Abstract
Objectives:
As the choice of suprastructure alloy to be combined with titanium for the oral cavity is still a much debated issue, the aim of this study was to investigate the electrochemical interaction of the suprastructure/implant couples under the determined experiment conditions.
Material and Methods:
The potentiodynamic polarization curves and open-circuit potentials (OCP) of four UCLA type suprastructures coupled with straight Swiss Plus implant fixtures were taken in Afnor type artificial saliva solution at 37°C. The concentration of ions leached into artificial saliva solutions was estimated with ICP-MS. SEM images of the margins of suprastructure/implant couples were obtained before and after the electrochemical tests.
Results:
The OCP value of titanium became passive at the most negative potential. The lowest difference between the initial and constant OCP value was exhibited by the Au based suprastructure. Suprastructures made greater contributions to the potentiodynamic polarization curves of the implant/suprastructure couples. According to the ICP-MS results, Pd based and Au based couples dissolved less than Co-Ni based and Co-Cr based couples.
Conclusions:
Within the conditions this study, it may be concluded that the titanium implant forms a stable passive oxide layer in artificial saliva exposed to open air and does not affect the corrosion properties of the suprastructures. Pd based and Au based couples have been found to be more corrosion-resistant than base alloy couples.
Keywords: Corrosion, Dental casting alloys, Titanium implant, Electrochemical properties, Galvanic corrosion
INTRODUCTION
Dental implants and abutments are generally produced from titanium because of its well documented biocompatibility and mechanical properties1. While the quality of implant materials made of titanium is unanimously acknowledged, the selection of suitable alloys for suprastructure is open to discussion. This selection must be done by considering the given alloy's resistance to corrosion when coupled with titanium, its biocompatibility, and the existing clinical studies of the relation between the metals and epithelial/subepithelial connective tissues21.
A galvanic current occurs when different metals come into contact with each other in an electrolytic medium, resulting in corrosion. The intensity of the galvanic current depends on different factors such as the electrode potential, polarization, surface area ratio of anode/cathode, distance between the electrodes, structure of the electrode surface, aeration, temperature, pH and composition of the electrolyte. In addition, there are some corrosion types to be taken into account, like the occurrence of crevice corrosion originating from its geometry17,21. Depending on the electrode potentials of the metals in the electrolytic medium, one acts as the anode and the other as the cathode. The more noble metal is the cathode and the more active one is the anode. Thus, a corrosion cell is formed. Only the anode goes into corrosion in this cell. In some cases, corrosion is expected, but in practice, the reaction does not occur or occurs at a very slow rate due to the formation of a passive layer. The corrosion rate of some metals is considerably low by reason of this passive layer formation.
Oral galvanism takes place when there is a difference in the electrical potential between dissimilar restorative metals5. With metals and alloys, corrosion is always accompanied by ion release and a current. When the materials in the oral cavity undergo corrosion, deterioration occurs in the mechanical and aesthetic properties. More importantly, corrosion products have local or systemic adverse effects on biological structures23,26. Besides, the current resulting from the galvanizing couple can cause bone resorption7,14,19. The extended presence of corrosion reactions can result in fractures at the interface of the alloy and abutment. Some researchers have reported that high stress concentrations between the suprastructure-implant interfaces may be responsible for implant failure and the stress corrosion fractures there may evolve13,24. The effects of galvanic corrosion over short or long periods could be decreased with the use of the appropriate suprastructure alloy13. It can be said that, in the ideally selection of an suprastructure alloy, the alloy should not cause galvanic corrosion when coupled with titanium therefore no ion release occur because of the galvanic corrosion at the titanium implant or at the alloy.
Even though numerous studies have been conducted, all of the mechanisms involved in such corrosion are not yet completely understood12. On the one hand, in vivo evaluations are not reproducible; on the other hand, the physiological conditions of in vitro experiments cannot be fully simulated. In order to measure how corrosion-resistant dental alloys are qualitatively, electrochemical tests such as open circuit potential measurements and potentiodynamic polarization tests can be used15. The potentiodynamic polarization technique used mostly in corrosion tests is an in vitro method8. Electrochemical potentiodynamic polarization techniques are often used, providing general information about the corrosion resistance and susceptibility, such as the general corrosion rate, the range of passivation, and the break-down potential. In these methods, corrosion curent (Icorr) and potential (Ecorr) can be obtained by extrapolating the anodic and cathodic branches of potentiodynamic polarization curves. Electrochemical methods have been successfully implemented in several investigations of various corrosion problems in dentistry.
The aim of this in vitro study was to investigate and compare the electrochemical interaction of four different commercially available dental casting alloys when used as implant suprastructure, while coupled with titanium implant.
