Abstract
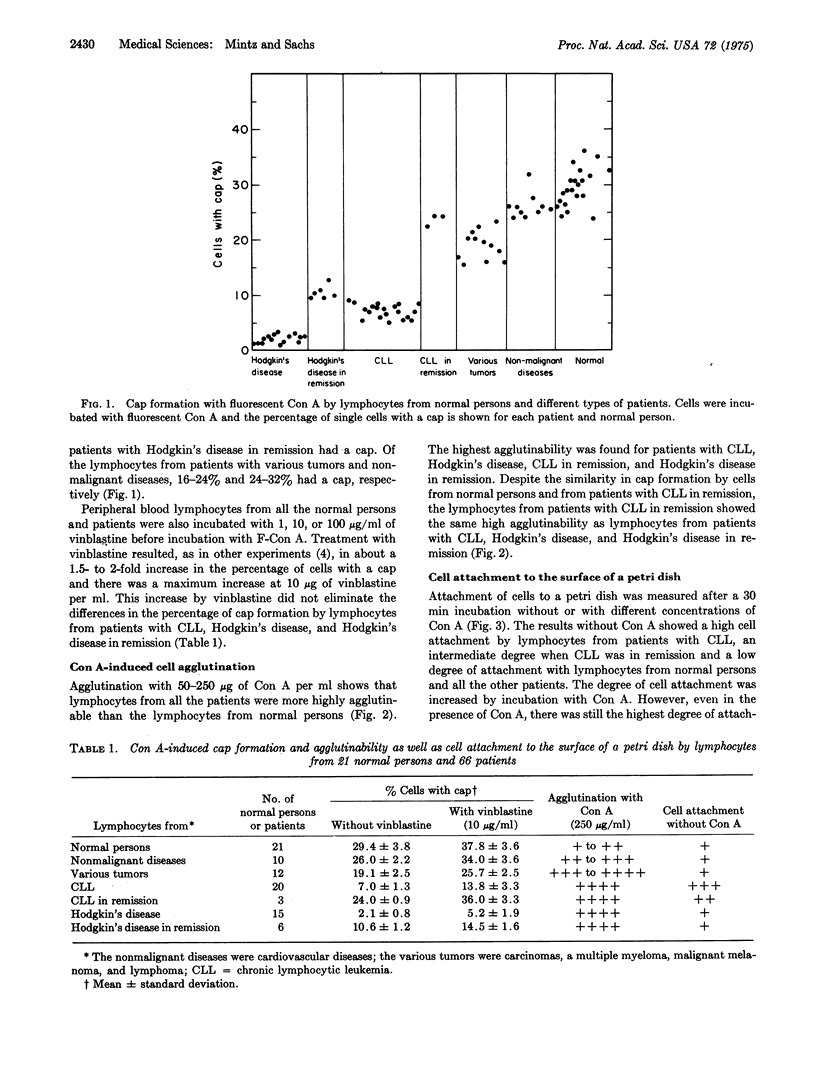
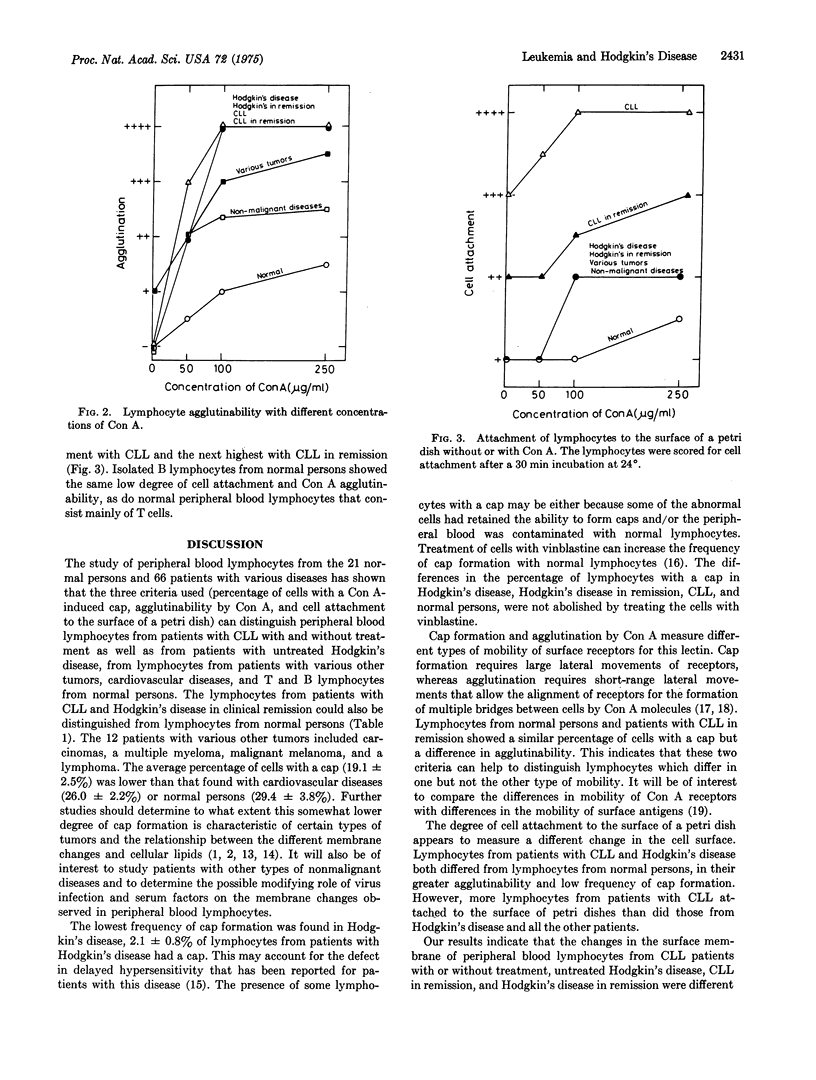
Lymphocytes were isolated from the peripheral blood of 21 normal persons and 66 patients with chronic lymphocytic leukemia (CLL), CLL in remission, Hodgkin's disease, Hodgkin's disease in remission, various other tumors, or cardiovascular diseases; The lymphocytes were studied for cap formation and agglutinability by concanavalin A, and for cell attachment to the surface of a petri dish. The frequency of cap formation was lowest in lymphocytes from patients with untreated Hodgkin's disease (2.1 plus or minus 0.8%), next lowest in lymphocytes from patients with CLL who were or were not under treatment (7,0 plus or minus 1;3%), and also low in Hodgkin's disease in remission (10.6 plus or minus 1.2%). The frequencies of cap formation by lymphocytes from patients with various other tumors (19.1 plus or minus 2.5%), with CLL in remission (24.0 plus or minus 0.9%), and with nonmalignant diseases (26.0 plus or minus 2.2%) were more similar to the frequency found in lymphocytes from normal persons (29.4 plus or minus 2.8%). Lymphocytes from all the patients, including those in remission, showed a higher degree of agglutinability by concanavalin A than lymphocytes from normal persons. Cell attachment to a petri dish was highest with CLL, next highest with CLL in remission, and low for normal persons and all the other patients. Lymphocytes from normal persons that consisted predominantly of thymus-derived cells gave similar results to isolated normal bone marrow-derived cells. The results indicate that there were different changes in the surface membrane of lymphocytes from patients with CLL, CLL in remission, Hodgkin's disease, and Hodgkin's disease in remission, and that the patients in clinical remission still showed abnormalities in their lymphocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Bassat H., Goldblum N., Manny N., Sachs L. Mobility of Concanavalin A receptors on the surface membrane of lymphocytes from normal persons and patients with chronic lymphocytic leukemia. Int J Cancer. 1974 Sep 15;14(3):367–371. doi: 10.1002/ijc.2910140310. [DOI] [PubMed] [Google Scholar]
- Cohnen G., Augener W., Buka A., Brittinger G. Rosette-forming lymphocytes in normals and patients with malignant lymphomas. Acta Haematol. 1974;51(2):65–75. doi: 10.1159/000208278. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltringham J. R., Kaplan H. S. Impaired delayed-hypersensitivity responses in 154 patients with untreated Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:107–115. [PubMed] [Google Scholar]
