Abstract
Objective
This study examined the effects of adjunctive aripiprazole therapy on metabolism in clozapine-treated patients with schizophrenia.
Method
In an 8-week randomized, double-blind, placebo-controlled study, subjects received either aripiprazole (15mg/day) or placebo. At baseline and week 8, metabolic parameters were assessed by the frequently sampled intravenous glucose tolerance test, nuclear magnetic resonance spectroscopy and whole-body dual-energy X-ray absorptiometry (DXA).
Results
Thirty subjects completed the study (16 in the aripiprazole group and 14 in the placebo group). Glucose effectiveness measured by the frequently sampled intravenous glucose tolerance test improved significantly in the aripiprazole group (0.003 ± 0.006 versus −0.005 ± 0.007/min, P = 0.010). The aripiprazole group showed significant reductions in both plasma low-density lipoprotein (LDL) levels (−15.1 ± 19.8 vs. 4.4 ± 22.5 mg/dl, P = 0.019) and LDL particle numbers (−376 ± 632 vs. −36 ± 301 nM, P= 0.035). Further, there was a significant reduction in lean mass (−1125 ± 1620 vs. 607 ± 1578 g, P= 0.011) measured by whole-body DXA scan in the aripiprazole group. All values were expressed as mean ± standard deviation, aripiprazole vs. placebo.
Conclusion
Adjunctive therapy with aripiprazole may have some metabolic benefits in clozapine-treated patients with schizophrenia.
Introduction
Cardiometabolic diseases remain the leading cause for medical morbidity and mortality among people with schizophrenia (1), and the rates of these diseases are much higher than in the general population (2). Because of its superior efficacy for psychosis (3) and suicidality (4), clozapine may reduce overall mortality but its elevation of cardiovascular risk is of great concern (5). Clinically significant weight gain has been well established with clozapine, mostly during the first 6–12 months of its use (6). Similarly, clozapine has been associated with dyslipidemia (7), hypertension (8), type 2 diabetes mellitus (9) and myocardial infarction (10).
The mechanism of clozapine’s metabolic risk is believed to be associated with its strong blockade effects on H1-histamine and 5HT2C receptors (11). Kroeze et al. (12) found that histamine receptor antagonism closely correlated with antipsychotic-induced weight gain. More recently, Kim et al. (13) reported that clozapine stimulates hypothalamic adenosine monophosphate-activated protein kinase (AMPK) – an enzyme that reverses the action of leptin – an anorexigenic hormone; this effect was abolished in H1 knockout mice. Other studies found 5-HT2C antagonism to be associated with antipsychotic-induced weight gain and diabetes (14); further, 5-HT2C knockout mice developed insulin resistance and impaired glucose tolerance and experienced severe weight gain (15). In contrast to clozapine, aripiprazole has no anti-histaminergic activity and is an agonist at 5-HT2C receptors (16, 17). In addition, aripiprazole has a unique 5-HT1A partial agonist property (18). It is generally believed that agonists at 5-HT1A receptors lower blood glucose levels, while antagonists decrease blood insulin levels, thereby leading to hyperglycemia. Decreased insulin levels associated with 5-HT1A antagonism are probably due to a decrease in pancreatic β-cell responsiveness to blood glucose levels (19, 20). Treatment with aripiprazole has been associated with minimal impact on metabolic parameters, including weight, glucose metabolism, and lipids in patients with schizophrenia (21). Given its agonist effects on both 5-HT2C receptors and 5-HT1A receptors, aripiprazole might be protective against weight gain, diabetes and other metabolic disturbances in clozapine treated patients with schizophrenia.
Our group conducted a 6-week, open-label trial to examine the effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia (22). The combination treatment was associated with a significant decrease in body weight, body mass index (BMI), fasting serum total cholesterol and triglycerides. In a randomized, double-blind, placebo-controlled 8-week study, Chang et al. (23) reported that aripiprazole augmentation of clozapine significantly reduced fasting serum triglycerides. A more recent 16-week, randomized, double-blind, placebo-controlled trial reported that adding aripiprazole to clozapine resulted in significant reductions in weight, BMI, waist circumference, and fasting serum total and low-density lipoprotein (LDL) cholesterol (24). Despite these encouraging findings, important questions remain with regard to the potential metabolic benefits of adjunctive aripiprazole in clozapine-treated patients: i) It is unclear how the combination treatment might affect specific aspects of glucose metabolism; ii) The LDL abnormality found in an insulin-resistant state may not appear on a standard lipoprotein profile. Insulin resistance results in cholesterol-depleted and therefore smaller and denser LDL particles (25). It is unclear whether the combination treatment might affect atherogenic LDL particle size and iii) the impact of the combination treatment on body composition, especially fat and lean body mass distribution, remains unknown.
The frequently sampled intravenous glucose tolerance test (FSIVGTT) is a gold standard procedure to assess insulin sensitivity and the effectiveness of glucose metabolism (26). Nuclear magnetic resonance (NMR) spectroscopy has been used to measure lipoprotein subclass concentration and size (27). NMR capitalizes on the fact that each lipoprotein subclass particle of a given size emits its own characteristic signal. Conversion factors relating signal amplitudes to subclass concentrations expressed in particle concentration units are then applied. In recent years, whole-body dual-energy X-ray absorptiometry (DXA) has been increasingly used to measure body composition including bone mineral content (BMC), fat mass and lean mass (28).
Aims of the study
We now present the results of an 8-week, randomized, placebo-controlled, double-blinded study to examine aripiprazole’s effects on glucose metabolism in clozapine-treated patients with schizophrenia using the frequently sampled intravenous glucose tolerance test. In addition, the study examined how adjunctive aripiprazole therapy affects lipid particle size and body composition using nuclear magnetic resonance (NMR) spectroscopy and whole-body dual-energy X-ray absorptiometry (DXA), respectively.