MATERIAL AND METHODS
In this study, 4 straight SwissPlus (Sulzer Dental Inc., Carlsbad, CA, USA) implant fixtures 10 mm in length, 4.1 mm in diameter and 4.8 mm in platform diameter were coupled with four UCLA type suprastructures made of four different commercial dental alloys. The cervical regions of the implants had a 2.5 mm high machined surface and 0.6 mm high beveled shoulders. The suprastructures and suprastructure/implant couples were named as M1, M2, M3, M4 and C1, C2, C3, C4, respectively.
Four cylindrical UCLA type suprastructures with a length of 6.5 mm and a diameter of 4.8 mm were obtained by using Swiss Plus plastic waxing coping (Sulzer Dental Inc.) from four different types of dental alloy. Table 1 shows the types and composition of the samples used. Each pattern was invested and melted in an induction casting machine (Bego Fornax 35 EM, Bremer, Germany), based on recommendations of the alloy manufacturers. All casts were sandblasted to remove the oxide films and residual investments. Finishing and polishing were done using light pressure under standard conditions with a high speed polishing motor. The polishing was completed using a soft brush and a 1-μm polishing paste. The specimens were washed in distilled water and ultrasonically cleaned in ethanol. The suprastructure samples were screwed into the fixtures using a manual torque wrench with a force of 30 Ncm.
TABLE 1. Chemical composition of materials used as weight %.
| Material | Au | Pt | Pd | Ni | Co | Cr | Cu | Ga | In | Mo | Zn | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 (Co-Ni based) | 61 | 26 | 11 | 1.5 | ||||||||
| xM2 (Co-Cr based) | 64.8 | 28.5 | 5.3 | 0.5 | ||||||||
| *M3 (Pd based) | 2 | 79 | 6.9 | 5.5 4.5 | ||||||||
| +M4 (Au based) | 85.9 | 11.7 | 1.5 | |||||||||
| Ti | Cold worked grade 4 commercially pure Ti (Ti=99, C<0.1, H < 0.015, Fe < 0.5, N < 0.05, O < 0.4) |
|||||||||||
Mn+C<1%;
Sn+Zn < %2;
Rh +Ir+Nb+Mn+In+Fe < %1 M1
Remanium CS, Dentaurum, Ispringen, Germany.
M2 Biosil F, DeguDent GmbH, Hanau, Germany.
M3 Cerapall 2, Metalor Métaux Préciux SA, Neuchatel, Switzerland
M4 V-Gnathos®Plus, Metalor Métaux Préciux SA, Neuchatel, Switzerland
Ti SwissPlus™, Sulzer Dental Inc., Carlsbad. CA, USA
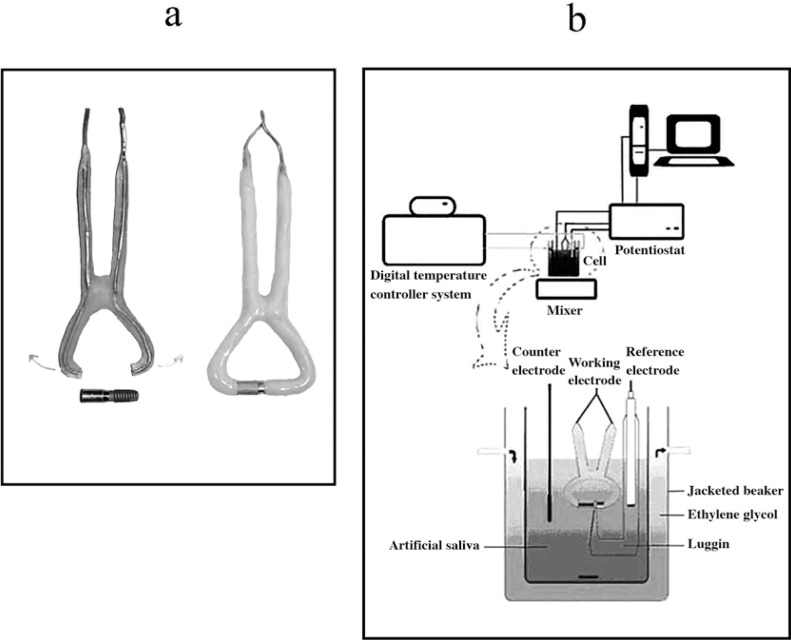
The 1.5 mm thick copper wire was cleaned using concentrated nitric acid, and then covered with polymethyl methacrylate resin. The clamp shaped copper wire with both its tips open and covered with resin was resistant to distortion and also slightly flexible. The gap between the two ends of the resin coated copper wire was 3 mm shorter than the coupled samples (Figure 1a). The coupled samples were tightened between the two ends of the copper wire. In this way, an electrical connection between the two ends of the sample was formed. Finally, the uncovered copper, the top of the cylindrical suprastructure and the MTX textured surface of fixture were covered with epoxy resin (Figure 1a).
