Abstract
Chronic post-thoracotomy pain syndrome (PTPS) can cause significant patient distress and is frequently difficult to manage. Percutaneous intercostal nerve (ICN) cryoablation by palpation of surface landmarks can be risky, as inaccurate probe placement can lead to hemo- or pneumothorax. Experience with image-guided ICN cryoablation with treatment planning and device navigation is limited. A patient with intractable PTPS was treated with ICN cryoablation under cone-beam computed tomography (CBCT) guidance with software-assisted needle trajectory planning and ablation zone simulation. This procedure provided the patient approximately 8 weeks of relief. This case demonstrated that ICN cryoablation is feasible under image-guidance with device navigation and ablation simulation, and may result in a few months of pain relief in cases of intractable PTPS.
Introduction
Chronic post-thoracotomy pain syndrome (PTPS) affects more than 50% of individuals following thoracic surgery and is difficult to prevent and manage [1]. A significant contributor to PTPS is intercostal nerve (ICN) trauma from incision, retraction/instrument placement, or suturing [1]. ICN cryoablation, which causes localized freezing to nerves [2], may provide long-term therapeutic relief. In one study, 60% of patients experienced immediate pain relief from cryoablation, and 50% continued to report significant pain relief at 3 month follow up [3]. When compared to other ablation modalities, like radiofrequency ablation, cryoablation may be better tolerated with decreased need for anesthetics during the procedure [4]. Furthermore, cryoablation is less likely to cause neuroma formation than surgical or thermal nerve ablations since it does not damage the perineurium or epineurium [5]. While needle placement for ICN ablation is conventionally estimated by palpation of bony landmarks, recent success of cryoanalgesia under ultrasound [6] and CT [7] guidance has raised interest for imaged-guided ICN cryoanalgesia for PTPS. Nevertheless, experience in device navigation and ablation simulation in combination with image-guidance is limited in the field of cryoanalgesia. The presented case describes the use of ICN cryoablation via cone beam computed tomography (CBCT) guidance, device navigation, and CBCT treatment planning and simulation software to manage a case of PTPS after thoracotomy and pleurectomy.
Case Report
A 51-year-old woman with a history of metastatic mesothelioma, who underwent a thoracotomy and pleurectomy, complained of chronic moderate to severe pain that radiated from her right mid axilla, ribs and flank to her anterior chest and abdomen. Her pain was transiently responsive to myofascial release with botulism toxin and local anesthesia. An ICN nerve block with ethanol provided 8 months of pain relief. The patient continued to have recurrent pain refractory to oral medications, acupuncture and massage therapy. The pain was described as sharp and constant and at a level of 7-8/10. She reported that the severity of the pain greatly impacted her quality of life and activities of daily living.
Cryoablation of T6 ICN was subsequently performed under CBCT guidance with device planning and navigation and ablation zone simulation. A 5-minute freeze of the T6 right mid-thoracic ICN was followed with a thaw and another 2-minute freeze (Endocare cryoablation system with a 15mm active tip) (Endocare; HealthTronics, Austin, Texas) [8]. Given ICN's close proximity to the ribs, freeze time was shortened to minimize risk for bone necrosis and other untoward effects of freezing a large volume. Total fluoroscopy time was 1.2 minutes.
The patient reported approximately 8 weeks of pain relief. She is currently being treated by with multiple oral medications and is planning for radiofrequency ablation at an outside institution.
Discussion
PTPS is notoriously difficult to manage, as demonstrated by this patient who had failed oral medications, myofascial release with botulism toxin, local anesthesia, and ethanol injection of intercostal nerves. Cryoanalgesia offers an alternative therapy for patients who have failed other treatments. During cryoanalgesia, nerves thought responsible for pain conduction are frozen to -60°C, damaging the myelin sheath and thereby interrupting nerve conduction. Because axons are not damaged, functional recovery of the nerve frequently occurs after the myelin sheath has regenerated, theoretically permitting recovery of muscular functions [2]. Percutaneous cryoablation for pain palliation has been performed in patients with neoplastic and metastatic disease in multiple organs, such as bone, kidney and prostate [2]. Cryoablation has also been used for patients with trigeminal [9] and genitofemoral neuralgia [5]. ICN cryoablation in particular has been described in randomized trials that compared surgical cryoanalgesia to narcotics during surgery or shortly after. These studies suggested that cryoanalgesia provided no less pain relief than narcotics, if not marginally more [2].
Percutaneous ICN cryoablation is comparatively less invasive than traditional surgical cryoablation, which dissects the nerve from pleura prior to ablation. However, it is typically guided by palpation of bony landmarks without direct visualization of the target anatomy [6]. Complications associated with this procedure (e.g. bleeding/hemothorax or pneumothorax) are related to needle placement and can be catastrophic. For example, the incidence of pneumothorax with percutaneous ICN cryoablation is about 7% [6].
