Abstract
Objective:
The aim of this investigation was to evaluate the cytotoxicity of two brands of root canal sealers, epoxy-resin based and zinc oxide-eugenol based, and one commercial calcium hydroxide paste on a monocyte cell line THP-1.
Material and methods:
Undiluted (crude extract) and diluted extracts to 10%, 1%, 0.1%, 0.01%, 0.001% and 0.0001% of the sealers were tested for cytotoxicity to THP-1 cells using the trypan blue assay. Extracts were obtained according to ISO standard. Data were analyzed statistically by the Kruskal-Wallis and Mann-Whitney tests at 5% significance level.
Results:
Crude extract of AH Plus and Fill Canal killed approximately 90% of THP-1 cells versus 36% of THP-1 cells killed by L&C crude extract (p<0.05). Ten-fold dilutions of L&C, Fill Canal and AH Plus killed 24, 35 and 61% of THP-1 cells (p<0.05), respectively. Dilutions lesser than 1% caused minimal cell death as compared to the control groups (p>0.05), except for L&C 1% extract.
Conclusions:
The results revealed that the L&C paste crude extract was less cytotoxic to THP-1 cells than AH Plus or Fill Canal crude extracts.
Keywords: AH Plus, Fill Canal, L&C, Cytotoxicity, Trypan blue
INTRODUCTION
Successful endodontic therapy depends on thorough cleaning and shaping followed by obturation of the root canal system. During obturation, root canal sealers serve to fill irregularities between the dentinal walls and the gutta-percha core, act as lubricants, fill lateral or accessory canals, and bond to gutta-percha and dentin11. Numerous root canal sealers and intracanal pastes are commercially available and contain different components, including epoxy resin, zinc oxide-eugenol and calcium hydroxide. Sealers and pastes are materials used in the root canal system, and may induce inflammatory reaction when they extrude through the apical foramen and contact periradicular tissues. The calcium hydroxide-based paste L&C paste is claimed to induce apexification by stimulating the periapical tissues to promote apical mineralization, and it is also indicated to stimulate mineralization during perforation repair14.
The ISO standards establish general guidelines for the investigation of dental materials9. For studies dealing with endodontic sealers, samples should be stored for 1 to 3 days in culture medium. Depending on the materials tested and its thickness, the ratio of sealer surface area to culture medium should be between 0.5 to 6.0cm2/ mL.
This in vitro study aimed to assess the cytotoxicity of an epoxy-resin sealer (AH Plus), a zinc oxide-eugenol sealer (Fill Canal), and a commercial calcium hydroxide-based paste (L&C) on THP-1 cells, measuring cell viability by Trypan Blue dye exclusion method.
MATERIAL AND METHODS
Cell Culture
A monocyte cell line THP-1 derived from acute monocyte leukemia was obtained from Fundação Oswaldo Cruz (Rio de Janeiro, Brazil). Cells were cultured in RPMI-1640 culture medium (Sigma-BioSciences, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 U/mL). Cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
Extracts preparation
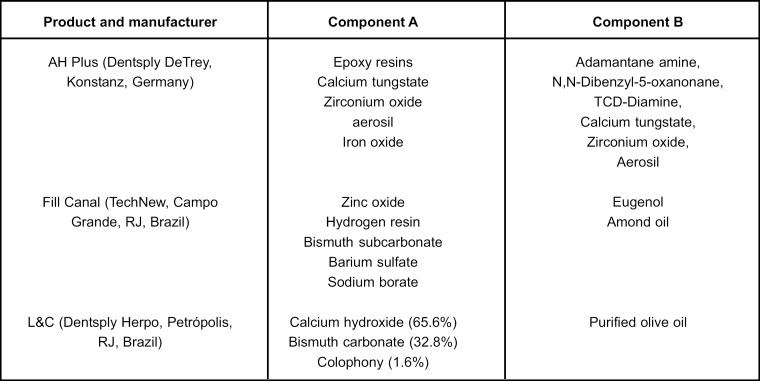
Figure 1 shows the tested materials and their compositions. Materials were mixed according to the manufacturer's instructions and 0.4 g of each sample was applied to the bottom of a round plastic Petri dish (35 mm × 10 mm) yielding a free specimen surface area of 55 mm2, according to ISO10993-59 standard. To prevent contamination, specimens were exposed to UV light for 30 min after manipulation. Thereafter, each specimen was covered with 4 mL of RPMI 1640 cell culture medium (Sigma-BioSciences) supplemented with penicillin (100 U/mL), streptomycin (100 U/mL) and sodium bicarbonate (2 g/L) at 37°C for a total period of 24 h. The supernatants were filtered through a 0.22μm membrane (Millipore Express PLUS Membrane, Millipore Inc., Billerica, MA, USA) and considered as crude extract (100%). Media alone stored under the same conditions were used as negative controls. Serial dilutions from the crude extract (100%) were made to obtain dilutions of 10%, 1%, 0.1%, 0.01%, 0.001%, and 0.0001% in supplemented RPMI medium. THP-1 cells were plated onto 24-well plates (FaL&Con, 3072, Becton Dickinson, Oxford, UK) at a density of 3.5 × 105 cells/well. After 24 h, 1 mL of extract for each serial dilution was added to each well and incubated for 24 h at 37°C in a humidified air atmosphere containing 5% CO2. All experiments were done in triplicate.