- Fibach E., Sachs L. Control of normal differentiation of myeloid leukemic cells. IV. Induction of differentiation by serum from endotoxin treated mice. J Cell Physiol. 1974 Apr;83(2):177–185. doi: 10.1002/jcp.1040830203. [DOI] [PubMed] [Google Scholar]
- Fröland S. S. Binding of sheep erythrocytes to human lymphocytes. A probable marker of T lymphocytes. Scand J Immunol. 1972;1(3):269–280. doi: 10.1111/j.1365-3083.1972.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Gottfried E. L. Lipids of human leukocytes: relation to celltype. J Lipid Res. 1967 Jul;8(4):321–327. [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Mintz U., Sachs L. Changes in the surface membrane of lymphocytes from patients with chronic lymphocytic leukemia and Hodgkin's disease. Int J Cancer. 1975 Feb 15;15(2):253–259. doi: 10.1002/ijc.2910150211. [DOI] [PubMed] [Google Scholar]
- Polliack A., Lampen N., Clarkson B. D., De Harven E., Bentwich Z., Siegal F. P., Kunkel H. G. Identification of human B and T lymphocytes by scanning electron microscopy. J Exp Med. 1973 Sep 1;138(3):607–624. doi: 10.1084/jem.138.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Sachs L. Receptor mobility and the mechanism of cell-cell binding induced by concanavalin A. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2456–2460. doi: 10.1073/pnas.71.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Regulation of membrane changes, differentiation, and malignancy in carcinogenesis. Harvey Lect. 1974;68:1–35. [PubMed] [Google Scholar]
- Seligmann M., Preud'Homme J. L., Brouet J. C. B and T cell markers in human proliferative blood diseases and primary immunodeficiencies, with special reference to membrane bound immunoglobulins. Transplant Rev. 1973;16:85–113. doi: 10.1111/j.1600-065x.1973.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Sachs L. Difference in the cellular cholesterol to phospholipid ratio in normal lymphocytes and lymphocytic leukaemic cells. Nature. 1974 Jul 5;250(461):67–68. doi: 10.1038/250067a0. [DOI] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yefenof E., Klein G. Antibody-induced redistribution of normal and tumor associated surface antigens. Exp Cell Res. 1974 Oct;88(2):217–224. doi: 10.1016/0014-4827(74)90234-1. [DOI] [PubMed] [Google Scholar]