Material and methods
Patients
The study was approved by the institutional review boards of the Massachusetts General Hospital (MGH) and the Massachusetts Department of Mental Health. Subjects were recruited from the Freedom Trial Clinic at the Erich Lindemann Mental Health Center and were studied at the Clinical Research Center (CRC) at MGH, Boston. The inclusion criteria were: i) age 18–65 years; ii) diagnosis of schizophrenia or schizoaffective disorder; iii) treatment with clozapine for at least 1 year; iv) stable dose of clozapine for at least 1 month; v) female subjects were eligible to participate in the study if they were of non-childbearing potential or of child-bearing potential and willing to practice appropriate birth control methods during the study. The exclusion criteria included: i) inability to provide informed consent; ii) current substance abuse; iii) significant medical illness including severe cardiovascular, hepatic, renal disease; iv) history of immunosuppression; v) current or recent radiation or chemotherapy treatment for cancer; vi) being pregnant or breastfeeding. Patients treated with the following medications known to affect glucose tolerance were also excluded: birth control pills containing norgestrel, steroids, beta-blockers, anti-inflammatory drugs (including aspirin and ibuprofen), thiazide diuretics and valproate sodium. Subjects with diabetes mellitus were not excluded from the study; however, insulin-dependent subjects, for safety reasons, did not have a FSIVGTT performed.
After providing written informed consent, subjects underwent a diagnostic evaluation by a research psychiatrist using the Structured Clinical Interview for DSM-IV (SCID) (29). Female patients were given instructions to practice appropriate birth control methods during the study period. Additionally, as insulin sensitivity has been found to decrease during the luteal phase (30), menstruating women underwent the FSIVGTT during the early follicular phase of their menstrual cycle (days 1–7) as identified by interview and menstrual log.
Baseline procedures
Eligible subjects completed a baseline assessment which included the Positive and Negative Syndrome Scale (PANSS) (31) and the Systematic Assessment for Treatment Emergent Events (SAFTEE) (32). In addition, extrapyramidal symptoms were evaluated at each visit using the Simpson-Angus Scale (33) and the Abnormal Involuntary Movement Scale (AIMS) (34).
Because dietary carbohydrate affects insulin sensitivity (35), subjects were given a diet plan calculated to maintain body weight and to provide a minimum of 250mg of carbohydrate for each of the 3 days prior to the FSIVGTT. Subjects were also instructed to fast for 12 hours preceding the FSIVGTT and to hold their morning medications the day of the test. Family, residential program staff and outreach workers assisted subjects to maintain a high-carbohydrate intake and to guarantee fasting. Subjects were admitted to the MGH CRC at 6:45 AM on the morning of the test. The nutrition assessment was conducted prior to the initiation of the FSIVGTT. Following the FSIVGTT, subjects were randomized, stratified by the presence or absence of diabetes, in a double-blind fashion to receive either 15mg aripiprazole per day or matching placebo.
Nutritional assessment
Height was measured using a Harpenden stadiometer, which was calibrated on a weekly basis. Subjects were weighed on a digital electronic scale, and weight was recorded to the nearest 0.1kg. Waist circumference was measured at the umbilicus waist. Body composition was determined using the DXA (Hologic QDR-4500; Hologic Inc, Waltham, MA, USA). DXA has been validated for body composition measurement (28). Body composition data were presented as BMC (in grams), fat mass (in grams), lean mass (in grams), and total body mass (BMC + fat mass + lean mass, in grams); in addition, fat percentage was calculated as fat mass (in grams) divided by total mass (in grams). Data were calculated separately for the trunk and total values.
FSIVGTT and Minimal Model Calculation
Two intravenous catheters were placed in antecubital veins (one in each arm). Baseline blood samples were drawn for fasting plasma glucose and serum insulin levels, HbA1c and lipids 10 min prior to the glucose infusion (time, 10 min). Glucose 0.3 g/kg in normal saline was administered intravenously for 30 s at time 0. Approximately 2-ml blood samples were withdrawn at −10, −5, 0, 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, 21, 22, 23, 24, 25, 27, 30, 35, 40, 45, 50, 55, 60, 65, 70, 80, 90, 100, 110, 120, 140, 160, and 180 min for measurement of plasma glucose and serum insulin concentrations. Twenty minutes after the glucose infusion, 500 mg of tolbutamide (Upjohn Co, Kalamazoo, MI, USA) was administered intravenously for 45 s. Vital signs and plasma glucose concentrations were monitored throughout the procedure. By using the MINMOD Millennium computer program developed by Richard Bergman, PhD (36), insulin sensitivity index (SI), glucose effectiveness (SG), acute insulin response to glucose (AIRG), and disposition index (DI) were calculated based on plasma glucose and serum insulin values obtained from the FSIVGTT procedure. The SI represents the increase in net fractional glucose clearance rate per unit change in serum insulin concentration after the intravenous glucose load. The SG represents the net fractional glucose clearance rate attributable to the increase in glucose independent of any increase in circulating insulin concentrations above baseline. The AIRG measures the acute (0–10 min) beta-cell response to a glucose load calculated by the areas under the curve higher than basal insulin values. The DI, which equals SI x AIRG), is an index of beta-cell function that takes account of prevailing insulin sensitivity and exploits the hyperbolic relationship between SI and AIRG (37). The HOMA-IR was calculated by the following formula: fasting serum insulin concentration x fasting plasma glucose concentration/22.5 (38).