Image guidance may reduce complications associated with cryoanalgesia of ICN. Cryoanalgesia therapy has been recently performed under ultrasound [6] and CT guidance [7]. While ultrasound may rapidly detect pneumothorax [6], iceball formation is better visualized on CT and CBCT [10]. CBCT procedures tend to use less radiation compared to CT procedures [11], and does not restrict the physical working space because of its rotating C-arm. When CBCT is equipped with 3D fluoroscopy (Allura FD20 angiography interventional system, Philips Healthcare, Best, Netherlands), real-time needle position on fluoroscopy is superimposed over intra-procedural CBCT images [12]. Continuous monitoring of needle position can potentially improve accuracy and prevent unintentional injury of adjacent vital structures (e.g. lung parenchyma, vessels). This combination of CBCT/fluoroscopy needle guidance has a comparable accuracy to that of CT/fluoroscopy [12]. In this patient, cryoablation of ICN was performed by CBCT with fluoroscopic guidance and CBCT-based navigation and ablation simulation.
The CBCT-navigation software (XperGuide, Philips Healthcare, Best, Netherlands) assists the physician by planning the needle trajectory and monitoring needle advancement (Figure 1). It automatically displays the planned trajectory in relation to the real-time needle location in two orthogonal perspectives. The “progress view” displays the viewpoint lateral to the needle (Figure 2); the “entry point view” displays the viewpoint down the shaft of the needle (Figure 3). Simultaneous display of both orthogonal perspectives provides the physician three-dimensional real-time feedback of needle position.
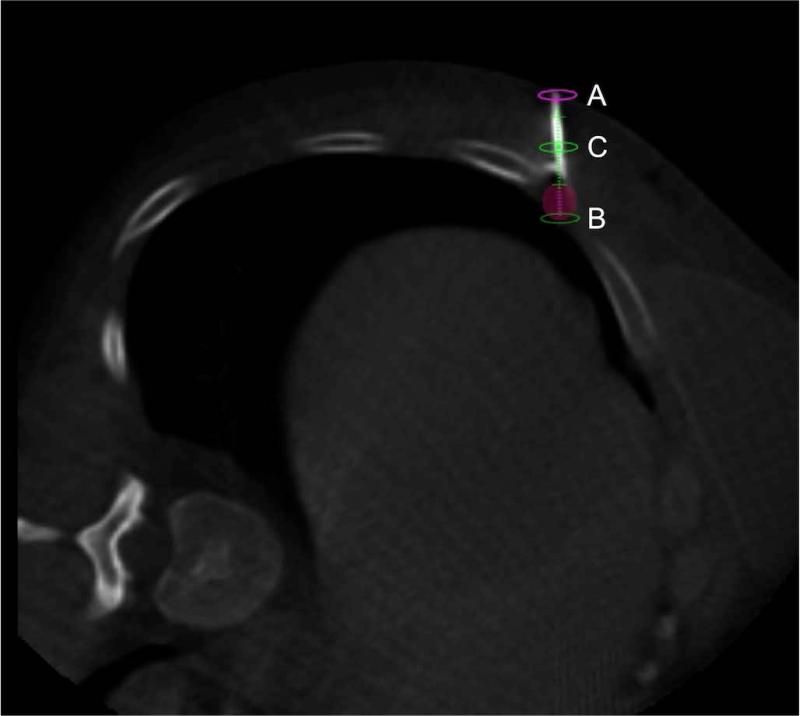
Figure 1.
Axial image of procedural cone-beam CT scan showing the actual needle with superimposed planned virtual trajectory (green line) and superimposed treatment zone (shaded pink sphere). Skin entry point (pink ellipse, A), planned target (green ellipse, B), and planned needle position as it traverses through this axial slice (green ellipse, C) are also displayed.
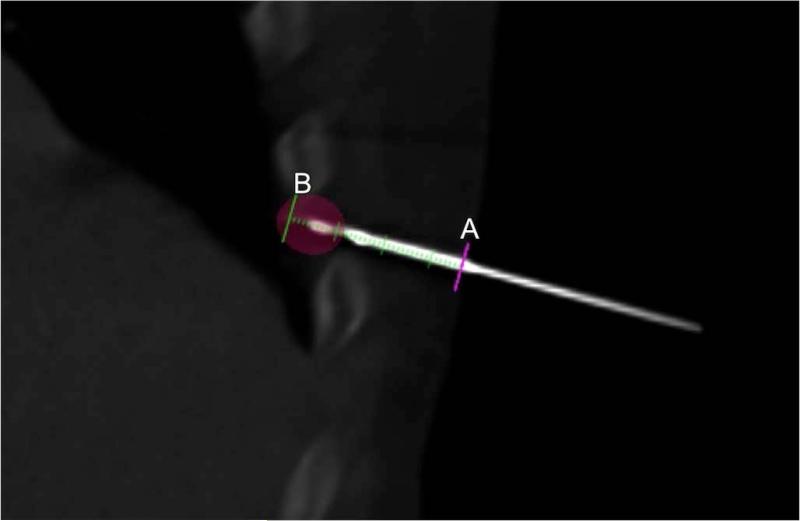
Figure 2.