FIGURE 1. (a) Two tips open, flexible, copper clamp and covered sample with epoxy resin. (b) Schematic view of electrochemical cell and experiment set up.
A jacketed beaker was used to maintain a constant temperature during the electrochemical measurements. The Afnor type6 artificial saliva solution (K2HPO4:0.2, KCl: 1.2, KSCN: 0.33, Na2HPO4: 0.26 NaCl: 0.7, NaHCO3:1.5, CO(NH2)2: 1.5 g.L-1) buffered to 6.7 and exposed to open air was used as the electrolytic medium. A digital temperature controller system (Model 9602; Polysciences Inc., Warrington, PA, USA) was used to maintain the temperature of the system containing 125 mL of artificial saliva constant at 37 °C. A potentiostat-galvanostat system (System Model 660B; Ch Instruments Inc., Austin, TX, USA) was connected to a computer and used for the purposes of the study. The electrochemical measurements were carried out in a single compartment three-electrode cell with the prepared alloy as the working electrode (1.33 cm2), platinum foil (1.00 cm2) as the counter electrode and the saturated calomel electrode (SCE) as the reference electrode. The SCE was drawn about 2 mm from the working electrode with a capillary tip (Luggin capillary). The potential was applied between the reference and working electrode, and the resulting current between the working electrode and the counter electrode was recorded. The entire set up of the experiment is shown in Figure 1b.
Prior to the potentiodynamic polarization test, the samples underwent 10 min of electrolysis at a constant potential of - 600 mV so that the effects of oxides and other impurities on the surface would be eliminated. Potentiodynamic polarization curves were obtained between a -800 mV and 1600 mV potential range with a scan rate of 1 mV s-1. Each experiment was performed with freshly prepared artificial saliva solution. At least three experiments were carried out in order to get similar potentiodynamic polarization curves for all of the samples.
After obtaining the potentiodynamic polarization curves of all samples coupled with titanium, the concentration of the ions that leached into the artificial saliva solutions was estimated with ICP-MS (Thermo Elemental X 7 ICP-MS, Winsford, England) in ppm (parts per million).
A scanning electron microscope (SEM) (JEOL JSM 6400, Jeol, Tokyo, Japan) was used to obtain images before and after the potentiodynamic polarization tests for the coupled samples, including the margins of the suprastructures and implants in order to determine resultant corrosion. All images were saved in a digital medium. Following the potentiodynamic polarization tests, the samples were washed several times with de-ionized water to remove any ions adsorbed to the surface and were kept in a nitrogen environment.
Following the SEM imaging, the suprastructures were removed from the implants, and cleaned and polished once again. The open circuit potential vs. time (nearly 14 h) curves and potentiodynamic polarization curves of each sample were obtained separately for all the suprastructures and titanium under the same experimental conditions.
RESULTS
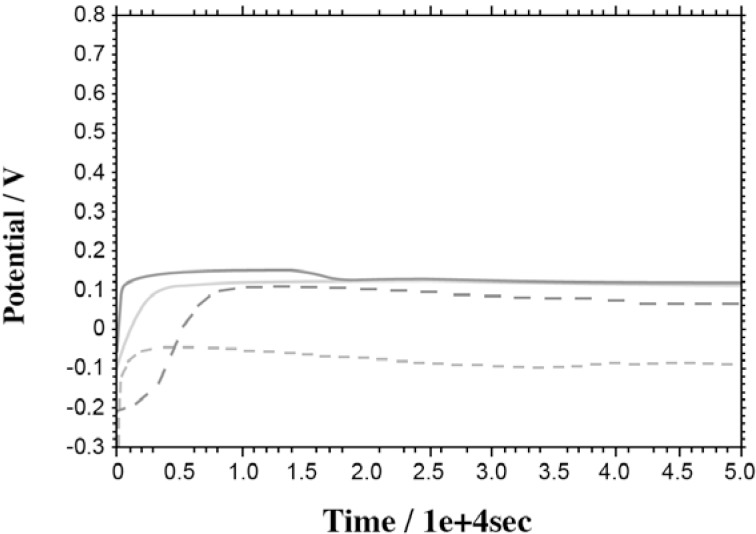
Open-Circuit Potentials (OCP): Figure 2 shows the OCP versus time curves of the suprastructures and titanium recorded during 5.0 x 104 seconds in Afnor type solution at 37°C. The OCP data obtained are summarized in Table 2.