Laboratory Assays
Laboratory assays were performed by the Chemistry Lab and the CRC Core Lab of the Massachusetts General Hospital. Laboratory assays were performed by the chemistry laboratory and the Mallinckrodt General Clinical Research Center Core Laboratory of Massachusetts General Hospital. Insulin immunometric assays were performed using an Immulite Analyzer (Diagnostic Product Corp; Los Angeles, CA, USA) with an intra-assay coefficient of variation of 4.2%–7.6%. Fasting plasma glucose level was measured with a hexokinase reagent kit (A-gent glucose test; Abbott, South Pasadena, CA, USA). Glucose assays were run in duplicate, and the intra-assay coefficient of variation ranged from 2% to 3%. Fasting total plasma cholesterol and triglyceride levels were measured enzymatically with an intra-assay coefficient of variation of 1.7%–2.7% and 0.9%–1.2%, respectively. The high-density lipoprotein (HDL) cholesterol fraction was measured after precipitation of low-density and very low–density lipoproteins (VLDLs) with dextran sulfate magnesium with an intra-assay coefficient of variation of 0.89%–1.82%. LDL cholesterol values were determined by the Direct LDL reagents (Roche Diagnostics, Indianapolis, IN, USA). Hemoglobin A1c (HbA1c) was measured with high performance liquid chromatography using an automated analyzer (normal range 4.5–6.5%) (SmithKline, Van Nuys, CA, USA). Lipoprotein subclass concentrations and the LDL particle size were determined using the NMR spectroscopy (LipoScience, Raleigh, NC, USA) (27). In the present study, the following lipoprotein subclasses were assessed: LDL particle, small LDL particle, large HDL particle, and large VLDL particle. Serum levels of C-reactive protein (CRP) were measured via a high-sensitivity latex-enhanced immunonephelometric assay on a BN II analyzer (Dade Behring, Newark, DE, USA).
Follow up assessment
Subjects met with a research assistant at weeks 2, 4 and 6. Visits varied from 1 to 2 h in length and consisted of the assessment of vital signs and side effects. Study medication was dispensed every 2 weeks. Subjects were asked to return their bottle of medication during each follow-up visit; extra pills in the bottle were counted to assess adherence. At week 8, all baseline assessments were repeated, including the rating scales, nutritional measures, the FSIVGTT, and laboratory assays.
Statistical analysis
Statistical analysis was performed using SAS (version 9.2, SAS Institute, Cary, NC, USA). Descriptive statistics were performed to describe demographic and clinical characteristics of the study sample. Group comparisons were performed using the independent t test for continuous variables, and the Fisher’s exact test or chi-square test for categorical variables. Analysis of covariance (ANCOVA) was used to compare change scores from baseline to week 8 between the two groups controlling for baseline scores. For all analyses, a P value <0.05 (two-tailed) was used for statistical significance. For those continuous variables with findings at a trend level, Cohen’s d effect sizes were calculated.
Results
Forty-eight subjects were screened. Among them, 44 were enrolled and 38 were randomized to aripiprazole or placebo treatment. A total of 30 subjects completed the study (16 in the aripiprazole group and 14 in the placebo group) (Fig. 1). For the entire sample, 22 were men and eight women; 24 were Caucasians, 4 African Americans, and 2 in ‘other’ category; 9 were diabetic and 21 non-diabetic. The two groups differed in race at a trend level (P = 0.084); all four African Americans were in the aripiprazole group. There were no significant differences between the two groups in age, gender, education level, diagnosis (schizophrenia or schizoaffective disorder), tobacco use, diabetes status, and the daily clozapine dosage (P values > 0.1) (Table 1).
Figure 1.
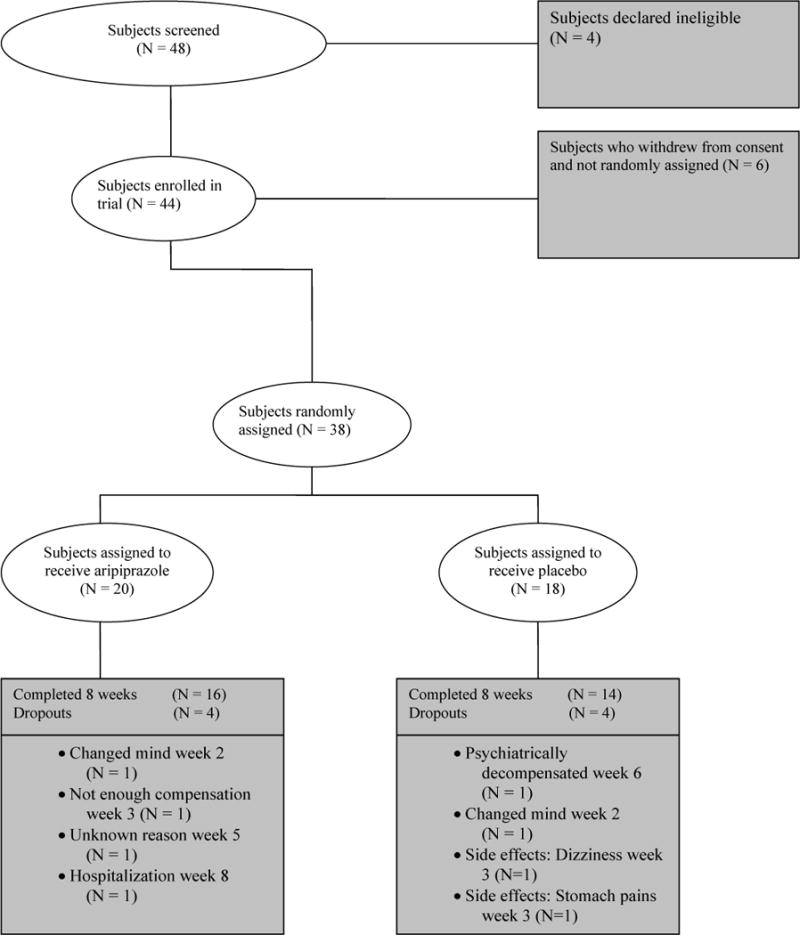
Patients who entered and completed the study
Table 1.
