Abstract
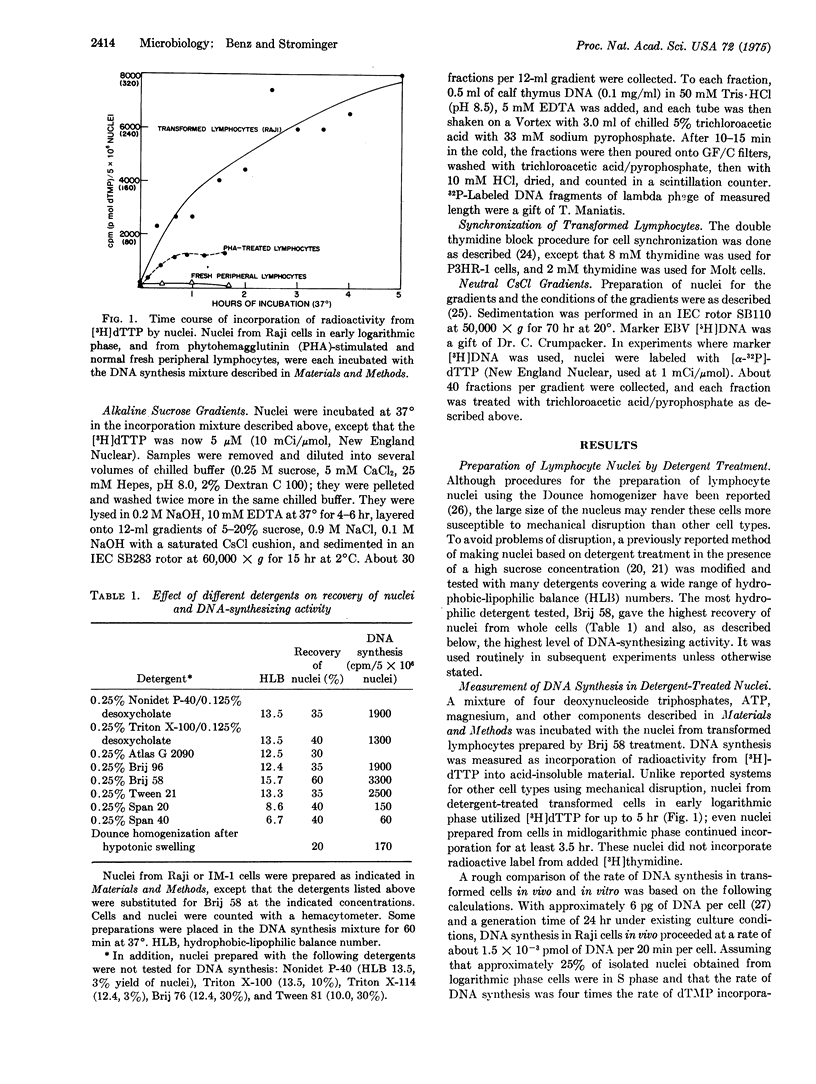
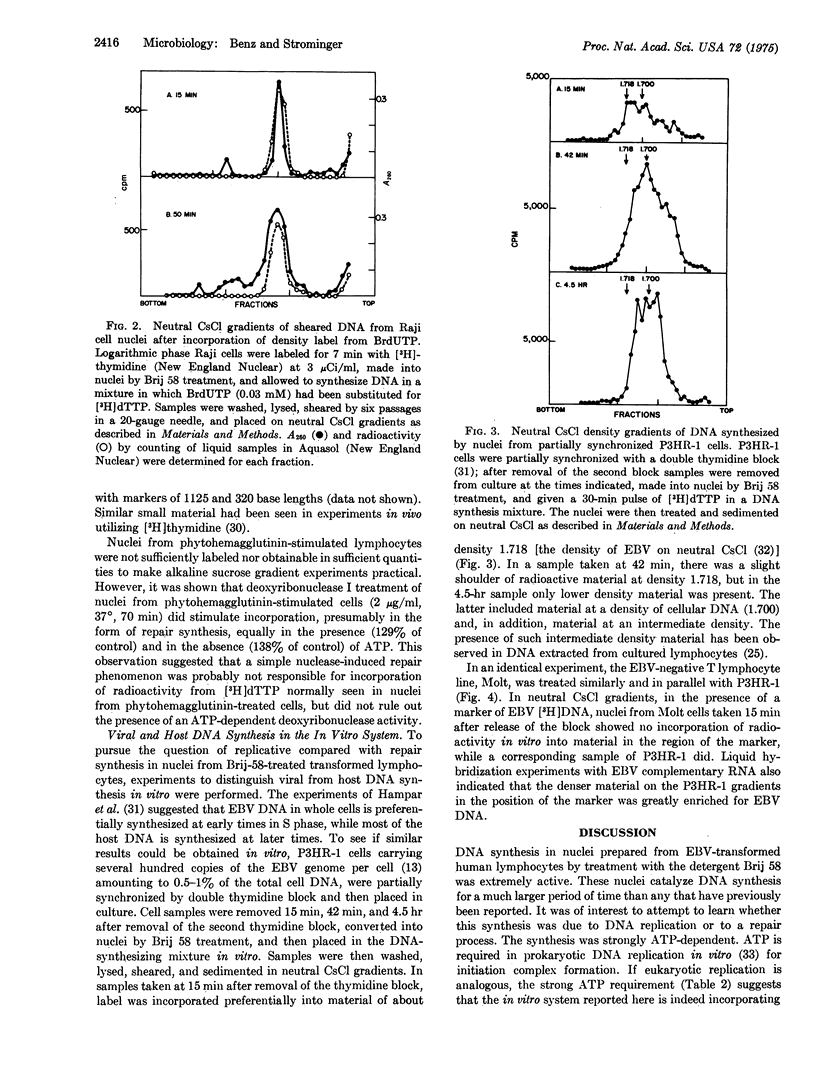
A DNA-synthesizing system in vitro, using nuclei prepared by treatment of human lymphocytes with the detergent Brij 58, was developed. Nuclei from cultured lymphocytes synthesized DNA for as long as 5 hr, and required ATP, deoxynucleoside triphosphates, magnesium, and a calcium chelator. In nuclei from a partially synchronized line of cultured lymphocytes carrying several hundred copies of the Epstein-Barr viral genome, synthesis in vitro was predominately viral in early S phase and cellular in late S phase. These and other data suggested that the DNA synthesis observed in vitro was predominately replicative.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Heterogeneity of patch size in repair replicated DNA in Escherichia coli. J Mol Biol. 1972 Jun 14;67(1):1–10. doi: 10.1016/0022-2836(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Derge J. G., Martos L. M., Tagamets M. A., Chang S. Y., Chakrabarty M. Identification of a critical period during the S phase for activation of the Epstein-Barr virus by 5-iododeoxyuridine. Nat New Biol. 1973 Aug 15;244(137):214–217. doi: 10.1038/newbio244214a0. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Fox R. M., Mendelsohn J., Barbosa E., Goulian M. RNA in nascent DNA from cultured human lymphocytes. Nat New Biol. 1973 Oct 24;245(147):234–237. doi: 10.1038/newbio245234a0. [DOI] [PubMed] [Google Scholar]
- Fridlander B. R., Medrano E., Mordoh J. Synthesis of DNA in human lymphocytes: possible control mechanism. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1128–1132. doi: 10.1073/pnas.71.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Tanaka A., Nonoyama M., Derge J. G. Replication of the resident repressed Epstein-Barr virus genome during the early S phase (S-1 period) of nonproducer Raji cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):631–633. doi: 10.1073/pnas.71.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hershey H. V., Stieber J. F., Mueller G. C. Dna synthesis in isolated HeLa nuclei. A system for continuation of replication in vivo. Eur J Biochem. 