FIGURE 1. Tested materials.
Cytotoxicity assay
After incubation for 10 min at room temperature, the effect of the different materials on THP-1 cells was evaluated by 0.2% (final concentration) trypan blue dye exclusion analysis. Briefly, the cell number was determined by counting the viable cells in a hemocytometer. The percentage of viable cells from each well after incubation with material extracts was obtained by applying the following equation: % viable cells = (VC/TC) X 100, where VC = viable cells counted and TC = total cells counted (stained plus unstained cells).
Statistical analysis
The difference between the control and experimental groups was analyzed statistically by the Kruskal-Wallis and Mann-Whitney tests (SPSS version 10.0; SPSS Inc. Chicago, IL, USA). A p-value <0.05 was considered statistically significant.
RESULT
The cytotoxic effects of the epoxy-resin-based sealer (AH Plus), zinc oxide-eugenol-based sealer (Fill Canal) and calcium hydroxide-based paste (L&C) are shown in Figure 2. Crude and 10% extracts of all materials reduced significantly cell viability when compared to the control groups (p<0.05). Crude extracts of AH Plus and Fill Canal killed 90% of cells versus 36% of cells killed by the L&C extract. Ten-fold dilutions of L&C, Fill Canal and AH Plus killed 24, 35 and 61% of THP-1 cells respectively. Dilutions of sealer extracts of 1% and lower caused minimal cell death as compared to the control groups (p>0.05), except for 1% L&C paste extract, which was significantly different from control (p<0.05) (Figure 2).
FIGURE 2. Effect of 24 h crude (100%) and diluted elutes of the epoxy-resin sealer (AH Plus), zinc oxide-eugenol sealer (Fill Canal), and commercial calcium hydroxide-based paste (L&C) on THP1 cells by trypan blue dye exclusion assay expressed as percentage of viable cells in control and test groups. Each bar represents the mean.
DISCUSSION
The cytotoxicity of root canal fillings can be evaluated by different methods10,13. THP-1 cells were chosen because they may differentiate into phagocytes22, which are the predominant cells in a chronic inflammatory response.
Extrusion of intracanal materials, such as sealers or pastes, beyond the apical foramen resulting in direct contact with periapical tissues is undesirable. Chronic inflammatory diseases of tooth-supporting structures are one of the most significant causes of tooth loss in adults and the most prevalent form of bone pathology in humans, in addition to acting as a modifying factor of patients' systemic health6. Procedures to induce apexogenesis, apexification and repair of perforations and resorptions usually involve direct contact of materials with tissue, which makes relevant the study of cytotoxicity of sealers and calcium hydroxide pastes.
The aim of this investigation was to evaluate the cytotoxicity of two brands of root canal sealers and one commercial calcium hydroxide-based paste on a monocyte cell line THP-1, derived from acute monocyte leukemia.
In this study, using a standardized cytotoxicity assay, it was determined that AH Plus and Fill Canal were highly toxic to THP-1 cells, whereas L&C had relatively lower toxicity. THP-1 cells may be converted into mature cells with macrophage functions during cultivation22. This cell line was selected due to the fact that macrophages are the major phagocytes for the elimination of bacteria, cellular debris and foreign materials that reach periradicular tissues. Macrophages are the foremost phagocytes present in chronic inflammatory processes8. Moreover, studies have reported that the behavior of primary human fibroblasts do not differ significantly from that of immortalized cell lines in response to toxic challenge of endodontic materials20. Some studies have questioned the clinical relevance of cell cultures used to test sealer toxicity since any material can be toxic to mammalian cells in vitro when a large surface area is exposed1,10. However, it has been reported that the surface area and shape of a material exposed to test cells should resemble the conditions found under clinical conditions.