Baseline demographic and clinical characteristics of the study sample
| Variable | Aripiprazole (N=16) | Placebo (N=14) | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 44.3 | 8.2 | 44.2 | 8.9 | 0.991 |
| Clozapine dosage (mg/day) | 397 | 144 | 400 | 139 | 0.952 |
| N | % | N | % | ||
| Gender | 0.417 | ||||
| Male | 13 | 81 | 9 | 64 | |
| Female | 3 | 19 | 5 | 36 | |
| Race | 0.084 | ||||
| Caucasian | 11 | 69 | 13 | 93 | |
| African American | 4 | 25 | 0 | 0 | |
| Other | 1 | 6 | 1 | 7 | |
| Education | 0.533 | ||||
| Middle school | 1 | 6 | 0 | 0 | |
| Some high school | 6 | 38 | 5 | 39 | |
| High school diploma/GED | 0 | 0 | 2 | 15 | |
| Some college | 6 | 37 | 3 | 23 | |
| College graduate | 3 | 19 | 3 | 23 | |
| Diagnosis | 0.122 | ||||
| Schizophrenia | 13 | 81 | 7 | 50 | |
| Schizoaffective disorder | 3 | 19 | 7 | 50 | |
| Tobacco use | 1.000 | ||||
| Yes | 9 | 56 | 8 | 57 | |
| No | 7 | 44 | 6 | 43 | |
| Diabetes | 0.694 | ||||
| Yes | 4 | 25 | 5 | 36 | |
| No | 12 | 75 | 9 | 64 | |
Glucose metabolism
The two groups differed significantly in week 8 change for SG [F(1,21) = 7.9, P = 0.010], demonstrating that the aripiprazole group had significant improvement in the effectiveness of glucose clearance compared to with the placebo group. There were between group differences at a trend level in week 8 changes for HOMA-IR [F(1,24) = 3.8, P = 0.065, effect size 0.75] and SI [F(1,21) = 3.3, P = 0.082, effect size 0.70], suggesting that the aripiprazole group might have some improvement in insulin sensitivity compared with the placebo group. There were no significant differences between the two groups in week 8 changes for plasma glucose, serum insulin, HbA1c, AIRG, or DI (P values > 0.1) (Table 2).
Table 2.
Changes in glucose metabolism, lipids and other metabolic parameters over 8-week study period
| Aripiprazole (N=16) | Placebo (N=14) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Baseline | Week 8 change | Baseline | Week 8 change | ||||||
|
|
|||||||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Fasting plasma glucose (mg/dL) | 112.6 | 26.5 | 0.9 | 15.6 | 97.1 | 18.4 | 7.5 | 26.4 | 0.206 |
| Fasting serum insulin (μIU/mL) | 12.7 | 7.7 | −1.5 | 8.6 | 11.1 | 7.7 | 3.5 | 10.8 | 0.195 |
| HOMA-IR | 3.8 | 3.4 | −0.7 | 2.8 | 2.9 | 2.5 | 2.6 | 5.6 | 0.065 |
| HbA1c (%) | 6.70 | 1.74 | −0.32 | 0.63 | 6.08 | 0.51 | 0.01 | 0.34 | 0.381 |
| SI (× 10 – 4 min−1) | 4.2 | 3.9 | 0.6 | 3.8 | 4.2 | 3.9 | 0.65 | 1.9 | 0.082 |
| SG (min−1) | 0.012 | 0.006 | 0.003 | 0.006 | 0.013 | 0.007 | −0.005 | 0.007 | 0.010 |
| AIRG (AUC, 0–10) (μU/mL per 10 min) | 240.7 | 251.2 | −64.1 | 131.8 | 292.0 | 362.0 | −78.1 | 134 | 0.737 |
| DI | 833 | 1,246 | −296 | 1,243 | 756 | 578 | −222 | 651 | 0.579 |
| Total cholesterol (mg/dL) | 181.0 | 46.4 | −15.3 | 33.3 | 179.1 | 23.7 | 5.6 | 34.0 | 0.125 |
| Direct LDL (mg/dL) | 101.0 | 32.5 | −15.1 | 19.8 | 101.6 | 29.2 | 4.4 | 22.5 | 0.019 |
| HDL (mg/dL) | 39.4 | 9.2 | −0.4 | 5.6 | 39.3 | 8.9 | −2.7 | 6.2 | 0.321 |
| Triglycerides (mg/dL) | 207.9 | 127.6 | −5.9 | 75.1 | 222.1 | 100.6 | −7.3 | 100.3 | 0.982 |
| LDL particle number (nmol/L) | 1,610 | 763 | −376 | 632 | 1,628 | 252 | −36 | 301 | 0.035 |
| LDL particle size (nm) | 21.02 | 0.98 | −0.19 | 0.80 | 20.33 | 0.52 | 0.41 | 0.26 | 0.271 |
| Small LDL particle number (nmol/L) | 1,065 | 772 | −246 | 675 | 1,213 | 266 | −148 | 316 | 0.241 |
| Large HDL particle number (nmol/L) | 6.12 | 3.93 | 0.66 | 2.23 | 5.13 | 3.16 | −0.36 | 1.77 | 0.281 |
| Large VLDL particle number (nmol/L) | 5.10 | 4.34 | −1.62 | 4.84 | 8.66 | 8.39 | −2.58 | 8.14 | 0.534 |
Week 8 change equals week 8 value minus baseline value.
P values were based on ANCOVA comparing between group differences in week 8 changes controlling for baseline values
HOMA-IR, homeostasis model of assessment of insulin resistance; HbA1c, hemoglobin A1c; SI, insulin sensitivity index; SG, glucose effectiveness; AIRG, acute insulin response to glucose; DI, disposition index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein
Lipids and other metabolic parameters
There were significant differences between the two groups in week 8 changes for both plasma LDL levels [F(1,23) = 6.4, P = 0.019] and LDL particle numbers [F(1,17) = 5.2, P = 0.035], suggesting that the aripiprazole group had significant reduction in plasma LDL levels compared with the placebo group. There were no significant differences between the two groups in week 8 changes for plasma total cholesterol, HDL, triglycerides, LDL particle size, small LDL particle number concentration, large HDL particle number concentration and large VLDL particle number concentration (P values > 0.1) (Table 2).
