Abstract
Refractoriness to growth factor therapy is commonly associated with inferior outcome in patients with low-risk myelodysplastic syndrome (LR-MDS) who require treatment for cytopenias. However, the mechanisms leading to refractoriness are unknown. Here we describe a clinically depressed 74-year-old male with refractory cytopenia with multilineage dysplasia (RCMD) and documented growth factor refractory anemia after erythropoeisis stimulating agent (ESA) therapy, who attained transfusion and growth factor independence after the addition of sertraline to his medication regimen. Our case demonstrates hematological improvement-erythroid (HI-E) in growth factor refractory, low risk MDS and highlights a potential mechanistic link between common inflammatory diseases and LR-MDS.
Keywords: Myelodysplasia, Transfusion dependence, Erythropoietin, Sertraline
Highlights
-
•
Our case shows HI-E with sertraline in growth factor refractory, low risk MDS.
-
•
We highlight a potential mechanism between common inflammatory diseases and LR-MDS.
-
•
This is the first report of potential therapeutic role for SSRIs in myelodysplasia.
1. Introduction
Myelodysplastic Syndrome (MDS) is a heterogeneous clonal disorder characterized by ineffective hematopoeisis and propensity for transformation into Acute Myelogenous Leukemia (AML). The Revised International Prognostic Scoring System (R-IPSS) is an accepted model used to predict overall survival and the risk of AML progression. Those with a lower risk R-IPSS score are treated supportively with ESAs, granulocyte colony stimulating factor (G-CSF), and transfusions as necessary. However, even when given in combination, response for growth factors remain approximately 35% [1]. Though high levels of endogenous erythropoietin (>500 µ/ml), an increased proportion of blast, and a high transfusion requirement are associated with a poor ESA response, the subsequent treatment of cytopenias refractory to growth factor therapy remains largely unexplored, thus highlighting the need for novel therapies to restore transfusion independence [2].
Patients with MDS have multiple somatically acquired genetic abnormalities leading to gene expression defect including deregulation of cytokine signaling pathways [3–6]. In rheumatoid arthritis (RA), a similar repertoire of deranged cytokines is responsible for chronic inflammation [7,8]. Patients with RA often also suffer from major depressive disorder, and interestingly, Selective Serotonin Reuptake Inhibitors (SSRIs) prescribed for these patients׳ depression have been proposed to modulate their co-morbid rheumatoid arthritis due to the pleiotropic effects on cytokine pathways. Indeed, sertraline was recently shown to result in a significant reduction in clinical arthritis in a mouse model accompanied by a measured decrease in serum TNF-α level [8].
We hypothesize that the use of SSRIs in patients with MDS has the potential to modulate manifestations of this disease through similar effects on cytokine derangements. Here, we report a case of sequential reversal of ESA refractoriness, transfusion and growth factor dependence in a patient with LR-MDS who received sertraline for treatment of underlying depression.
2. Case report
A 74-year male was seen in our clinic with an 18-month history of transfusion dependent refractory anemia (8 units RBC in 8 weeks). His bone marrow biopsy showed no blasts or ring sideroblast. Erythroid and megakaryocytic dysplasia with a myeloid: erythroid (M:E) ratio of 10:1 consistent with RCMD by WHO 2008 classification was detected (Fig. 1A). Standard G-band karyotyping revealed 45, X,-Y in 4 of 20 metaphases analyzed. His resultant R-IPSS score was 1.5 [Cytogenetic=0; blast %=0; Hb [7 g/dL]=1.5; Platelets [135,000/µL]=0; ANC[1500/µL]=0]. Initial erythropoietin (EPO) and ferritin levels were 449 IU/L and 1600 NG/ML, respectively. With these baseline characteristics, his predicted likelihood of response to EPO therapy was 23% based on a currently validated model for patients with LR-MDS [9].
Fig. 1.
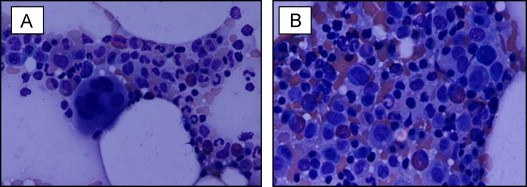
Sources: Hematoxylin and eosin stain: original magnification ×40. Image A shows bone marrow at diagnosis with erythroid and myeloid dysplasia. Predominant erythroid precursors are seen (myeloid: erythroid [ME]=10:1). Image B shows bone marrow 12 weeks after ESA discontinuation (Week 120) with ME ratio normalization (2:1).