Oblique multiplanar reconstruction cone-beam CT shows “progress view” of the needle along the superimposed planned trajectory (green line with markings). The planned trajectory displays the skin entry site (A), the planned needle tip target (B), and the distance along the planned trajectory via millimeter demarcations. Ablation zone (pink shaded ellipse) for planned -40°C isotherm (13 mm diameter, 15 mm length) is superimposed.
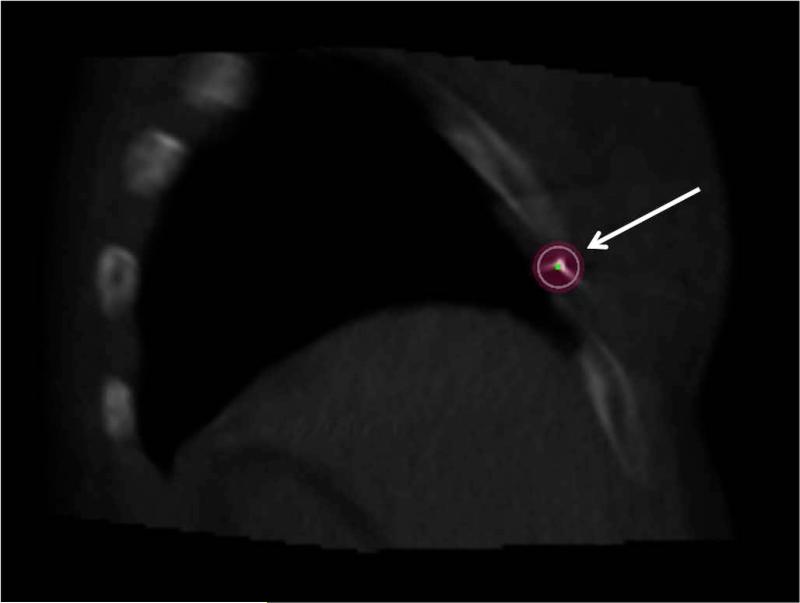
Figure 3.
Procedural reconstructed cone-beam CT shows “entry point view,” which is the view down the needle shaft toward the nerve target. Expected ablation zone (arrow) for -40°C isotherm (13 mm diameter) is superimposed over the needle.
Accurate prediction of the ablation zone coverage, which varies by tissue, needle type, needle trajectory and ablation time can potentially facilitate complete nerve ablation with minimal collateral damage. Conventional method requires the physician to imagine the ablation zone. This process depends upon the physician's spatial awareness and is inevitably prone to human error. The CBCT ablation simulation and treatment planning software can use needle-specific ablation parameters, and superimpose estimated ablation coverage over CBCT scans (Figures 1-3). It can also use post-procedural CBCT scans to compare the formed ice ball to the planned ablation zone [13,14]. In the past, this software has been successfully used to facilitate solid tumor ablations [14], but it is relatively novel in cryoanalgesia. Simultaneous fluoroscopy with real-time needle navigation and ablation simulation may have improved cryoanalgesia success in this case.
Cryoablation of the sixth ICN provided pain relief for this patient for 8 weeks. Currently, there is no consensus on the expected duration of pain relief from cryoablation of ICNs because the pathophysiology of neuropathic pain recurrence remains poorly understood and frequently involves a combination of multiple mechanisms [15]. The duration of cryoanalgesia's effectiveness may in part be explained by regeneration of myelin sheath that leads to continued nerve conduction [2]. Additionally, had cryoablation terminated the nerve axons, aberrant regeneration of pain fibers may still lead to persistent pain [15]. Furthermore, the pain may return as the disease progresses to the tissue outside of the area covered by the cryoablative treatment. This case of PTPS also differs from historical randomized trials involving cryoanalgesia, in that this therapy was performed years after, instead of during or days after, thoracotomy.
In conclusion, cryoanalgesia under image-guidance may benefit intractable PTPS for a few months. This case demonstrated the feasibility of using a combination of fused CBCT/fluoroscopy, device navigation and ablation simulation for ICN cryoablation.
Acknowledgments
Grant # ZIA BC 011242-03CCRO
The authors wish to thank Dr. Nadine Abi-Jaoudeh for contributing the images used in this publication.