Anthropometric measures
There were no significant differences between the two groups in week 8 changes for body weight, BMI, or waist circumference (P values > 0.1). The DXA assessment demonstrated significant differences between the two groups in week 8 changes for lean mass [trunk, F(1,24)=7.9, P=0.010; total body, F(1,24)=7.6, P=0.011) and total mass [trunk, F(1,24)=4.9, P=0.037; total body, F(1,24)=6.7, P=0.016], suggesting that aripiprazole treatment significantly reduced lean mass and total mass in clozapine-treated patients with schizophrenia. However, there were no significant differences between the two groups in week 8 changes for BMC, fat mass, or fat percentage in either trunk or total body (P values > 0.1) (Table 3).
Table 3.
Changes in anthropometric assessment over 8-week study period
| Aripiprazole (N=16) | Placebo (N=14) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Baseline | Week 8 change | Baseline | Week 8 change | ||||||
|
|
|||||||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body weight (kg) | 90.9 | 19.7 | −1.5 | 2.3 | 103.9 | 23.8 | 0.3 | 2.3 | 0.109 |
| BMI (kg/m2) | 30.66 | 5.99 | −0.52 | 0.79 | 35.41 | 7.29 | 0.03 | 0.85 | 0.229 |
| Waist circumference (umbilicus, cm) | 108.8 | 15.2 | −0.4 | 2.3 | 120 | 16.0 | −0.8 | 4.2 | 0.754 |
| DXA BMC (g) | |||||||||
| Trunk | 623 | 135 | 8 | 17 | 697 | 199 | −16 | 48 | 0.317 |
| Total body | 2,473 | 482 | 28 | 46 | 2,566 | 493 | −4 | 61 | 0.145 |
| DXA fat mass (g) | |||||||||
| Trunk | 14,740 | 6,162 | −326 | 699 | 19,828 | 6,698 | 19 | 822 | 0.514 |
| Total body | 29,054 | 10,489 | −633 | 1,055 | 37,691 | 12,211 | 2 | 1,137 | 0.321 |
| DXA fat percentage (%) | |||||||||
| Trunk | 31.5 | 8.0 | −0.1 | 1.4 | 38.4 | 7.9 | −0.2 | 0.8 | 0.521 |
| Total body | 30.7 | 6.7 | −0.2 | 0.8 | 36.8 | 7.8 | −0.2 | 0.6 | 0.617 |
| DXA lean mass (g) | |||||||||
| Trunk | 29,631 | 4,463 | −641 | 903 | 30,166 | 5,050 | 253 | 689 | 0.010 |
| Total body | 61,314 | 12,637 | −1,125 | 1,620 | 60,602 | 10,574 | 607 | 1,578 | 0.011 |
| DXA total mass (g) | |||||||||
| Trunk | 44,995 | 9,381 | −960 | 1,032 | 50,691 | 9,962 | 256 | 1,306 | 0.037 |
| Total body | 92,841 | 19,948 | −1,730 | 2,132 | 100,859 | 18,903 | 602 | 2,273 | 0.016 |
Week 8 change equals week 8 value minus baseline value.
P vales were based on ANCOVA comparing between group differences in week 8 changes controlling for baseline values.
BMI, body mass index; DXA, dual-energy X-ray absorptiometry; BMC, bone mineral content.
Psychopathology and side effect assessment
There were no significant differences between the two groups in week 8 changes for the PANSS total score (mean±SD, −5.6±8.8 in the aripiprazole group and −2.6±6.3 in the placebo group, P=0.660). There were no serious adverse events during the study. The side effects reported in more than 5% of the subjects in the aripiprazole group and that occurred at least twice as commonly as in the placebo group were over-arousal, drowsiness, itching, headache, chest pain, back pain, nasal congestion, hypersalivation, nausea, vomiting and stomach discomfort (Table 4). There were no significant differences between the two groups for listed side effects (P values > 0.1). No subject in the aripiprazole group withdrew from the study because of these side effects.
Table 4.
Side effect assessment*
| Aripiprazole (N=16) | Placebo (N=14) | p | |||
|---|---|---|---|---|---|
| Adverse event | N | % | N | % | |
| Over-arousal | 3 | 19 | 0 | 0 | 0.271 |
| Drowsiness | 4 | 25 | 1 | 7 | 0.413 |
| Itching | 2 | 13 | 0 | 0 | 0.527 |
| Headache | 3 | 19 | 1 | 7 | 0.689 |
| Chest pain | 3 | 19 | 1 | 7 | 0.689 |
| Back pain | 2 | 13 | 0 | 0 | 0.527 |
| Nasal congestion | 5 | 31 | 1 | 7 | 0.235 |
| Hypersalivation | 3 | 19 | 0 | 0 | 0.271 |
| Nausea | 4 | 25 | 1 | 7 | 0.413 |
| Vomiting | 3 | 19 | 0 | 0 | 0.271 |
| Stomach discomfort | 4 | 25 | 0 | 0 | 0.142 |
Occurred in more than 5% of the subjects taking aripiprazole and was at least twice as common as in the placebo group.
Repeated data analysis with Caucasian subjects only
As there was a between group difference at a trend level in race (four African Americans in the aripiprazole group and none in the placebo group; one in ‘other’ category in each group), group comparisons were repeated with Caucasians only (11 subjects in the aripiprazole group and 13 in the placebo group). The results were similar as reported previously (data not shown).
