0.00) | 2.83d(0.05) | 2.77c (0.03) | 2.73ab (0.01) | 2.78c (0.01) | 2.77c (0.00) | 2.75bc (0.02) |
| Ketac Fil Plus | 2.7 a (0.00) | 4.28 e(0.05) | 3.74d (0.03) | 3.61d (0.02) | 3.59c (0.01) | 3.53b (0.02) | 3.49b (0.03) |
| Ketac Molar | 2.7 a (0.00) | 3.83 e (0.05) | 3.50d (0.02) | 3.47cd (0.02) | 3.48cd (0.02) | 3.44bc (0.03) | 3.41b (0.01) |
| Fuji IX | 2.7 a (0.00) | 4.08 e (0.07) | 3.59d (0.03) | 3.56cd (0.03) | 3.55cd (0.04) | 3.50bc (0.03) | 3.45b (0.02) |
| Vitro Molar | 2.7 a (0.00) | 4.85 c (0.34) | 4.22c (0.16) | 4.12c (0.10) | 4.06c (0.06) | 4.06c (0.04) | 4.04b (0.05) |
| Magic Glass | 2.7 a (0.00) | 4.82 e(0.12) | 4.78e (0.13) | 4.72de (0.31) | 4.56cd (0.20) | 4.44c (0.22) | 4.21b (0.12) |
Values are expressed as mean (standard deviation). Same letter indicates no statistically significant difference in rows.
Mass change data (Table 2) showed statistical significance for material and material versus time interaction (p<0.05). Fuji IX showed mass gain in the first week, which was stabilized from the second week. Conversely, Magic Glass lost mass in the first week, stabilizing from the second week. Vitro Molar showed a discrete gain of mass in the first week and mass loss in the fourth week. Ketac Molar, Ketac Fil Plus and Filtek Z250 showed insignificant changes in the evaluated period. There were no statistically significant differences among Fuji IX, Magic Glass and Ketac Fil Plus.
TABLE 2. Mass of the control materials and ART-GICs exposed to the aqueous lactic acid storage solution over a 6-week period.
| Material | Initial | 1 week | 2 week | 3 week | 4 week | 5 week | 6 week | p value |
|---|---|---|---|---|---|---|---|---|
| Filtek Z250 | 0.2086a (0.0009) | 0.2130 a (0.0124) | 0.2132 a (0.0124) | 0.2133 a (0.0124) | 0.2134 a (0.0124) | 0.2134 a (0.0123) | 0.2134 a (0.0122) | p>0.05 |
| Ketac Fil Plus | 0.2422 a (0.0141) | 0.2326 a (0.0187) | 0.2322 a (0.0187) | 0.2317 a (0.0187) | 0.2311 a (0.0187) | 0.2303 a (0.0189) | 0.2298 a (0.0188) | p>0.05 |
| Ketac Molar | 0.2343 a (0.0118) | 0.2398a (0.0160) | 0.2431a (0.0147) | 0.2433 a (0.0147) | 0.2427 a (0.0144) | 0.2427 a (0.0143) | 0.2426 a (0.0142) | p>0.05 |
| Fuji IX | 0.1983 a (0.0141) | 0.2341b (0.0121) | 0.2339 b (0.0121) | 0.2337 b (0.0120) | 0.2334 b (0.0121) | 0.2327 b (0.0123) | 0.2323 b (0.0122) | p<0.05 |
| Vitro Molar | 0.2117 ab (0.0185) | 0.2155 c (0.0187) | 0.2149 bc (0.0189) | 0.2159 c (0.0187) | 0.2112 a (0.0179) | 0.2135 abc (0.0187) | 0.2131 abc (0.0189) | p>0.05 |
| Magic Glass | 0.2328 a (0.0184) | 0.2011 b (0.0131) | 0.1993 b (0.0134) | 0.1978 b (0.0134) | 0.1960 b (0.0134) | 0.1941 b (0.0137) | 0.1924 b (0.0133) | p<0.05 |
ART = atraumatic restorative treatment; GIC: glass-ionomer cement. Values are expressed as mean (standard deviation). Same letter indicates no statistically significant difference in the same row.
For surface roughness analysis (Table 3), all materials were rougher after 6 weeks (p<0.05). No statistically significant difference (p>0.05) was observed for interaction between material and time. Filtek Z250 and Ketac Molar presented the least alterations while Vitro Molar showed the greatest alterations on surface roughness. There were no statistically significance differences (p>0.05) among Fuji IX, Magic Glass and Ketac Fil Plus.