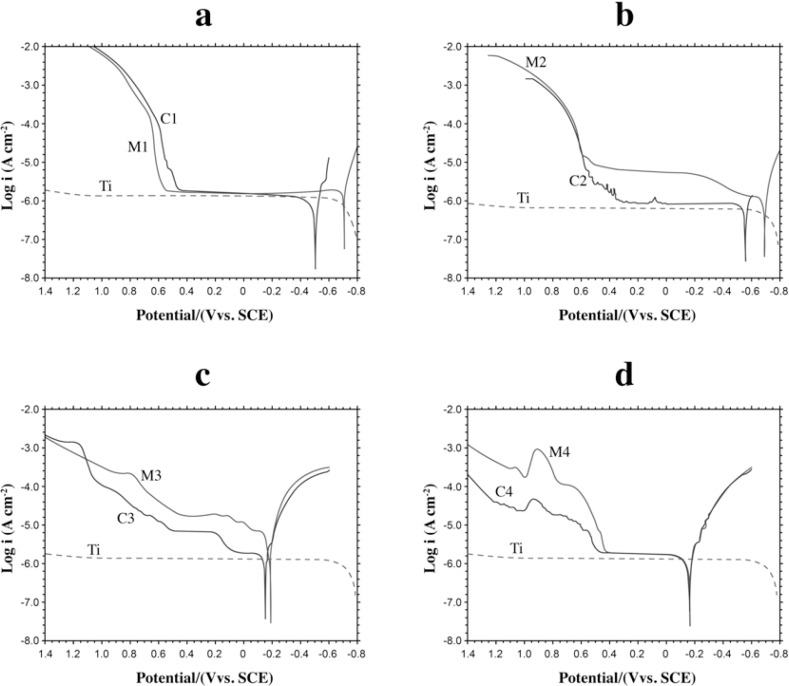
The effect of the titanium and the suprastructure in the Ti/suprastructure couple on the potentiodynamic polarization curves: Figures 3a, b, c, d show the potentiodynamic polarization curves of Titanium (Ti), the suprastructures (M1, M2, M3, M4) and the Ti/suprastructure couples (C1, C2, C3, C4) in Afnor type solution at 37°C, respectively. According to these curves it was observed that suprastructures contribute more to the potentiodynamic polarization curves of the implant/suprastructure couples (C1, C2, C3, C4) compared to the potentiodynamic polarization curves of the suprastructures (M1, M2, M3, M4) and titanium (Ti) (Figures 3a, b, c, d) because no considerable rise at the anodic current branch of titanium was observed between -60.0 mV and 1200 mV. The most important pitting potentials of the couples and their suprastructures are; C1: 626 mV, M1: 630 mV; C2: 588 mV, M2: 601 mV; C3: 779, M3: 696 mV; C4: 521 mV, and M4: 530.
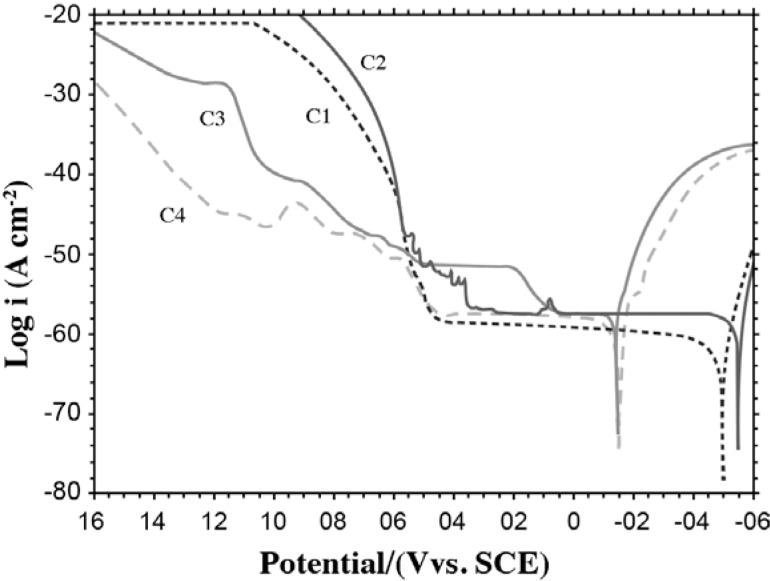
Comparison of the potentiodynamic polarization curves of the Ti/suprastructure samples: The potentiodynamic polarization curves of the couples (C1, C2, C3, and C4) are shown in Figure 4. The Ecorr and Epotentials; Icorr and current values at 250 mV, 800 mV are also given in Table 3 as obtained from these curves.
Ion concentration leached into the solution from the Ti/suprastructure couples: After obtaining the potentiodynamic polarization curves of the samples coupled with titanium, the concentration of the ions that leached into the artificial saliva solutions were estimated with ICP-MS and are illustrated in Table 4.