Discussion
Our results demonstrated that adjunctive aripiprazole treatment in clozapine-treated patients with schizophrenia improved certain aspects of glucose metabolism including insulin sensitivity and especially the effectiveness of insulin-independent glucose clearance rate (SG). In a previous study, we found that rosiglitazone, a thiazolidinedione used for diabetes treatment, also improved SG in clozapine-treated patients with schizophrenia (39). Although insulin resistance is the main determinant of impaired glucose tolerance, the contribution of SG to total glucose disposal becomes critical in the presence of insulin resistance; small changes in SG are likely to produce significant effects (40). In previous studies, SG has been shown to independently predict the development of diabetes (41–43). Increased SG might reflect not only an enhanced activity of the insulin-independent glucose transporters GLUT1 and GLUT2, but also an accelerated glucose-induced recruitment of the insulin-dependent GLLUT4 transporters (44, 45). Studies have shown that clozapine blocks glucose transporters in rat pheochromocytoma (PC12) cells (46). Our study suggests that perhaps this blocking effect could be counteracted by adjunctive aripiprazole therapy.
Aripiprazole treatment reduced plasma LDL levels and LDL particle numbers but there was no change in LDL particle size and no changes in other lipid measures. Dyslipidemia associated with insulin resistance includes hypertriglyceridemia, an increase in VLDL secretion from the liver, an increase in the atherogenic LDL and a decrease in HDL cholesterol (47–49). As patients become more insulin resistant, the LDL phenotype is characterized by smaller, denser LDL particles as opposed to the larger, more buoyant LDL particles. Small, dense LDL particles are associated with a significant increase in risk for coronary heart disease (50). LDL particle number has been suggested as a more reliable measure of atherogenicity than plasma LDL level, and an independent predictor of cardiovascular disease (51, 52). Because the cholesterol content per LDL particle exhibits large inter-individual variation, the information provided by plasma LDL level and the information from LDL particle number are not equivalent. Individuals with the same level of plasma LDL may have higher or lower numbers of LDL particles and, as a result, may differ in terms of absolute cardiovascular disease risk. Adjunctive aripiprazole therapy may reduce cardiovascular risk in clozapine treated patients with schizophrenia by reducing both LDL levels and LDL particle numbers.
Our findings suggested that the adjunctive aripiprazole treatment significantly reduced lean body mass and total body mass as measured by DXA. Even though the link between abdominal body fat and insulin resistance has been well established, studies have also suggested that lean body mass might contribute to the development of insulin resistance and metabolic disturbances. You et al. (53) reported that a higher lean body mass was associated with an increased risk for metabolic syndrome in postmenopausal women with visceral obesity. In another study, Brochu et al. (54) found that, also in postmenopausal women with visceral obesity, a higher lean body mass was associated with elevated levels of serum insulin and CRP, and decreased glucose disposal as measured by the euglycemic hyperinsulinemic clamp technique. Lean body mass reduction may decrease ectopic fat accumulation, which is associated with insulin resistance (55). Lean body mass reduction with adjunctive aripiprazole therapy might contribute to the metabolic improvement in clozapine-treated patients with schizophrenia.
The present study showed that the adjunctive aripiprazole treatment was well tolerated; no subjects who received aripiprazole withdrew from the study because of side effects. However, no definitive conclusions should be drawn with regard to tolerability and safety of using aripiprazole as an adjunct to clozapine treatment given the small sample size in the present study.
The strengths of this study include the rigorous study design and the use of state of the art, sensitive techniques including FSIVGTT, DXA and NMR spectroscopy to assess insulin sensitivity and glucose metabolism, body composition and lipid particle sizes, respectively. There were several limitations of this study: i) the sample size was relatively small and therefore the study might be underpowered; ii) the intervention time period (8 weeks) was relatively short; and iii) there might be a lack of generalizability of our findings to patients with schizophrenia treated by antipsychotic agents other than clozapine.
In summary, our study is the first to suggest that adjunctive aripiprazole therapy can improve glucose metabolism, decrease LDL particle numbers and reduce lean and total body mass in clozapine-treated patients with schizophrenia. The results from our study are generally consistent with findings from previous studies combining aripiprazole and clozapine with regard to potential metabolic benefits, but our results shed new light on how this combination strategy affects various specific metabolic parameters. The metabolic benefits of the combination therapy, if sustained, may reduce the risk of cardiovascular morbidity and mortality. Future studies to examine long-term effects of adjunctive aripiprazole therapy on metabolism and safety profile in a large sample of clozapine-treated patients with schizophrenia are needed.
Significant outcomes.
Our results demonstrated that adjunctive aripiprazole treatment in clozapine-treated patients with schizophrenia improved certain aspects of glucose metabolism including insulin sensitivity and especially the effectiveness of insulin-independent glucose clearance rate.
Aripiprazole treatment reduced plasma low-density lipoprotein levels and low-density lipoprotein particle numbers, but there was no change in low-density lipoprotein particle size and no changes in other lipid measures.
Our findings suggested that the adjunctive aripiprazole treatment significantly reduced lean body mass and total body mass as measured by whole-body dual-energy X-ray absorptiometry scan.
Limitations.
The sample size was relatively small; therefore, the study might be underpowered.
The intervention time period (8 weeks) of the study was relatively short.
There might be a lack of generalizability of our findings to patients with schizophrenia treated by antipsychotic agents other than clozapine.
Acknowledgments
This study was supported by Grant 5R01MH072635 from the National Institutes of Health (Dr. Henderson), Grant M01-RR-01066 and Grant UL1 RR025758-01 from the National Institutes of Health, National Center for Research Resources General Clinical Research Centers Program (MGH Clinical research Center).