TABLE 3. Mean initial and final surface roughness (Ra) of the control materials and ART-GICs exposed to the aqueous lactic acid storage solution over a 6-week period.
| Material | Initial roughness (μm) | Final roughness (μm) | Tukey's tests |
|---|---|---|---|
| Filtek Z250 | 0.25 a (0.12) | 0.62 b (0.30) | A |
| Ketac Fil Plus | 0.54 a (0.13) | 1.19 b (0.42) | B,C |
| Ketac Molar | 0.47 a (0.22) | 0.83 b (0.38) | A,B |
| Fuji IX | 0.63 a (0.16) | 0.95 b (0.28) | A,B,C |
| Vitro Molar | 0.82 a (0.29) | 1.37 b (0.28) | C |
| Magic Glass | 0.46 a (0.15) | 1.26 b (0.12) | B,C |
ART = atraumatic restorative treatment; GIC: glass-ionomer cement. The values are expressed as mean (standard deviation). Same superscript letter indicates no statistically significant difference in the same row by paired t-test. Same uppercase letter indicates no statistically significant difference for final roughness among the materials.
DISCUSSION
The anticariogenic capacity is a relevant property of GICs and it is thus expected that the interaction of these materials with an acidic environment will lead to pH increase10,17-19 Under the present experimental conditions, all tested GICs were able to increase the initial pH of the acid lactic solution, though a progressive decline of this ability occurred for all materials over time. These results are in accordance with those of Nicholson, et al.19, who subjected polyacid-modified composite resins to similar experimental conditions and also verified their ability to neutralize acidic solution. The profile in the same period of evaluation also indicated a decline of this property over time. Nomoto and McCabe21 verified that the initial pH of the tested solution (2.74) increased almost 1 unit in 7 days. Nicholson, et al.17 investigated the rate of change of the pH of lactic acid exposed to some ART-GICs and their results corroborate the findings of the present study.
The pH increase of acid storage solution is attributed to the acid-basic setting reaction of dental cements with salt formation. A possible explanation is that lactic acid reacts directly with basic glass filler to form calcium and aluminum lactate salts. Lactic acid is a stronger organic acid and it reacts with these salts forming lactate dominant salts20,21.
Despite the benefits of increasing the pH of acid environments as an important mechanism of caries protection for GICs, these materials can be damaged in this interaction. Different methodologies, such as surface roughness28, microhardness4,11, mass change17,20 and scanning electron microscopy8, have been applied to investigate the influence of acidic conditions on different restorative materials. In the present study, mass and surface roughness analyses were performed. The results showed that the increase of the pH of the acidic solution led to minimal material loss, which could be related to an erosive process1,18,20,21. Generally, mass change was not statistically significant, which does not mean that no phenomena occurred. It is more likely that the expected mass gain by water sorption was similar to the loss of material eroded from the surface1. Only Fuji IX revealed a significant initial mass gain, while Magic Glass lost mass at the same period. This difference can be attributed to differences in the materials' formulations. Regardless of their performance, the degradation of these materials can involve loss of sodium, fluoride ions and silica or matrix biodegradation18. On the other hand, mass gain can also result from the formation of insoluble glass-lactate cement.
It is important to state that the low pH of acidic solutions optimizes surface damage to the specimens caused by water content, since previous studies showed that an aqueous environment has a significant effect on the surfaces of the materials11. Similar results have been reported by Turssi, et al. 28 No specific qualitative analysis of the surfaces was performed in the present study, but all GIC specimens presented a chalky surface at the end of the experimental period, which may be suggestive of an erosive process18. Other methodologies can be used to investigate the performance of dental restorative materials under erosive conditions, such as the method of dripping/spraying acid solution and measuring volume rather than mass21.
In order to confirm the occurrence of any surface alteration, this study proposed a quantitative investigation using a roughness tester. An increase of surface roughness was evident for all tested materials. Nomoto and McCabe21 also verified the capacity of acid to erode GIC surfaces. Turssi, et al.28 investigated the role of the immersion solution on the roughness of resin-based restorative materials and concluded that micromorphological changes occurred in an acid-challenging situation. The concern about increased surface roughness is that rough surfaces can predispose restorations to staining, plaque and food accumulation, and gingival irritation6,16,27.