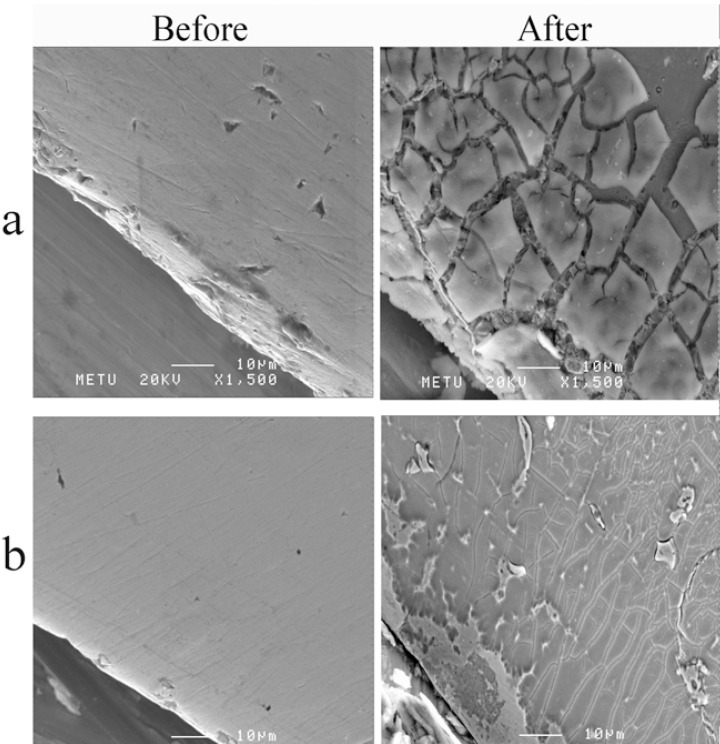
SEM Images: When SEM images taken before and after potentiodynamic polarization curves were compared, extensive breakdowns was observed at surface of the suprastructures of samples C1 and C2. It was clearly observed that segregations extensively observed on the whole surfaces, however there were still little unaffected areas could be detected appearing as weakly attached to the surface (Figures 5a, b). When surfaces of Ti, C3 and C4 materials were investigated, almost no visible effect was revealed (Figures 6a, b).
FIGURE 2. Open circuit potential (OCP) versus time curves of suprastructures (M1, M2, M3, M4) and Ti recorded in Afnor type solution at 37°C.
TABLE 2. Open-circuit potentials data for Ti and the suprastructures.
| Material | Einitial (mV) | Econstant (mV) |
|---|---|---|
| M1 | -185 | 120 |
| M2 | -220 | 105 |
| M3 | -100 | 105 |
| M4 | 60.0 | 135 |
| Ti | -200 | -50.0 |
FIGURE 3. Potentiodynamic polarization curves at 37 °C in Afnor type solution (scan rate: 1 mV sec-1). (a) M1, C1 and Ti (b) M2, C2 and Ti (c) M3, C3 and Ti (d) M4, C4 and Ti.
FIGURE 4. Potentiodynamic polarization curves of C1, C2, C3 and C4 at 37 °C in Afnor type solution (scan rate: 1 mV sec-1).
TABLE 3. Potential and current values obtained from potentiodynamic the polarization curves of Ti/suprastructure couples.
| Sample | Ecorr/mV | Epitting/mV | Icorr/pA cm-2 | I250mV/μA cm-2 | I800mV/MA cm-2 |
|---|---|---|---|---|---|
| C1 | -517 | 626 | 0.4341 | 1.222 | 1154 |
| C2 | -568 | 588 | 0.7502 | 1.297 | 2986 |
| C3 | -160 | 140, 779 | 0.7086 | 5.012 | 25.87 |
| C4 | -171 | 521 | 0.5522 | 1.236 | 12.54 |
TABLE 4. Concentration of the ions leached into the artificial saliva solutions from the Ti/suprastructure couples.
| Sample | Individual ion concentrations/ppm | Total |
|---|---|---|
| C1 | Ni: 0.950; Cr: 0.399; Mo: 0.214; Si: -; Ti: 0.184 | 1.75 |
| C2 | Co: 1.21; Cr: 0.479; Mo: 0.122; Mn: 0.011; Si: (-); Ti: 0.181 | 2.01 |
| C3 | Au: 0.002; Pd: 0.048; Cu: 0.011; Sn: 0.016; Ga: (-); In: (-); Ti: 0.179 | 0.256 |
| C4 | Au: 0.038; Pt: 0.006; Rh: 0.001; Zn: (-); Ti: 0.182 | 0.227 |
FIGURE 5. SEM images; before and after the potentiodynamic polarization tests (a) C1 (b) C2.
FIGURE 6. SEM images; before and after the potentiodynamic polarization tests (a) C3 (b) C4.