The patient initially received 40,000 IU of subcutaneous (SQ) R-H-EPO per week. In view of his co-morbid major depressive disorder, he was initiated on sertraline at 100 mg orally daily on week (W) 8 of treatment. Clinical response to ESA therapy was assessed according to internal working group (IWG) 2000 criteria. After 3 months of weekly SQ R-H-EPO W1-W12 (Fig. 2), 480 mcg of SQ G-CSF 5 times a week was added due to ESA refractoriness (ESA+G-CSF schedule) (W12–W26). Transfusion independence was observed by W23 of combined R-H-EPO+G-CSF with concurrent sertraline (Fig. 2). Progressive hemoglobin stabilization then resulted in G-CSF dose reduction to twice a week from W28 to W37 (Fig. 2). Sequential resolution of ESA dependence was observed from W37 to W90. Over the entire treatment period, the absolute increase in Hb level was 4.2 g/dL from baseline. Restoration of ESA erythroid response to exogenous EPO was observed by W26–W37. Improved Hb levels were maintained after G-CSF discontinuation (W37) and ESA dose de-escalation/cessation while on sertraline treatment. To date, the patient has remained on single agent sertraline for 11 months with median hemoglobin values of 10.4 g/dL (Fig. 2). His most recent EPO level, measured more than 32 months from the start of therapy and nearly a year after ceasing EPO injections was 70.3 IU/L. His bone marrow evaluation 12 weeks after ESA discontinuation (W120) showed trilineage hematopoeisis, mild erythroid dysplasia and M:E normalization with a ratio of 2:1 (Fig. 1B).
Fig. 2.
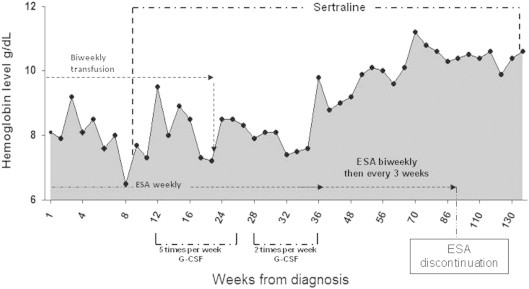
Hemoglobin levels during sequential treatment with erythropoeitin stimulating agent, granulocyte stimulating factor and sertraline.
3. Discussion
Our case demonstrates feasibility for improvement in erythropoiesis in a patient with poor predictor of ESA/G-CSF response/growth factor refractory, low-risk MDS who was treated with sertraline for co-morbid depression. Within 100 days concurrent administration of sertraline, ESA and G-CSF, the patient experienced resolution of transfusion dependence. In addition, sensitivity to endogenous EPO was demonstrated with rise in hemoglobin by more than 4 points and fall in endogenous EPO levels from 449 IU/L to 70.3 IU/L. To the best of our knowledge, this is the first description of a potential therapeutic role for SSRIs in patients with MDS.
MDS is clinically heterogeneous with a variable risk AML progression, an observation that can be partially explained by the underlying genetic heterogeneity among patients [10]. Gene expression profiling of patients with early MDS has identified multiple derangements in cell signaling pathways, including immunoregulation, apoptosis, and chemokine secretion [5]. Abnormal levels of pro-inflammatory cytokines such as IL-12, IL-7, IFN-γ, and RANTES have been observed in marrow samples from these patients. These changes were associated with an increased number of inflammatory CD3+ CD4+ Th17 cells, leading to amplification in marrow apoptotic signaling and resulting in ineffective hematopoiesis and eventual marrow failure [4].
Similar imbalances in inflammatory cytokines underlie the pathophysiology of major depression. Recent work found higher levels of pro-inflammatory cytokines such as IL-2, IL-12, and TNF-α and lower levels of anti-inflammatory cytokines such as IL-4 in patients with major depressive disorder as compared to normal controls. An 8-week course of sertraline treatment resulted in an objective improvement in depression as well as normalization of many of the cytokine derangements [7]. Serotonin is known to have diverse immunoregulatory actions including activation of helper T cells, enhancement of NK cell activity, and induction of B-cell proliferation; thus the anti-inflammatory effect of SSRI treatment are likely directly related to their modulation of inflammatory process. These effects of SSRI treatment have been explored in relation to other related disease processes, namely rheumatoid arthritis, a state of chronic inflammation. In a recent study, the use of sertraline showed a significant reduction in clinical arthritis in a mouse model accompanied by a measured decrease in TNF-α serum levels [8].