Standardized testing methods provide highly reproductive protocols to evaluate the cytotoxicity of materials9. Recently, Camps and About2 compared cytotoxic testing by ISO methods and a new root-dipping technique. They showed that while Sealapex exhibited a highly cytotoxic effect causing cell death ranging from 91% to 96% when evaluated according to ISO standards, the cytotoxicity of Sealapex ranged from 0% to 9% using the root-dipping technique. This discrepancy between the testing methods is more striking because they choose the lowest ratio between the surface of the sample and the volume of culture medium given by ISO standards (0.5 cm2/mL).
Trypan blue dye exclusion technique was chosen for the present experiment because it is easy to perform and allows for distinguishing non-viable from viable cells by microscopic analysis. Although accurate procedures for determination of cell viability are reported in literature3, the analysis by trypan blue assay reveals the disruption of cell membrane integrity. Trypan blue staining of non-viable cells is a common procedure used in cell culture research, and it relies on the premise that vital cells will not allow the stain to penetrate through cell membranes5,18. A previous scanning electron microscopic study23 designed to verify the accuracy of trypan blue in distinguishing vital and non-vital cells showed that cells permeated by the stain (non-vital cells) presented disruption of organelles, whereas vital cells presented integrity of membranes and organelles, as confirmed by ultra structural analysis.
In the present study, freshly mixed materials were analyzed because previous reports have shown that the cytotoxicity of sealers is higher immediately after mixing4,17. Schwarze, et al.19 reported that different sealers had a moderate to severe effect on human fibroblasts, periodontal ligament cells and immortal 3T3 fibroblasts immediately and 24 h after mixing.
AH Plus formulation is similar to that of AH26, except for the formaldehyde release21. In this investigation, L&C paste were less cytotoxic than Fill Canal and AH Plus. These results did not support the findings of Camp and About2, Tai, et al.21, Schwarze, et al.19 and Eldiniz, et al.4, but corroborate the findings of Willerhausen, et al.24. The differences could be accounted to the use of different cells types (L9292, V79, BF, GF21, 3T3, PDL19 and HGF4, and distinct methodologies (MTT2,4,19,21, trypan blue4). As observed in the present study, a previous study using neutral red assay for cytotoxicity found that AH Plus was cytotoxic immediately after mixing7, but its cytotoxicity decreased 4 h after mixing.
Except for L&C paste, concentrations below 1% of freshly prepared sealers did not promote significant cell death when compared to control groups. This suggested a dose-dependent cytotoxic effect, even using freshly prepared extracts.
With regard to AH Plus extracts, a previous study has shown only a moderate toxic effect up to one hour after mixing, whereas extracts obtained 5 and 24 h after mixing showed no toxic effect on mitochondrial activity19. In the same investigation, Apexit, a calcium hydroxide-based sealer, did not show toxic effects on PDL and 3T3 fibroblast cells, as observed in this study for L&C paste on THP-1 cells.
In agreement with our results, zinc oxide-eugenol-based sealers have been shown to be cytotoxic, which has been attributed to the eugenol present in different formulations15,16. Lee, et al.12 demonstrated that eugenol suppressed IL-1â and TNFá production in LPS-treated macrophages. Those authors12 believed that this inhibition of cytokine production may contribute to decreasing the impact of cytokine-mediated host destructive process during pulp and periapical infection. Thus, eugenol-based sealers may have a potential anti-inflammatory effect from a clinic point of view.
Considerations for choosing an adequate root canal sealer include its physical properties and biocompatibility. Further studies are necessary to ensure that the clinical use of root canal sealers are well tolerated by periapical tissues, produce the low cytotoxic effects and facilitate successful clinical endodontic outcomes.
CONCLUSION
Based on the results of this study, it may be concluded that the calcium hydroxide paste (L&C) crude extract was less cytotoxic than the epoxy-resin sealer (AH Plus) or zinc oxide-eugenol sealer (Fill Canal) crude extracts.
ACKNOWLEDGEMENTS
This research was supported by grants from FAPERJ (Process #E-26 171.176/05 / E-/152.642/2005) and CAPES (Process # 2993-06-6). The authors would like to express their appreciation to Professor José Mauro Granjeiro from Department of Cell and Molecular Biology of Federal Fluminense University, for critical reading and discussion of the manuscript. The authors also acknowledge Dr. Helio dos Santos Dutra from the Laboratory of Blood Marrow Transplantation of the Cell Bank of Rio de Janeiro, Federal University of Rio de Janeiro, for making possible part of this work.