1973 Apr;34(2):383–394. doi: 10.1111/j.1432-1033.1973.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Francke B. In vitro polyoma DNA synthesis: characterization of a system from infected 3T3 cells. J Virol. 1974 Jan;13(1):125–139. doi: 10.1128/jvi.13.1.125-139.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus L. H. A novel system for DNA synthesis in isolated nuclei. FEBS Lett. 1973 Sep 1;35(1):166–168. doi: 10.1016/0014-5793(73)80602-7. [DOI] [PubMed] [Google Scholar]
- Lynch W. E., Umeda T., Uyeda M., Lieberman I. Nature of the doexyribonucleic acid made by isolated liver nuclei. Biochim Biophys Acta. 1972 Nov 16;287(1):28–37. doi: 10.1016/0005-2787(72)90327-9. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo F., Brega A., Falaschi A. DNA replication in mammalian cells. I. The size of newly synthesized helices. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1017–1024. doi: 10.1073/pnas.65.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. State of newly synthesized HeLa DNA. Nature. 1969 Mar 29;221(5187):1215–1217. doi: 10.1038/2211215a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Pegrum G. D., Thompson E. Inhibition by lymphocyte nuclei of DNA and RNA synthesis in stimulated lymphocytes and leukaemic cells. Br J Exp Pathol. 1971 Oct;52(5):560–564. [PMC free article] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Takakusu A., Lazarus H., Levine M., McCoy T. A., Foley G. E. Studies on the nuclei of cultured human leukemic lymphoblasts (CCRF-CEM cells): method of isolation. Exp Cell Res. 1968 Jan;49(1):226–229. doi: 10.1016/0014-4827(68)90540-5. [DOI] [PubMed] [Google Scholar]
- Wickner W., Kornberg A. DNA polymerase 3 star requires ATP to start synthesis on a primed DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3679–3683. doi: 10.1073/pnas.70.12.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Epstein-Barr virus in human tumor cells. Int Rev Exp Pathol. 1972;11:233–258. [PubMed] [Google Scholar]