The relationship among factors related to caries onset and progression is controversial because of several events are involved. However, it is known that a low pH is favorable to establish a condition that facilitates demineralization of hard dental tissue11,19,23. In the present study, an increased pH of acidic storage solution was observed over time after contact with the tested materials. This performance is an important predictive that GICs present a real potential to arrest caries. In the oral environment, however, the proportion between dental material and acidic fluid is different. Yet, although other factors should be associated to permit a correct interpretation, an enhanced performance of all materials is expected clinically.
CONCLUSION
According to the proposed methodology and based on the obtained results, the null hypotheses tested in this study were partially rejected. All GICs increased the pH of the lactic acid solution, though this ability declined over time. All materials presented higher surface roughness at the end of the lactic acid challenge. Minimal mass change occurred throughout the experiment. These findings can be helpful to predict the performance of these materials under clinical conditions. A protective action against the carious process with significant surface damage due to erosion may be expected.
ACKNOWLEDGEMENTS
The authors thank Thelma Lopes da Silva and Veralúcia dos Santos for laboratory assistance. We also thank 3M/ESPE Dental Products, DFL and, Vigodent for material support.
REFERENCES
- 1.Bapna MS, Mueller HJ. Relative solubilities of hybrid ionomers and compomers by acid impingement. J Oral Rehabil. 1999;26(10):786–790. doi: 10.1046/j.1365-2842.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 2.Barata TJE, Bresciani E, Mattos MCR, Lauris JRP, Ericson D, Navarro MFL. Comparison of two minimally invasive methods on the longevity of glass ionomer cement restorations: short-term results of a pilot study. J Appl Oral Sci. 2008;16(2):155–160. doi: 10.1590/S1678-77572008000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw DJ, Marsh PD. Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res. 1998;32(6):456–462. doi: 10.1159/000016487. [DOI] [PubMed] [Google Scholar]
- 4.Chadwick RG, McCabe JF, Walls AWG, Storer R. The effect of storage media upon the surface microhardness and abrasion resistance of three composites. Dent Mater. 1990;6(2):123–128. doi: 10.1016/s0109-5641(05)80042-9. [DOI] [PubMed] [Google Scholar]
- 5.Ewoldsen N, Covey D, Lavin M. The physical and adhesive properties of dental cements used for atraumatic restorative treatment. Spec Care Dentist. 1997;17(1):19–24. doi: 10.1111/j.1754-4505.1997.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 6.Forss H, Jokinen J, Spets-Happonen S, Seppä L, Luoma H. Fluoride and mutans streptococci in plaque grown on glass ionomer and composite. Caries Res. 1991;25(6):454–458. doi: 10.1159/000261410. [DOI] [PubMed] [Google Scholar]
- 7.Frencken JE, Songpaisan Y, Phantumvanit P, Pilot T. An atraumatic restorative treatment (ART) technique: evaluation after one year. Int Dent J. 1994;44(5):460–464. [PubMed] [Google Scholar]
- 8.Gao F, Matsuya S, Ohta M, Zhang J. Erosion process of light-cured and conventional glass ionomer cements in citrate buffer solution. Dent Mater J. 1997;16(2):170–179. doi: 10.4012/dmj.16.170. [DOI] [PubMed] [Google Scholar]
- 9.Gao W, Smales RJ, Gale MS. Fluoride release/uptake from newer glass-ionomer cements used with the ART approach. Am J Dent. 2000;13(4):201–204. [PubMed] [Google Scholar]
- 10.Garcez RMV, Buzalaf MAR, Araújo PA. Fluoride release of six restorative materials in water and pH-cycling solutions. J Appl Oral Sci. 2008;15(5):406–411. doi: 10.1590/S1678-77572007000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geurtsen W, Leyhausen G, Garcia-Godoy F. Effect of storage media on the fluoride release and surface microhardness of four polyacid-modified composite resins ("compomers") Dent Mater. 1999;15(3):196–201. doi: 10.1016/s0109-5641(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 12.Hojo S, Komatsu M, Okuda R, Takahashi N, Yamada T. Acid profiles and pH of carious dentine in active and arrested caries. J Dent Res. 1994;73(12):1853–1857. doi: 10.1177/00220345940730121001. [DOI] [PubMed] [Google Scholar]