This research was supported by the NIH Intramural Research Program, the NIH Center for Interventional Oncology, and through a Cooperative Research and Development Agreement (CRADA) between the NIH Center for Interventional Oncology and Philips Healthcare (Best, Netherlands). NIH may have intellectual property in the area. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The year-long research fellowship for Y.K. was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of Interest Disclosure Statement:
YK: The year-long research fellowship for Y.K. was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
AJM: No conflicts of interest.
BJW: This research was supported by the NIH Intramural Research Program, the NIH Center for Interventional Oncology, and through a Cooperative Research and Development Agreement (CRADA) between the NIH Center for Interventional Oncology and Philips Healthcare (Best, Netherlands). NIH may have intellectual property in the area. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006 Mar;104(3):594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Detterbeck FC. Efficacy of Methods of Intercostal Nerve Blockade for Pain Relief After Thoracotomy. The Annals of Thoracic Surgery. 2005 Oct;80(4):1550–9. doi: 10.1016/j.athoracsur.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 3.Green CR, de Rosayro AM, Tait AR. The role of cryoanalgesia for chronic thoracic pain: results of a long-term follow up. J Natl Med Assoc. 2002 Aug;94(8):716–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain Control Requirements for Percutaneous Ablation of Renal Tumors: Cryoablation versus Radiofrequency Ablation--Initial Observations. Radiology. 2005 Aug 26;237(1):366–70. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- 5.Campos NA, Chiles JH, Plunkett AR. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician. 2009 Nov;12(6):997–1000. [PubMed] [Google Scholar]
- 6.Byas-Smith MG, Gulati A. Ultrasound-Guided Intercostal Nerve Cryoablation. Anesthesia & Analgesia. 2006 Oct;103(4):1033–5. doi: 10.1213/01.ane.0000237290.68166.c2. [DOI] [PubMed] [Google Scholar]
- 7.Dar SA, Love Z, Prologo JD, Hsu DP. CT-guided cryoablation for palliation of secondary trigeminal neuralgia from head and neck malignancy. Journal of NeuroInterventional Surgery. 2012 Mar 30; doi: 10.1136/neurintsurg-2012-010265. [DOI] [PubMed] [Google Scholar]
- 8.Moore W, Kolnick D, Tan J, Yu HS. CT Guided Percutaneous Cryoneurolysis for Post Thoracotomy Pain Syndrome. Academic Radiology. Elsevier Ltd. 2010 May 1;17(5):603–6. doi: 10.1016/j.acra.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Dar SA, Love Z, Prologo JD, Hsu DP. CT-guided cryoablation for palliation of secondary trigeminal neuralgia from head and neck malignancy. Journal of NeuroInterventional Surgery. 2012 Mar. doi: 10.1136/neurintsurg-2012-010265. [DOI] [PubMed] [Google Scholar]
- 10.Ahrar K, Littrup PJ. Is Cryotherapy the Optimal Technology for Ablation of Lung Tumors? J Vasc Interv Radiol. Elsevier Inc. 2012 Mar 1;23(3):303–5. doi: 10.1016/j.jvir.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 11.FRCS IH, FRCS DE, FRCS OM, FRCS VA. Video-assisted intercostal nerve cryoablation in managing intractable chest wall pain. The Journal of Thoracic and Cardiovascular Surgery. The American Association for Thoracic Surgery. 2010 Mar 1;139(3):774–5. doi: 10.1016/j.jtcvs.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Braak SJ, van Strijen MJL, van Leersum M, van Es HW, van Heesewijk JPM. Real-Time 3D Fluoroscopy Guidance During Needle Interventions: Technique, Accuracy, and Feasibility. American Journal of Roentgenology. 2010 Apr 21;194(5):W445–51. doi: 10.2214/AJR.09.3647. [DOI] [PubMed] [Google Scholar]
- 13.Abi-Jaoudeh N, Kruecker J, Kadoury S, Kobeiter H, Venkatesan AM, Levy E, Wood BJ. Multimodality Image Fusion–Guided Procedures: Technique, Accuracy, and Applications. Cardiovasc Intervent Radiol. 2012 Aug 1; doi: 10.1007/s00270-012-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abi-Jaoudeh N, Mielekamp P, Noordhoek N, Venkatesan AM, Millo C, Radaelli A, Carelsen B, Wood BJ. Cone-Beam Computed Tomography Fusion and Navigation for Real-Time Positron Emission Tomography–guided Biopsies and Ablations: A Feasibility Study. J Vasc Interv Radiol. Elsevier Inc. 2012 Jun 1;23(6):737–43. doi: 10.1016/j.jvir.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. The Lancet Neurology. Elsevier Ltd. 2010 Aug 1;9(8):807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]