Our case leads us to hypothesize that the anti-inflammatory effect of sertraline could similarly be used to modulate the immune dysfunction proposed to underlie MDS, thus resolving cytokine derangements, normalizing the pro-inflammatory T-cell environment in the bone marrow, and improving ineffective hematopoiesis. However, there are several limitations of this case report. First, G-CSF was added after sertraline prior to the development of transfusion independence in our patient. Though sertraline may have a synergistic effect in combination with growth factors, this may affect our ability to assign an independent role to SSRIs in the eventual achievement of hemoglobin stabilization. Another limitation is the delayed six month time course required to see hemoglobin stabilization in our patient, though earlier administration of G-CSF in combination with sertraline and ESA may have allowed this effect to occur more quickly. In addition, given our patient high M:E ratio, atypical CML should be considered within differential diagnosis. Further studies are needed to elucidate the specific immunologic mechanisms that underpin the pathophysiology of MDS. In addition, retrospective cohort studies and eventually, prospective clinical trials are necessary to validate these therapeutic possibilities for patients with and without growth factor refractory LR-MDS.
Conflict of interest
There are no conflicts of interest to report.
References
- 1.Balleari E., Rossi E., Clavio M., Congiu A., Gobbi M., Grosso M. Erythropoietin plus granulocyte colony-stimulating factor is better than erythropoietin alone to treat anemia in low-risk myelodysplastic syndromes: results from a randomized single-centre study. Ann Hematol. 2006;85(3):174–180. doi: 10.1007/s00277-005-0044-6. (Mar) [DOI] [PubMed] [Google Scholar]
- 2.Musto P., Villani O., Martorelli M.C., Pietrantuono G., Guariglia R., Mansueto G. Response to recombinant erythropoietin alpha, without the adjunct of granulocyte-colony stimulating factor, is associated with a longer survival in patients with transfusion-dependent myelodysplastic syndromes. Leuk Res. 2010;34(8):981–985. doi: 10.1016/j.leukres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Pardanani A., Finke C., Lasho T.L., Al-Kali A., Begna K.H., Hanson C.A. IPSS-independent prognostic value of plasma CXCL10, IL-7 and IL-6 levels in myelodysplastic syndromes. Leukemia. 2012;26(4):693–699. doi: 10.1038/leu.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordasti S.Y., Afzali B., Lim Z., Ingram W., Hayden J., Barber L. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145(1):64–72. doi: 10.1111/j.1365-2141.2009.07593.x. (Apr) [DOI] [PubMed] [Google Scholar]
- 5.Theilgaard-Monch K., Boultwood J., Ferrari S., Giannopoulos K., Hernandez-Rivas J.M., Kohlmann A. Gene expression profiling in MDS and AML: potential and future avenues. Leukemia. 2011;25(6):909–920. doi: 10.1038/leu.2011.48. [DOI] [PubMed] [Google Scholar]
- 6.Pellagatti A., Cazzola M., Giagounidis A., Perry J., Malcovati L., Della Porta M.G. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia. 2010;24(4):756–764. doi: 10.1038/leu.2010.31. [DOI] [PubMed] [Google Scholar]
- 7.Sutcigil L., Oktenli C., Musabak U., Bozkurt A., Cansever A., Uzun O. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baharav E., Bar M., Taler M., Gil-Ad I., Karp L., Weinberger A. Immunomodulatory effect of sertraline in a rat model of rheumatoid arthritis. Neuroimmunomodulation. 2012;19(5):309–318. doi: 10.1159/000339109. [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom-Lindberg E., van de Loosdrecht A. EPO stimulating agents and other growth factors in low-risk MDS. Best Pract Res Clin Haematol. 2013;23(4) doi: 10.1016/j.beha.2013.09.007. (410-410) [DOI] [PubMed] [Google Scholar]
- 10.Bejar R., Stevenson K.E., Caughey B.A., Abdel-Wahab O., Steensma D.P., Galili N. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30(27):3376–3382. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]