DISCUSSION
In this study, UCLA type abutment was used to connect a titanium implant body directly to a suprastructure. With UCLA type abutment implants, removable crowns can be attached directly to the bodies of the implants by using screws9,29. A disadvantage while using this type of abutment is the possibility of corrosion due to the connection of two different metals and fitting problems resulted at casting29.
The resistance a metal or an alloy exhibits against corrosion depends not only on its own properties but also on its interactions with its surroundings4. When metals and alloys are exposed to high temperature (0.0 °C - 70 °C), such as during eating, their pH changes (2-11)10. As opposed to liquids in other types of tissue, the concentration of electrolyte and oxygen in the oral cavity varies. For this reason; dental implants, open to the oral cavity, are more exposed to galvanic corrosion than orthopedic implants13. Moreover, saliva is exposed to open air, which contains gases like SOx, NOx, chlorides, oxygen and sulfide, resulting in atmospheric corrosion22.
In biomedical applications, titanium is known to have good corrosion properties because of the stable, smooth and strongly adherent passive oxide film forming rapidly on the metal surface3 when the oxide layer brakes down mechanically. Since resistance to corrosion is directly proportional to the oxide layer formation, a strong passive film formation on the metal surface enhances resistance to corrosion. It follows from this that the worst situation is the prevention of passive layer formation for a biomedical metallic material. The concentration of the metal ions released into the solution is related to the nature, composition and thickness of the metal oxides and adherence force to the alloy18.
Information about the general interaction at the surface of the alloy can be obtained by evaluating the changes at open circuit potential values. OCP curve values shifting towards to positive potentials show that the oxidation current of the alloy will reduce and form a passive surface layer. Shifting the values of the curve towards to more negative potential indicates that the oxidation current density gradually increases, in other words ion release is taking place. The potential becomes constant against time and this behavior shows that electrochemical reactions at the alloy surface are in equilibrium. In this study, the potential of all samples shifted to more positive values and became constant after a period of time (Figure 2, Table 2). The surface of the titanium became passive at the most negative potential. The open circuit potentials of M1 and M2 became constant after 280 and 145 min because of the slowly forming unstable oxide layer. On the other hand, M3 and M4 had open circuit potentials constant at 65 and 50 min, respectively. With the smallest difference between the constant and initial open circuit potential values, M4 was found to be the most suitable suprastructure alloy in the alloys tested in this study.
According to the ICP-MS results, the titanium concentration transferring to the solution from all of the Ti/suprastructure couples was the almost the same. The investigation of the total ion concentrations leached into the artificial saliva solution shows that C3 (0.256 ppm) and C4 (0.227 ppm) dissolved less than the base alloys, C1 (1.75 ppm) and C2 (2.01 ppm) while the potentiodynamic polarization curves were being obtained (Table 4). These results are in accordance with those of the potentiodynamic polarization curves. This is because C3 (79% Pd) and C4 (86% Au) contain large amounts of noble metals. On the other hand, the C1 and C2 base alloys were made up of Ni, Cr and Co, Cr respectively. It can also be said that because of the unstable oxide layer composition, a larger ion concentration transferred to the solution from the C1 and C2 suprastructure alloys.
The corrosion current and corrosion potential were determined by extrapolation of the linear portions of the anodic and cathodic potentiodynamic polarization curves27. When a comparison was made between the potentiodynamic polarization curves of the suprastructures (M1, M2, M3, M4) and titanium implant (Ti) with those of the implant/suprastructure couples (C1, C2, C3, C4), it was observed that the contribution to the couples was mostly from the suprastructure (Figs. 3a, b, c, d). This was because the anodic current density of the titanium implant had a very small value at higher potentials and no rise was observed at the anodic branch. Although titanium has a negative corrosion potential value (Ecorr), its surface is covered with oxide layers (TiO2 and a small amount of Ti2O3 and TiO), protecting it against corrosion10. These results are consistent with those of previous studies on the formation of a passive layer on the titanium surface, which increases corrosion strength20.
The Ecorr values of C3 and C4 (-160 V and -171 V) were found to be at more positive values than those of C1 and C2 (-517 V and -568 V). The current densities of C3 and C4 also had low values at 800 mV when compared with those of the other samples because of the noble metals present in C3 (79% Pd) and C4 (86% Au) (Table 3). These results show that C1 and C2 are weaker materials when their corrosion performance is compared. The total ion concentration passing onto the solution from C1 and C2 showed that they had dissolved more than the other alloys, which is in agreement to the above result. The nickel and cobalt present in the structure of C1 formed a complex with the thiocyanate ion from saliva, and nickel could make complexes or compounds with urea16,28. Thus, the formation of complexes and compounds could contribute to the weakness of the alloys.