Footnotes
Declaration of interest
Dr. Fan has received research support or honoraria from Eli Lilly, AstraZeneca, Bristol-Myer-Squibb, Janssen, and Pfizer. Dr. Freudenreich has received research support or honoraria from AstraZeneca, Bristol-Myer-Squibb, Janssen, Eli Lilly, Cephalon and Pfizer. Dr. Goff has received research support or honoraria from Bristol-Myers Squibb, Indevus Pharmaceuticals, H. Lundbeck, Schering-Plough, Eli Lilly, Takeda, Biovail, Sovay, Hoffman-La Roche, Cypress, Dainippon Sumitomo, Abbott Laboratories, Genentech, Pfizer, Janssen, Novartis, PamLab, and GlaxoSmithKline and Endo Pharmaceuticals. He served on a DSMB for Otsuka. Dr. Henderson has received research support or honoraria from Takeda, Janssen, Solvay, Novartis, Covance, Alkermis, and Pfizer. Dr. Borba, Dr. Copeland and Mr. Hayden report no competing interests.
References
- 1.GOFF DC, CATHER C, EVINS AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–194. doi: 10.4088/jcp.v66n0205. [DOI] [PubMed] [Google Scholar]
- 2.MASAND PS, CULPEPPER L, HENDERSON D, et al. Metabolic and endocrine disturbances in psychiatric disorders: a multidisciplinary approach to appropriate atypical antipsychotic utilization. CNS Spectr. 2005;10(suppl14):1–15. [PubMed] [Google Scholar]
- 3.MCEVOY JP, LIEBERMAN JA, STROUP TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American J of Psychiatry. 2006;163:600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- 4.KERWIN RW, BOLONNA AA. Is clozapine antisuicidal? Expert Review of Neurotherapeutics. 2004;4:187–190. doi: 10.1586/14737175.4.2.187. [DOI] [PubMed] [Google Scholar]
- 5.NIELSEN J, DAMKIER P, LUBLIN H, TAYLOR D. Optimizing clozapine treatment. Acta Psychiatrica Scandinavica. 2011;123:411–422. doi: 10.1111/j.1600-0447.2011.01710.x. [DOI] [PubMed] [Google Scholar]
- 6.UMBRICHT DS, POLLACK S, KANE JM. Clozapine and weight gain. J Clin Psychiatry. 1994;55(Suppl B):157–160. [PubMed] [Google Scholar]
- 7.OLFSON M, MARCUS SC, COREY-LISLE P, TUOMARI AV, HINES P, L’ITALIEN GJ. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–1825. doi: 10.1176/ajp.2006.163.10.1821. [DOI] [PubMed] [Google Scholar]
- 8.HENDERSON DC, DALEY TB, KUNKEL L, RODRIGUES-SCOTT M, KOUL P, HAYDEN D. Clozapine and hypertension: a chart review of 82 patients. J Clin Psychiatry. 2004;65:686–689. doi: 10.4088/jcp.v65n0514. [DOI] [PubMed] [Google Scholar]
- 9.HENDERSON DC, CAGLIERO E, COPELAND PM, et al. Elevated hemoglobin A1c as a possible indicator of diabetes mellitus and diabetic ketoacidosis in schizophrenia patients receiving atypical antipsychotics. J Clin Psychiatry. 2007;68:533–541. doi: 10.4088/jcp.v68n0407. [DOI] [PubMed] [Google Scholar]
- 10.HENDERSON DC, NGUYEN DD, COPELAND PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–1121. doi: 10.4088/jcp.v66n0905. [DOI] [PubMed] [Google Scholar]
- 11.NASRALLAH HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Molecular Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- 12.KROEZE WK, HUFEISEN SJ, POPADAK BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 13.KIM SF, HUANG AS, SNOWMAN AM, TEUSCHER C, SNYDER SH. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proceedings of the National Academy of Sciences. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MATSUI-SAKATA A, OHTANI H, SAWADA Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metabolism and Pharmacokinetics. 2005;20:368–378. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- 15.NONOGAKI K, STRACK AM, DALLMAN MF, TECOTT LHCINNMO, PMID Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nature medicine. 1998 Oct;4:1152–6. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 16.HERRICK-DAVIS K, GRINDE E, TEITLER M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. The Journal of pharmacology and experimental therapeutics. 2000 Oct;295:226–32. [PubMed] [Google Scholar]
- 17.ZHANG JY, KOWAL DM, NAWOSCHIK SP, LOU Z, DUNLOP J. Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms. Biochemical pharmacology. 2006 Feb 14;71:521–9. doi: 10.1016/j.bcp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.BOLONNA AA, KERWIN RWCINBJPA, PMID Partial agonism and schizophrenia. The British journal of psychiatry: the journal of mental science. 2005 Jan;186:7–10. doi: 10.1192/bjp.186.1.7. [DOI] [PubMed] [Google Scholar]
- 19.UVNAS-MOBERG K, AHLENIUS S, ALSTER P, HILLEGAART V. Effects of selective serotonin and dopamine agonists on plasma levels of glucose, insulin and glucagon in the rat. Neuroendocrinology. 1996;63:269–274. doi: 10.1159/000126970. [DOI] [PubMed] [Google Scholar]
- 20.WOZNIAK KM, LINNOILA M. Hyperglycemic properties of serotonin receptor antagonists. Life Sciences. 1991;49:101–109. doi: 10.1016/0024-3205(91)90023-5. [DOI] [PubMed] [Google Scholar]
- 21.MCQUADE RD, STOCK E, MARCUS R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry. 2004;65(Suppl18):47–56. [PubMed] [Google Scholar]
- 22.HENDERSON DC, KUNKEL L, NGUYEN DD, et al. An exploratory open-label trial of aripiprazole as an adjuvant to clozapine therapy in chronic schizophrenia. Acta Psychiatr Scand. 2006;113:142–147. doi: 10.1111/j.1600-0447.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 23.CHANG JS, AHN YM, PARK HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:720–731. doi: 10.4088/jcp.v69n0505. [DOI] [PubMed] [Google Scholar]
- 24.FLEISCHHACKER WW, HEIKKINEN ME, OLIE JP, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13:1115–1125. doi: 10.1017/S1461145710000490. [DOI] [PubMed] [Google Scholar]