REFERENCES
- 1.Browne RM. The in vitro assessment of the cytotoxicity of dental materials, does it have a role? Int Endod J. 1988;2:50–58. doi: 10.1111/j.1365-2591.1988.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 2.Camps J, About I. Cytotoxicity testing of endodontic sealers: a new method. J Endod. 2003;20:83–86. doi: 10.1097/00004770-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Costa AO, Assis MC, Marques EA, Plotkowski MC. Comparative analysis of three methods to assess viability of mammalian cells in culture. Biocell. 1999;23:65–72. [PubMed] [Google Scholar]
- 4.Eldiniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of resin, calcium hydroxide and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cells lines. Int Endod J. 2007;40:329–337. doi: 10.1111/j.1365-2591.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 5.Freshney RI. Culture of animal cells: a manual of basic technique. 4th ed. New York: Wiley-Liss; 2000. [Google Scholar]
- 6.Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 7.Gheshlaghi NA, Heidari M, Bahrami ZS, Shokri F. In vitro cytotoxicity of a new epoxy resin root canal sealer. J Endod. 2000;26:462–465. doi: 10.1097/00004770-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Haglund R, He J, Jarvis J, Safavi KE, Spångberg LS W, Zhu Q. Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:739–745. doi: 10.1067/moe.2003.231. [DOI] [PubMed] [Google Scholar]
- 9.International Organization for Standardization. Biological evaluation of medical devices. Part 5: Tests for cytotoxicity: In vitro methods. Geneva: The Organization; 1999. ISO standards 10993-5. [Google Scholar]
- 10.Langeland K, Guttusa J, Langeland L, Tobon G. Methods in the study of biologic responses to endodontic materials. Oral Surg. 1969;27:522–542. [Google Scholar]
- 11.Lee KW, Williams MC, Campos J, Pashley D. Adhesion of endodontics sealers to dentin and gutta-percha. J Endod. 2002;28:684–688. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lee YY, Hung SL, Pai SF, Lee YH, Yang SF. Eugenol suppresses the expression of lipopolysaccharide induce proinflammatory mediators in human macrophages. J Endod. 2007;33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Leonardo MR, Silva LAB, Almeida WA, Utrilla LS. Tissue response to an epoxy-resin-based root canal sealer. Endod Dent Traumatol. 1999;15:28–32. doi: 10.1111/j.1600-9657.1999.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 14.Siqueira JF, Jr, Roçás I, Lopers HP. Lopes HP, Siqueira JF., Jr. Endodontia: biologia e técnica. 2. ed. Rio de Janeiro: Editora Medsi; 2004. Materiais obturadores; pp. 619–643. [Google Scholar]
- 15.Nakamura H, Sakakibara F, Matsumoto Y, Hirano S, Hayakawa H, Sakai K, et al. Study on the cytotoxicity of root canal filling materials. J Endod. 1986;4:156–160. doi: 10.1016/S0099-2399(86)80054-1. [DOI] [PubMed] [Google Scholar]
- 16.Rapport HM, Lilly GE, Kapsimalis P. Toxicity of endodontic filling materials. Oral Surg Oral Med Oral Pathol. 1964;8:785–802. doi: 10.1016/0030-4220(64)90480-3. [DOI] [PubMed] [Google Scholar]
- 17.Safavi KE, Spangberg LS, Costa NS, Sapounas G. An in vitro method for longitudinal evaluation of toxicity of endodontic sealers. J Endod. 1990;15:484–486. doi: 10.1016/s0099-2399(89)80029-9. [DOI] [PubMed] [Google Scholar]
- 18.Scelza MFZ, Oliveira LRL, Carvalho FB, Faria SCR. In vitro evaluation of macrophage viability after incubation in orange oil, eucalyptol and chloroform. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:24–27. doi: 10.1016/j.tripleo.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Schwarze T, Fiedler I, Leyhausen G, Geurtsen W. The cellular compatibility of five endodontic sealers during the setting period. J Endod. 2002;28:784–786. doi: 10.1097/00004770-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Spangberg L. Biological effects of root canal filling materials. Odontol Rev. 1969;29:427–437. [PubMed] [Google Scholar]
- 21.Tai KW, Huang FM, Chang YC. Cytotoxic evaluation of root canal filling materials on primary human oral fibroblast cultures and a permanent Hamster cell line. J Endod. 2001;27:571–573. doi: 10.1097/00004770-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 23.Van Bezooijen C, Van Noord M, Knook D. The viability of parenchymal liver cells isolated from young and old rats. Mech Ageing Dev. 1974;43:107–119. doi: 10.1016/0047-6374(74)90009-8. [DOI] [PubMed] [Google Scholar]
- 24.Willershausen B, Marroquín BB, Schäfer D, Schulze R. Citotoxicity of root canal filling materials to three different human cell lines. J Endod. 2000;26:703–707. doi: 10.1097/00004770-200012000-00007. [DOI] [PubMed] [Google Scholar]