- 13.Hojo S, Takahashi N, Yamada T. Acid profiles in carious dentin. J Dent Res. 1991;70(3):182–186. doi: 10.1177/00220345910700030501. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya Y, García-Godoy F. Bonding mechanism of Ketac-Molar Aplicap and Fuji IX GP to enamel and dentin. Am J Dent. 1998;11(5):235–239. [PubMed] [Google Scholar]
- 15.International Organization for Standardization. ISO 9917. Specification for dental water-based dental cements. Geneva: The Organization; 2003. [Google Scholar]
- 16.Lindquist B, Emilson CG. Distribution and prevalence of mutans streptococci in the human dentition. J Dent Res. 1990;69(5):1160–1166. doi: 10.1177/00220345900690050801. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson JW, Aggarwal A, Czarnecka B, Limanowska-Shaw H. The rate of change of pH of lactic acid exposed to glass-ionomer dental cements. Biomaterials. 2000;21(19):1989–1993. doi: 10.1016/s0142-9612(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson JW, Czarnecka B, Limanowska-Shaw H. A preliminary study of the effect of glass-ionomer and related dental cements on the pH of lactic acid storage solutions. Biomaterials. 1999;20(2):155–158. doi: 10.1016/s0142-9612(98)00153-7. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson JW, Czarnecka B, Limanowska-Shaw H. The interaction of glass-ionomer cements containing vinylphosphonic acid with water and aqueous lactic acid. J Oral Rehabil. 2003;30(2):160–164. doi: 10.1046/j.1365-2842.2003.00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson JW, Millar BJ, Czarnecka B, Limanowska-Shaw H. Storage of polyacid-modified resin composites ("compomers") in lactic acid solution. Dent Mater. 1999;15(6):413–416. doi: 10.1016/s0109-5641(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 21.Nomoto R, McCabe JF. A simple acid erosion test for dental water-based cements. Dent Mater. 2001;17(1):53–59. doi: 10.1016/s0109-5641(00)00058-0. [DOI] [PubMed] [Google Scholar]
- 22.Platt JA, Rhodes B. Microleakage of high-strength glass ionomer: resin composite restorations in minimally invasive treatment. J Indiana Dent Assoc. 2002;80(4):20–22. [PubMed] [Google Scholar]
- 23.Prakki A, Cilli R, Mondelli RFL, Kalachandra S, Pereira JC. Influence of pH environment on polymer based dental materials properties. J Dent. 2005;33(2):91–98. doi: 10.1016/j.jdent.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Rahimtoola S, Amerongen E van, Maher R, Groen H. Pain related to different ways of minimal intervention in the treatment of small caries lesions. ASDC J Dent Child. 2000;67(2):123–127. [PubMed] [Google Scholar]
- 25.Smales RJ, Gao W, Ho FT. In vitro evaluation of sealing pits and fissures with newer glass-ionomer cements developed for the ART technique. J Clin Pediatr Dent. 1997;21(4):321–323. [PubMed] [Google Scholar]
- 26.Smales RJ, Gao W. In vitro caries inhibition at the enamel margins of glass ionomer restoratives developed for the ART approach. J Dent. 2000;28(4):249–256. doi: 10.1016/s0300-5712(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg D, Eyal S. Early formation of Streptococcus sobrinus biofilm on various dental restorative materials. J Dent. 2002;30(1):47–51. doi: 10.1016/s0300-5712(01)00058-6. [DOI] [PubMed] [Google Scholar]
- 28.Turssi CP, Hara AT, Serra MC, Rodrigues AL., Jr Effect of storage media upon the surface micromorphology of resin-based restorative materials. J Oral Rehabil. 2002;29(9):864–871. doi: 10.1046/j.1365-2842.2002.00926.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Lopes LG, Bresciani E, Lauris JRP, Mondelli RFL, Navarro MFL. Evaluation of Class I ART restorations in Brazilian schoolchildren: three-year results. Spec Care Dentist. 2004;24(1):28–33. doi: 10.1111/j.1754-4505.2004.tb01676.x. [DOI] [PubMed] [Google Scholar]
- 30.Yip HK, Smales RJ, Ngo HC, Tay FR, Chu FCS. Selection of restorative materials for the atraumatic restorative treatment (ART) approach: a review. Spec Care Dentist. 2001;21(6):216–221. doi: 10.1111/j.1754-4505.2001.tb00257.x. [DOI] [PubMed] [Google Scholar]