When the ICP-MS and Ecorr values of C1, C2, C3 and C4 were compared, it was expected that C3 and C4 would have lower current values at 250 mV and 800 mV (Table 3). Another reason for this expectation was the fact that C3 and C4 have large amounts of noble metals, palladium and gold, respectively. When dissolving total ion concentrations were compared, C3 was found as one of the alloys showing least dissolution (0.256 ppm). In addition, when the values of Ecorr were compared, C3 was observed to oxidize at the most positive potential. In contrast to these results, C3 started to dissolve at 140 mV and had a current value of 250 mV, which was higher than expected. This could be due to the presence of the small amount of Cu and Sn, making the alloy vulnerable to corrosion even though it contains high amounts of a noble metal like palladium. The presence of the small amount of Cu in the alloy prevents the material from being resistant against corrosion. Bayramoglu et al also mentioned in their study that titanium dissolves less because of its oxide layer while Sn and Cu containing alloys dissolve more2.
In this study, the sample C4 with Au was found to have dissolved the least according to the ICP-MS results, which was parallel with the results of the electrochemical test. C4 seems to have dissolved less when a comparison was made in terms of the I800mV values. The SEM images of C4 also support these data (Figure 5b). However, the potassium thiocyanate present in the saliva solution in high concentrations is a disadvantage25. Gold in C4 could form a complex with thiocyanate ions, posing a problem, as also reported for these types of solutions11. Even beginning to dissolve at a more negative potential (Epitting= 521 mV), which is a disadvantage for gold containing samples in artificial saliva solution, this sample was concluded to be superior to the others since it had dissolved less (I800mV=12.54 μA cm2). The low current density at 800 mV also confirmed that it was superior material comparing to the others. In conclusion, the formation of complexes with thiocyanate ion could contribute to the weakness of the C4 suprastructure alloy, but this alloy was appeared superior in terms of resistance to corrosion among the samples tested.
A comparison of the SEM images taken before and after the potentiodynamic polarization tests showed that the surfaces of the C1 and C2 suprastructures were extremely prone to corrosion. It was seen that the aggregations weakly attached to the surface had fallen off, and a strong presence of metal was observed (Figures 5a, b). The segregations on the surface of the alloys showed that the corrosion had started from the grain boundaries. On the other hand, no significant change was observed in the SEM images of Ti, C3 and C4 before and after corrosion (Figures 6a,b).
CONCLUSIONS
Under the circumstances of this in vitro experimental study it may be concluded that: 1. The titanium implants rapidly formed a stable, passive layer. 2. In the titanium implant and suprastructure alloy combinations, the titanium implant had virtually no effect on the pitting potential due to the passive layer formed on the surface of the implant. The corrosion properties were highly related to the suprastructure alloys. 3. The gold and palladium-based, noble alloys were found to have dissolved less than the nickel-chromium and cobalt-chromium alloys.
REFERENCES
- 1.Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10(6):387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 2.Bayramoglu G, Alemdaroglu T, Kedici S, Aksut AA. The effect of pH on the corrosion of dental metal alloys. J Oral Rehabil. 2000;27(7):563–575. doi: 10.1046/j.1365-2842.2000.00549.x. [DOI] [PubMed] [Google Scholar]
- 3.Berg E, Wagnere WC, Davik G, Dootz ER. Mechanical properties of laser-welded cast and wrought titanium. J Prosthet Dent. 1995;74(3):250–257. doi: 10.1016/s0022-3913(05)80131-3. [DOI] [PubMed] [Google Scholar]
- 4.Bergman M. Corrosion in the oral cavity: potential local and systemic effects. Int Dent J. 1986;36(1):41–44. [PubMed] [Google Scholar]
- 5.Certosimo AJ, O'Connor RP. Oral electricity. Gen Dent. 1996;44(4):324–326. [PubMed] [Google Scholar]
- 6.Cortada M, Giner L, Costa S, Gil FJ, Rodriguez D, Planell JA. Galvanic corrosion behavior of titanium implants coupled to dental alloys. J Mater Sci Mater Med. 2000;11(5):287–293. doi: 10.1023/a:1008905229522. [DOI] [PubMed] [Google Scholar]