- 25.REAVEN GM, CHEN YD, JEPPESEN J, MAHEUX P, KRAUSS RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993;92:141–146. doi: 10.1172/JCI116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HENDERSON DC, CAGLIERO E, COPELAND PM, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 27.OTVOS JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clinical Laboratory. 2002;48:171–180. [PubMed] [Google Scholar]
- 28.GLICKMAN SG, MARN CS, SUPIANO MA, DENGEL DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Applied Physiology. 2004;97:509–514. doi: 10.1152/japplphysiol.01234.2003. [DOI] [PubMed] [Google Scholar]
- 29.SPITZER RL, WILLIAMS JB, GIBBON M, FIRST MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 30.VALDES CT, ELKIND-HIRSCH KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72:642–646. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 31.KAY SR, OPLER LA, LINDENMAYER JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989;7:59–67. [PubMed] [Google Scholar]
- 32.LEVINE J, SCHOOLER NR. SAFTEE: A technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- 33.SIMPSON GM, ANGUS JW. A rating scale for extrapyramidal side effects. Acta psychiatry Scand Supplementum. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 34.MUNETZ MR, BENJAMIN S. How to examine patients using the Abnormal Involuntary Movement Scale. Hospital & Community Psychiatry. 1988;39:1172–1177. doi: 10.1176/ps.39.11.1172. [DOI] [PubMed] [Google Scholar]
- 35.CHEN M, BERGMAN RN, PORTE D., JR Insulin resistance and beta-cell dysfunction in aging: the importance of dietary carbohydrate. J Clin Endocrinol Metab. 1988;67:951–957. doi: 10.1210/jcem-67-5-951. [DOI] [PubMed] [Google Scholar]
- 36.BERGMAN RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 37.KAHN SE, PRIGEON RL, MCCULLOCH DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 38.HERMANS MP, LEVY JC, MORRIS RJ, TURNER RC. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia. 1999;42:678–687. doi: 10.1007/s001250051215. [DOI] [PubMed] [Google Scholar]
- 39.HENDERSON DC, FAN X, SHARMA B, et al. A double-blind, placebo-controlled trial of rosiglitazone for clozapine-induced glucose metabolism impairment in patients with schizophrenia. Acta psychiatr Scand. 2009;119:457–465. doi: 10.1111/j.1600-0447.2008.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.BEST JD, KAHN SE, ADER M, WATANABE RM, NI TC, BERGMAN RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19:1018–1030. doi: 10.2337/diacare.19.9.1018. [DOI] [PubMed] [Google Scholar]
- 41.GOLDFINE AB, BOUCHE C, PARKER RA, et al. Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc Natl Acad Sci USA. 2003;100:2724–2729. doi: 10.1073/pnas.0438009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.OSEI K, RHINESMITH S, GAILLARD T, SCHUSTER D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care. 2004;27:1439–1446. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 43.LORENZO C, WAGENKNECHT LE, REWERS MJ, et al. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:2098–2103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CARRUTHERS A. Facilitated diffusion of glucose. Physiol Rev. 1990;70:1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- 45.NOLTE LA, RINCON J, WAHLSTROM EO, CRAIG BW, ZIERATH JR, WALLBERG-HENRIKSSON H. Hyperglycemia activates glucose transport in rat skeletal muscle via a Ca(2+)-dependent mechanism. Diabetes. 1995;44:1345–1348. doi: 10.2337/diab.44.11.1345. [DOI] [PubMed] [Google Scholar]
- 46.ARDIZZONE TD, BRADLEY RJ, FREEMAN AM, 3RD, DWYER DS. Inhibition of glucose transport in PC12 cells by the atypical antipsychotic drugs risperidone and clozapine, and structural analogs of clozapine. Brain Research. 2001;923:82–90. doi: 10.1016/s0006-8993(01)03026-8. [DOI] [PubMed] [Google Scholar]
- 47.GARG A. Insulin resistance in the pathogenesis of dyslipidemia. Diabetes Care. 1996;19:387–389. doi: 10.2337/diacare.19.4.387. [DOI] [PubMed] [Google Scholar]
- 48.KARHAPAA P, MALKKI M, LAAKSO M. Isolated low HDL cholesterol. An insulin-resistant state. Diabetes. 1994;43:411–417. doi: 10.2337/diab.43.3.411. [DOI] [PubMed] [Google Scholar]
- 49.FAN X, LIU EY, HOFFMAN VP, POTTS AJ, SHARMA B, HENDERSON DC. Triglyceride/high-density lipoprotein cholesterol ratio: a surrogate to predict insulin resistance and low-density lipoprotein cholesterol particle size in nondiabetic patients with schizophrenia. J Clin Psychiatry. 2011;72:806–812. doi: 10.4088/JCP.09m05107yel. [DOI] [PubMed] [Google Scholar]
- 50.WATSON KE, HOROWITZ BN, MATSON G. Lipid abnormalities in insulin resistant states. Rev Cardiovasc Med. 2003;4:228–236. [PubMed] [Google Scholar]
- 51.SUPERKO HR, GADESAM RR. Is it LDL particle size or number that correlates with risk for cardiovascular disease? Curr Atheroscler Rep. 2008;10:377–385. doi: 10.1007/s11883-008-0059-2. [DOI] [PubMed] [Google Scholar]
- 52.CROMWELL WC, OTVOS JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6:381–387. doi: 10.1007/s11883-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 53.YOU T, RYAN AS, NICKLAS BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 54.BROCHU M, MATHIEU ME, KARELIS AD, et al. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity. 2008;16:1085–1093. doi: 10.1038/oby.2008.23. [DOI] [PubMed] [Google Scholar]
- 55.SINHA R, DUFOUR S, PETERSEN KF, et al. Assessment of skeletal muscle triglyceride content by nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]