- 7.Geis-Gerstorfer J, Weber H, Sauer KH. In vitro substance loss due to galvanic corrosion in Ti implant/Ni-Cr supraconstruction systems. Int J Oral Maxillofac Implants. 1989;4(2):119–123. [PubMed] [Google Scholar]
- 8.Holland RI. Corrosion testing by potentiodynamic polarization in various electrolytes. Dent Mater. 1992;8(4):241–245. doi: 10.1016/0109-5641(92)90093-r. [DOI] [PubMed] [Google Scholar]
- 9.Jaime APG, Vasconcellos DK, Mesquita AMM, Kimpara ET, Bottino MA. Effect of cast rectifiers on marginal fit of UCLA abutments. J Appl Oral Sci. 2007;15(3):169–174. doi: 10.1590/S1678-77572007000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedici SP, Aksut AA, Kilicarslan MA, Bayramoglu G, Gokdemir K. Corrosion behaviour of dental metals and alloys in different media. J Oral Rehabil. 1998;25(10):800–808. doi: 10.1046/j.1365-2842.1998.00305.x. [DOI] [PubMed] [Google Scholar]
- 11.Kononova ON, Kholmogorov AG, Danilenko NV, Kachin YS, Kononov YS, Dmitrieva ZV. Sorption of gold and silver on carbon adsorbents from thiocyanate solutions. Carbon. 2005;43:17–22. [Google Scholar]
- 12.Laurent F, Grosgogeat B, Reclaru L, Dalard F, Lissac M. Comparison of corrosion behaviour in presence of oral bacteria. Biomaterials. 2001;22(16):2273–2282. doi: 10.1016/s0142-9612(00)00416-6. [DOI] [PubMed] [Google Scholar]
- 13.Lemons JE, Dietsh-Mish F. Biomaterials for dental implants. In: Misch CE, editor. Contemporary implant dentistry. St Louis: Mosby; 1999. pp. 271–302. [Google Scholar]
- 14.Lucas LC, Lemons JE. Biodegradation of restorative metallic systems. Adv Dent Res. 1992;6:32–37. doi: 10.1177/08959374920060011301. [DOI] [PubMed] [Google Scholar]
- 15.Mareci D, Nemtoi G, Aelenei N, Bocanu C. The electrochemical behaviour of various non-precious Ni and Co based alloys in artificial saliva. Eur Cell Mater. 2005;10:1–7. [PubMed] [Google Scholar]
- 16.Mavis B, Akin M. Kinetics of urea decomposition in the presence of transition metal ions: Ni2+ J Am Ceram Soc. 2006;89:471–477. [Google Scholar]
- 17.Oh KT, Kim KN. Electrochemical properties of suprastructures galvanically coupled to a titanium implant. J Biomed Mater Res B Appl Biomater. 2004;70(2):318–331. doi: 10.1002/jbm.b.30046. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki Y, Gotoh E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials. 2005;26(1):11–21. doi: 10.1016/j.biomaterials.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Olmedo D, Fernandez MM, Guglielmotti MB, Cabrini RL. Macrophages related to dental implant failure. Implant Dent. 2003;12(1):75–80. doi: 10.1097/01.id.0000041425.36813.a9. [DOI] [PubMed] [Google Scholar]
- 20.Ravnholt G. Corrosion current and pH rise around titanium coupled to dental alloys. Scand J Dent Res. 1988;96(5):466–472. doi: 10.1111/j.1600-0722.1988.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 21.Reclaru L, Meyer JM. Study of corrosion between a titanium implant and dental alloys. J Dent. 1994;22(3):159–168. doi: 10.1016/0300-5712(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer PA. Corrosion engineering handbook. New York: Marcel Dekker Inc; 1996. pp. 167–167. [Google Scholar]
- 23.Sun ZL, Wataha JC, Hanks CT. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J Biomed Mater Res. 1997;34(1):29–37. doi: 10.1002/(sici)1097-4636(199701)34:1<29::aid-jbm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Tagger Green N, Machtei EE, Horwitz J, Peled M. Fracture of dental implants: literature review and report of a case. Implant Dent. 2002;11(2):137–143. doi: 10.1097/00008505-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Tsuge K, Kataoka M, Seto Y. Cyanide and thiocyanate levels in blood and saliva of healthy adult volunteers. J Health Sci. 2000;46:343–350. [Google Scholar]
- 26.Wataha JC. Alloys for prosthodontic restorations. J Prosthet Dent. 2002;87(4):351–363. doi: 10.1067/mpr.2002.123817. [DOI] [PubMed] [Google Scholar]
- 27.Yagan A, Ozcicek Pekmez N, Yýldýz A. Electropolymerization of poly(N-ethyl aniline) on mild steel: synthesis, characterization and corrosion protection. Electrochim Acta. 2006;51:2949–2955. [Google Scholar]
- 28.Yang H, Whitten JL. Chemisorption of OCN on Ni(100): an ab initio study. Surf Sci. 1998;401:312–321. [Google Scholar]
- 29.Yildirim M, Edelhoff D, Hanisch O, Spiekermann H. Ceramic abutments: a new era in achieving optimal esthetics in implant dentistry. Int J Periodontics Restorative Dent. 2000;20(1):81–91. [PubMed] [Google Scholar]